Abstract
Cholesterol-rich microdomains (also called lipid rafts), where platforms for signaling are provided and thought to be associated with microbe-induced pathogenesis and lead to cancer progression. After treatment of cells with cholesterol disrupting or usurping agents, raft-associated proteins and lipids can be dissociated, and this renders the cell structure nonfunctional and therefore mitigates disease severity. This review focuses on the role of cholesterol in disease progression including cancer development and infectious diseases. Understanding the molecular mechanisms of cholesterol in these diseases may provide insight into the development of novel strategies for controlling these diseases in clinical scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Metabolism of cholesterol
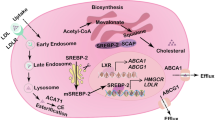
1.1. Biosynthesis of cholesterol
Cholesterol is an extremely important biological molecule as it is a precursor for the synthesis of steroid hormones, bile acids, and vitamin D [1]. The human body manufactures around 1 g of cholesterol each day and approximately 20-25% of total daily cholesterol production occurs in the liver [2]. Synthesis of cholesterol is a series process and starts with acetyl CoA and acetoacetyl-CoA, which are hydrated to form 3-hydroxy-3- methylglutaryl CoA (HMG-CoA). This molecule is subsequently reduced to mevalonate by the enzyme HMG-CoA reductase [3]. This is the regulated, rate-limiting, and irreversible step in cholesterol biosynthesis and is the target of action for statin drugs (HMGCoA reductase competitive inhibitors) [4].
1.2. Association of abnormal cholesterol levels with diseases
Both dietary cholesterol and synthesized de novo are transported by lipoprotein particles through the circulatory system. The four major types of lipoproteins are chylomicron, very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and highdensity lipoprotein (HDL). Chylomicrons and VLDL deliver triacylglycerol to cells in the body, whereas LDL delivers cholesterol to cells in the body. Meanwhile, HDL is involved in reverse cholesterol transport. The synthesis and utilization of cholesterol must be tightly regulated in order to prevent over-accumulation and abnormal depositing within the body. There are two manifestations of cholesterol disorders, hyperlipidemia and hypolipidemia. The reasons for cholesterol disorders include dietary issues, genetic disorders, and other diseases [5-7]. For example, due to a genetic disorder caused by a defect on chromosome 19, cholesterol continues to be produced despite there already being an excess of cholesterol in the blood (lack of uptake by LDL receptor), and this may cause familial hypercholesterolemia [8]. In contrast, hypo-cholesterol level may result from liver disease, hypothyroidism, and genetic disorders such as familial hypobetalipoproteinemia and Smith-Lemli-Opitz syndrome (7-dehydrocholesterol reductase deficiency) [9].
The level of cholesterol in the body being too high or too low may cause varies symptoms, syndromes, or diseases. Excessive cholesterol is associated with several cardiovascular diseases and such levels are easily attained with an unhealthy diet. In fact, it should be noted that it is not essential for cholesterol to be obtained from one’s diet as it is easily synthesized in the body. Whereas, low cholesterol is associated with mental disorders, neuropsychiatric diseases, and mortality in elderly [10]. Some critical diseases related to cholesterol levels are listed in Table 1.
1.3. The cholesterol lowering agents
The most important drugs for the treatment of dyslipidemia are statins which have been shown in multiple clinical trials to reduce cardiovascular events and mortality [25]. Statins can inhibit HMG-CoA reductase and design to subsequently inhibit enzyme activity in the liver [26]. Inhibition of cholesterol synthesis further decreases circulating LDL, which reduces levels of cholesterol in the hepatocyte and therefore lead to up-regulated expressions of LDL receptors. Some other drugs have been developed to treat dyslipidemia in specific subsets of patients. For instance, fibrates, which bind to the nuclear receptor PPAR-alpha, can increase HDL levels and decrease triglyceride levels [27]. Fibrates were originally used to address the primary problem of high levels of triglycerides. Another example is niacin (nicotinic acid), which increases HDL levels and decreases triglyceride and LDL levels at high doses (much higher than required for its role as a vitamin) [28, 29]. And there is ezetimibe, which inhibits cholesterol absorption in the small intestine and effectively lowers LDL cholesterol [30].
2. Role of cholesterol in cancer progression
2.1. Cholesterol and cancer development
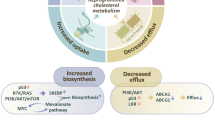
Cholesterol is known as a main component of lipid rafts and has been documented to regulate cell membrane proteins, receptor trafficking, signal transduction, as well as influence cell membrane fluidity [31]. Moreover, cholesterol and other lipid-components participate in the production of hormones [32] and energy [33]. However, when large concentrations of cholesterol accumulate in the human body, especially in the organs and blood stream, the risk of various diseases increases (Table 2). Notably, studies have revealed that an increased cholesterol level participates in cancer cell malignancy, and the dysfunction of cholesterol metabolism may also influence cancer progression [34-36]. For example, mevalonate, a cholesterol synthesis precursor, promotes breast cancer cell proliferation in vivo and in vitro [37, 38]. Additionally, 27- hydroxycholesterol, which is a metabolite from cholesterol, is expressed much higher in the estrogen receptor-positive breast cancer patient site, when compared with both normal breast tissue and a patient’s cancer-free region control [39, 40]. In oral cancer, cholesterol was found to be significantly increased in tumor tissue compared to normal tissue [41]. Moreover, previous studies have reported that elevated cholesterol in the circulatory system promotes Akt signaling, decreases apoptosis activity in LNCap prostate cell line, and enhances tumor aggressiveness in a xenograft animal model [42, 43]. Further, it has been reported that serum cholesterol is a positive factor in colon cancer development [44, 45]. Other cancers, including female reproductive organ cancers, lung cancer, and melanoma are also documented to correlate with high levels of cholesterol [46].
2.2. Reducing cholesterol inhibits cancer progression
In addition to the correlation between cholesterol and cancer progression, disruption of cell membrane lipid rafts or cholesterol components and interference of cholesterol synthesis are considered as treating prospects toward cancer treatment [64, 65]. Therefore, clinical use of cholesterol-controlling medicines has been implied to possess chemoprotective effects [66]. Statins, HMG-CoA reductase inhibitors, are cholesterol-lowering agents [67], and the total consumption of statins has been increasing in recent years [46]. Statins are documented to decrease the proliferation of cancer cells [49, 63], reduce the risk of cancer incidence rate [61], and even influence the mortality rate in cancer patients [68]. However, the findings of statins use in the treatment of cancer have revealed inconsistencies. Some reports have even claimed that the use of statin may increase the risk of cancer [51, 57], or have no correlation in the treatment of cancer [50, 69]. Therefore, the benefits of the cholesterol-controlling aspect in the treatments of lipid rafts-related cancers, animal models, and the details of their underlying mechanisms may need further investigations.
Despite arising number of reports that support the claim that the use of statin significantly reduces the incidence of cancer, not all of the statistical results are consistent with such a claim [48, 70]. Research into cholesterol-related cancer progression and the use of cholesterol-lowering drugs are mostly of the database analysis variety. However, the results may differ according to participant sample selection, sample size, and related confounding factors. Therefore, additional studies with cellular or animal models, long-term vs. short-term statin users follow-up, and even studies consisting of large sample sizes with multiple confounders would help further elucidate this issue.
3. Association of cholesterol with pathogen infections
Cholesterol is the most important component of lipid rafts in eukaryotic cells. Lipid rafts are also considered a critical factor in host-pathogen interaction and colonization of hosts by several pathogens including bacteria, viruses, as well as prions. Most of the studies we refer to here describe a few examples of the role of cholesterol in promoting pathogenic infections (Table 3).
3.1. Lipid rafts serve as platforms for bacterial pathogens
In order to promote their internalization into host, bacterial pathogens may utilize host cells to enhance their own adherence and survival abilities [83, 84]. Adhesion to host cells by pathogens is the first step in their invasion process and may be associated with lipid rafts. The most commonly described cellular target of intestinal pathogens is Campylobacter jejuni, which attach to host epithelial cells via membrane cholesterol [85-87]. In addition, the major virulence factor expressed by C. jejuni is cytolethal distending toxin (CDT) [74], which also can be produced by various common Gram-negative bacteria, including Aggregatibacter actinomycetemcomitans [88], Escherichia coli [89], Haemophilus ducreyi [76], Helicobacter hepaticus [90], and Shigella dysenteriae [91]. It has been reported that C. jejuni CDT-induced pathogenesis of host cells is dependent on membrane cholesterol levels. By using cholesterol-depleting agents such as methylh-β-cyclodextrin (MβCD) which markedly decreased the intoxication of cells [74, 92]. Further evidence of the role of lipid rafts in both C. jejuni and A. actinomycetemcomitans CDT-induced genotoxicity of host cells have been demonstrated through the cholesterol recognition/interaction amino acid consensus (CRAC) region of the CdtC subunit [71, 75]. These findings indicate that membrane cholesterol provides an essential component for CDT binding to the cell membrane and also serves as a portal for CdtB delivery into host cells for the induction of cell intoxication. Moreover, in this case, the virulence protein cytotoxin-associated gene A (CagA) of Helicobacter pylori, is delivered into the target cells by the type IV secretion system [93] and utilizes membrane cholesterol to lead to the activation of pro-inflammatory signaling pathways within gastric cells [75, 78, 94, 95]. Furthermore, a dramatic demonstration of the dissociation of infectivity and pathology is H. pylori within encoding glucosyltransferase, which is indispensable for cholesterol glucosylation and promotes H. pylori-induced phagocytosis escape and subsequent immune responses [77, 96]. Similar to C. jejuni and H. pylori, the recent description of the combination of apoE-deficiency and a high cholesterol diet in mice facilitated Anaplasma phagocytophilum infection in vivo and induced proinflammatory responses [73]. However, not all pathogens require lipid rafts to gain entry into host cells. Recently, it has been shown that cholesterol-mediated invasions and intracellular replication are not required for Chlamydia trachomatis and Salmonella enterica serovar Typhimurium infection of mice embryonic fibroblasts (MEFs) [97]. Together, these examples illustrate that lipid rafts provide several advantages for bacteria, including virulence factors in modulating internalization and transport of extracellular proteins as well as signaling platforms.
3.2. Conversion of prions is associated with lipid rafts
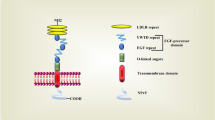
Neurodegenerative disorders caused by prions have been linked to the variant Creutzfeldt-Jakob Disease (vCJD) in humans [98]. The cellular prion protein (PrPC) is called a normal cell surface glycoprotein by means of a glycosylphosphatidylinositol (GPI)- anchor. GPI-anchored PrPC is presented in lipid rafts where are microdomains enriched in cholesterol [99]. It is widely known that PrPC is found in membrane cholesterol and plays a crucial role in the development of prion-related diseases by changing its conformation to a pathological isoform (PrPSc) [82]. PrPSc is an essential part of the prion, causing fatal and transmissible neurodegenerative prion diseases [82]. Several lines of evidence suggest that lipid rafts are highly essential for the transport of PrPC and the toxicity of PrPSc in neuronal cells [100, 101]. Altogether, these studies indicate the critical role of lipid rafts, which maintain the cell surface localization of GPI-anchor attachment of PrPC and are involved in prion conversion and neurotoxicity.
3.3. Lipid rafts facilitate virus infection
Human immunodeficiency virus (HIV) is the retrovirus that is well known to cause acquired immunodeficiency syndrome (AIDS) [102]. Previous clinical evidence indicated that the level of cholesterol may be a potential factor for controlling the spread or fusion of many viruses [103, 104] which are involved in HIV production and infectivity [81]. It has been reported that the negative effector (Nef) protein from HIV can enhance cholesterol uptake and biosynthesis by activating the transcription of the sterol-responsive element binding factor 2 (SREBF-2) and SREBF- 2-regulated genes [105]. In addition, the Nef inhibits the activity of the cellular cholesterol transporter ATP-binding cassette A1 (ABCA1) [106], which in response binds to cholesterol and delivers it to the lipid rafts. Conversely, reduction of cellular cholesterol by ABCA1 activation has been shown to potently inhibit HIV replication [107, 108]. Taken together, these results reveal that HIV requires cholesterol for its egress from and entry into cells.
4. Conclusions and perspectives
Cholesterol-enriched microdomains, which provide platforms for signaling, are thought to be associated with the development of various types of cancers. It has also been clear that the role of cholesterol in pathogen-host interactions contributes to further ensure the pathogens’ survival and virulence delivery into host. These findings indicate that an adequate regulation of cholesterol may prevent cancer progression as well as mitigate microbeinduced the pathogenesis of hosts. Fully unveiling the role of cholesterol in diseases’ manifestations may shed light on the possibility to develop a novel approach to the retardation or possible prevention of cancer development and the treatment of infectious diseases.
Acknowledgments
The authors would like to thank Dr. Ming-Chei Maa and Chang-Mei Lin for their valuable suggestions and editorial assistance. This work was funded by the Ministry of Science and Technology (103-2633-B-039-001 and 103-2991-I-005-507), China Medical University (CMU103-S-15 and CMU103-S-18), and the Tomorrow Medicine Foundation.
Declaration of interest
The authors declare no conflicts of interest for this work.
References
Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry 1992; 31: 4737–49.
Lewis GF. Determinants of plasma HDL concentrations and reverse cholesterol transport. Curr Opin Cardiol 2006; 21: 345–52.
Hampton R, Dimster-Denk D, Rine J. The biology of HMG-CoA reductase: the pros of contra-regulation. Trends Biochem Sci 1996; 21: 140–5.
Barrios-Gonzalez J, Miranda RU. Biotechnological production and applications of statins. Appl Microbiol Biotechnol 2010; 85: 869–83.
Porter FD, Herman GE. Malformation syndromes caused by disorders of cholesterol synthesis. J Lipid Res 2011; 52: 6–34.
Khosla P, Hayes KC. Dietary palmitic acid raises plasma LDL cholesterol relative to oleic acid only at a high intake of cholesterol. Biochim Biophys Acta 1993; 1210: 13–22.
Pollin TI, Quartuccio M. What We Know About Diet, Genes, and Dyslipidemia: Is There Potential for Translation? Curr Nutr Rep 2013; 2: 236–42.
Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol 2014; 7: 107–17.
Jira P. Cholesterol metabolism deficiency. Handb Clin Neurol 2013; 113: 1845–50.
Martinez-Carpio PA, Barba J, Bedoya-Del Campillo A. Relation between cholesterol levels and neuropsychiatric disorders. Rev Neurol 2009; 48: 261–4.
Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res 2014; 114: 205–13.
Lisak M, Demarin V, Trkanjec Z, Basic-Kes V. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat 2013; 52: 458–63.
Kratzer A, Giral H, Landmesser U. High-density lipoproteins as modulators of endothelial cell functions: alterations in patients with coronary artery disease. Cardiovasc Res 2014.
Sibley C, Stone NJ. Familial hypercholesterolemia: a challenge of diagnosis and therapy. Cleve Clin J Med 2006; 73: 57–64.
Moghadasian MH, Salen G, Frohlich JJ, Scudamore CH. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol 2002; 59: 527–29.
Puntoni M, Sbrana F, Bigazzi F, Sampietro T. Tangier disease: epidemiology, pathophysiology, and management. Am J Cardiovasc Drugs 2012; 12: 303–11.
Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, et al. Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J Neurosci 2005; 25: 9932–39.
Block RC, Dorsey ER, Beck CA, Brenna JT, Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. J Clin Lipidol 2010; 4: 17–23.
Rodriguez-Luna D, Rubiera M, Ribo M, Coscojuela P, Pagola J, Pineiro S, et al. Serum low-density lipoprotein cholesterol level predicts hematoma growth and clinical outcome after acute intracerebral hemorrhage. Stroke 2011; 42: 2447–52.
Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta-analysis. Stroke 2013; 44: 1833–9.
Ancelin ML, Carriere I, Boulenger JP, Malafosse A, Stewart R, Cristol JP, et al. Gender and genotype modulation of the association between lipid levels and depressive symptomatology in communitydwelling elderly (the ESPRIT study). Biol Psychiatry 2010; 68: 125–32.
Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav 2002; 32: 333–46.
Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Ann N Y Acad Sci 1997; 836: 288–301.
Duits N, Bos FM. Psychiatric disorders with use of simvastatin. Ned Tijdschr Geneeskd 1993; 137: 1312–5.
Koba S. Statin therapy for atherogenic hypertriglyceridemia. Nihon Rinsho 2013; 71: 1655–60.
Sirtori CR. The pharmacology of statins. Pharmacol Res 2014.
Remaley AT, Norata GD, Catapano AL. Novel concepts in HDL pharmacology. Cardiovasc Res 2014.
Ginsberg HN, Reyes-Soffer G. Niacin: a long history, but a questionable future. Curr Opin Lipidol 2013; 24: 475–9.
Song WL, FitzGerald GA. Niacin, an old drug with a new twist. J Lipid Res 2013; 54: 2586–94.
Sano M. An inhibitor of intestinal cholesterol transporter. Nihon Rinsho 2013; 71: 1661–6.
Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta 2003; 1610: 174–83.
Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev 1988; 9: 295–318.
Rui L. Energy metabolism in the liver. Compr Physiol 2014; 4: 177–97.
Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol- modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood 2003; 101: 3628–3634.
Cruz PM, Mo H, McConathy WJ, Sabnis N, Lacko AG. The role of cholesterol metabolism and cholesterol transport in carcinogenesis: a review of scientific findings, relevant to future cancer therapeutics. Front Pharmacol 2013; 4: 11–9.
Liu HH, Tsai YS, Lai CL, Tang CH, Lai CH, Wu HC, et al. Evolving personalized therapy for castration-resistant prostate cancer. Bio- Medicine 2013; 4: 7–15.
Duncan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem 2004; 279: 33079–84.
dos Santos CR, Domingues G, Matias I, Matos J, Fonseca I, de Almeida JM, et al. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis 2014; 13: 1–6.
Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013; 342: 1094–8.
Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep 2013; 5: 637–45.
Kolanjiappan K, Ramachandran CR, Manoharan S. Biochemical changes in tumor tissues of oral cancer patients. Clin Biochem 2003; 36: 61–5.
Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest 2005; 115: 959–68.
Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem 2004; 91: 54–69.
van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, et al. Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Gut 2011; 60: 1094–102.
Jacobs RJ, Voorneveld PW, Kodach LL, Hardwick JC. Cholesterol metabolism and colorectal cancers. Curr Opin Pharmacol 2012; 12: 690–5.
Boudreau DM, Yu O, Johnson J. Statin use and cancer risk: a comprehensive review. Expert Opin Drug Saf 2010; 9: 603–21.
Hu J, La Vecchia C, de Groh M, Negri E, Morrison H, Mery L. Dietary cholesterol intake and cancer. Ann Oncol 2012; 23: 491–500.
Goldstein MR, Mascitelli L, Pezzetta F. Do statins prevent or promote cancer? Curr Oncol 2008; 15: 76–7.
Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, et al. Breast cancer growth prevention by statins. Cancer Res 2006; 66: 8707–14.
Desai P, Chlebowski R, Cauley JA, Manson JE, Wu C, Martin LW, et al. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol Biomarkers Prev 2013; 22: 1868–76.
McDougall JA, Malone KE, Daling JR, Cushing-Haugen KL, Porter PL, Li CI. Long-term statin use and risk of ductal and lobular breast cancer among women 55 to 74 years of age. Cancer Epidemiol Biomarkers Prev 2013; 22: 1529–37.
Agnoli C, Grioni S, Sieri S, et al. Colorectal cancer risk and dyslipidemia: a case-cohort study nested in an Italian multicentre cohort. Cancer Epidemiol 2014; 38: 144–51.
Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol 2011; 29: 1592–8.
Melvin JC, Seth D, Holmberg L, Garmo H, Hammar N, Jungner I, et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev 2012; 21: 1381–4.
Saito N, Sairenchi T, Irie F, Iso H, Iimura K, Watanabe H, et al. Low serum LDL cholesterol levels are associated with elevated mortality from liver cancer in Japan: the Ibaraki Prefectural health study. Tohoku J Exp Med 2013; 229: 203–11.
Jagtap D, Rosenberg CA, Martin LW, Pettinger M, Khandekar J, Lane D, et al. Prospective analysis of association between use of statins and melanoma risk in the Women’s Health Initiative. Cancer 2012; 118: 5124–31.
Mascitelli L, Pezzetta F, Goldstein MR. The epidemic of nonmelanoma skin cancer and the widespread use of statins: Is there a connection? Dermatoendocrinol 2010; 2: 37–8.
Chawda JG, Jain SS, Patel HR, Chaduvula N, Patel K. The relationship between serum lipid levels and the risk of oral cancer. Indian J Med Paediatr Oncol 2011; 32: 34–37.
Lohe VK, Degwekar SS, Bhowate RR, Kadu RP, Dangore SB. Evaluation of correlation of serum lipid profile in patients with oral cancer and precancer and its association with tobacco abuse. J Oral Pathol Med 2010; 39: 141–8.
Srinivas GV, Namala S, Ananthaneni A, Puneeth HK, Devi BS. Evaluation and correlation of serum lipid profile in oral and gastrointestinal cancer patients. J Int Oral Health 2013; 5: 72–7.
Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev 2007; 16: 2213–7.
Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol 2012; 12: 751–9.
Yokomizo A, Shiota M, Kashiwagi E, Kuroiwa K, Tatsugami K, Inokuchi J, et al. Statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate 2011; 71: 298–304.
Silvente-Poirot S, Poirot M. Cholesterol metabolism and cancer: the good, the bad and the ugly. Curr Opin Pharmacol 2012; 12: 673–6.
Buchwald H. Cholesterol inhibition, cancer, and chemotherapy. Lancet 1992; 339: 1154–6.
Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005; 5: 930–42.
Clendening JW, Penn LZ. Targeting tumor cell metabolism with statins. Oncogene 2012; 31: 4967–78.
Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367: 1792–802.
Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010; 376: 1670–81.
Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res 2011; 71: 1763–71.
Boesze-Battaglia K, Besack D, McKay T, et al. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal-distending toxin. Cell Microbiol 2006; 8: 823–36.
Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, et al. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem 2009; 284: 10650–8.
Xiong Q, Wang X, Rikihisa Y. High-cholesterol diet facilitates Anaplasma phagocytophilum infection and up-regulates macrophage inflammatory protein-2 and CXCR2 expression in apolipoprotein Edeficient mice. J Infect Dis 2007; 195: 1497–503.
Lin CD, Lai CK, Lin YH, Hsieh JT, Sing YT, Chang YC, et al. Cholesterol depletion reduces entry of Campylobacter jejuni cytolethal distending toxin and attenuates intoxication of host cells. Infect Immun 2011; 79: 3563–75.
Lai CH, Lai CK, Lin YJ, Hung CL, Chu CH, Feng CL, et al. Characterization of putative cholesterol recognition/interaction amino acid consensus-like motif of Campylobacter jejuni cytolethal distending Toxin C. PLoS One 2013; 8: e6620–2.
Cope LD, Lumbley S, Latimer JL, Klesney-Tait J, Stevens MK, Johnson LS, et al. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci U S A 1997; 94: 4056–61.
Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, et al. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med 2006; 12: 1030–8.
Lai CH, Chang YC, Du SY, Wang HJ, Kuo CH, Fang SH, et al. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect Immun 2008; 76: 3293–303.
Lai CH, Hsu YM, Wang HJ, Wang WC. Manipulation of host cholesterol by Helicobacter pylori for their beneficial ecological niche. BioMedicine 2013; 3: 27–33.
Ricci V, Galmiche A, Doye A, Necchi V, Solcia E, Boquet P. High cell sensitivity to Helicobacter pylori VacA toxin depends on a GPIanchored protein and is not blocked by inhibition of the clathrinmediated pathway of endocytosis. Mol Biol Cell 2000; 11: 3897–909.
Cui HL, Grant A, Mukhamedova N, Pushkarsky T, Jennelle L, Dubrovsky L, et al. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J Lipid Res 2012; 53: 696–708.
Prusiner SB. Prions. Proceedings of the National Academy of Sciences of the United States of America 1998; 95: 13363–83.
Bhavsar AP, Guttman JA, Finlay BB. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007; 449: 827–34.
Duncan MJ, Shin JS, Abraham SN. Microbial entry through caveolae: variations on a theme. Cellular Microbiology 2002; 4: 783–91.
Wooldridge KG, Williams PH, Ketley JM. Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog 1996; 21: 299–305.
Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NF, et al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun 2012; 80: 4089–98.
Hu L, McDaniel JP, Kopecko DJ. Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81-176. Microb Pathog 2006; 40: 91–100.
Sugai M, Kawamoto T, Peres SY, Ueno Y, Komatsuzawa H, Fujiwara T, et al. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun 1998; 66: 5008–19.
Peres SY, Marches O, Daigle F, Nougayrede JP, Herault F, Tasca C, et al. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol 1997; 24: 1095–107.
Young VB, Knox KA, Schauer DB. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun 2000; 68: 184–91.
Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog 1995; 18: 167–72.
Lai CK, Su JC, Lin YH, Chang CS, Feng CL, Lin HJ, et al. Involvement of cholesterol in Campylobacter jejuni cytolethal distending toxin-induced pathogenesis. Future Microbiol 2015; 10: 489–501.
Ramarao N, Gray-Owen SD, Backert S, Meyer TF. Helicobacter pylori inhibits phagocytosis by professional phagocytes involving type IV secretion components. Mol Microbiol 2000; 37: 1389–404.
Lai CH, Wang HJ, Chang YC, Hsieh WC, Lin HJ, Tang CH, et al. Helicobacter pylori CagA-mediated IL-8 induction in gastric epithelial cells is cholesterol-dependent and requires the C-terminal tyrosine phosphorylation-containing domain. FEMS Microbiol Lett 2011; 323: 155–63.
Lu DY, Chen HC, Yang MS, Hsu YM, Lin HJ, Tang CH, et al. Ceramide and Toll-like receptor 4 are mobilized into membrane rafts in response to Helicobacter pylori infection in gastric epithelial cells. Infect Immun 2012; 80: 1823–33.
Du SY, Wang HJ, Cheng HH, Chen SD, Wang LH, Wang WC. Cholesterol glucosylation by Helicobacter pylori delays internalization and arrests phagosome maturation in macrophages. J Microbiol Immunol Infect 2014.
Gilk SD, Cockrell DC, Luterbach C, Hansen B, Knodler LA, Ibarra JA, et al. Bacterial colonization of host cells in the absence of cholesterol. PLoS Pathog 2013; 9: e1003107.
Biasini E, Turnbaugh JA, Unterberger U, Harris DA. Prion protein at the crossroads of physiology and disease. Trends in Neurosciences 2012; 35: 92–103.
Gilch S, Kehler C, Schatzl HM. The prion protein requires cholesterol for cell surface localization. Molecular and Cellular Neuroscience 2006; 31: 346–53.
Gilch S, Kehler C, Schatzl HM. The prion protein requires cholesterol for cell surface localization. Mol Cell Neurosci 2006; 31: 346–53.
Botto L, Cunati D, Coco S, Sesana S, Bulbarelli A, Biasini E, et al. Role of lipid rafts and GM1 in the segregation and processing of prion protein. PLoS One 2014; 9: e98344.
Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med 2009; 60: 471–84.
Daya M, Cervin M, Anderson R. Cholesterol enhances mouse hepatitis virus-mediated cell fusion. Virology 1988; 163: 276–83.
Danthi P, Chow M. Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry. Journal of Virology 2004; 78: 33–41.
van’t Wout AB, Swain JV, Schindler M, Rao U, Pathmajeyan MS, Mullins JI, et al. Nef induces multiple genes involved in cholesterol synthesis and uptake in human immunodeficiency virus type 1-infected T cells. Journal of Virology 2005; 79: 10053–8.
Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006; 4: e365.
Dubrovsky L, Van Duyne R, Senina S, Guendel I, Pushkarsky T, Sviridov D, et al. Liver X receptor agonist inhibits HIV-1 replication and prevents HIV-induced reduction of plasma HDL in humanized mouse model of HIV infection. Biochem Biophys Res Commun 2012; 419: 95–98.
Jiang H, Badralmaa Y, Yang J, Lempicki R, Hazen A, Natarajan V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids Health Dis 2012; 11: 69.
Author information
Authors and Affiliations
Corresponding authors
Additional information
* Corresponding author. Department of Life Sciences, National Chung Hsing University, Taichung 402, Taiwan.
** Corresponding author, Department of Urology, University of Texas Southwestern Medical Center, Dallas, Texas 75235, USA.
*** School of Medicine, China Medical University, Taichung 404, Taiwan.
**** Corresponding author, Department of Otolaryngology-Head and Neck Surgery, China Medical University and Hospital, Taichung 404, Taiwan.
† Equal contribution to this work. E-mail addresses: hlin@dragon.nchu.edu.tw (H. Lin), JT.Hsieh@UTSouthwestern.edu (J.-T. Hsieh), chl@mail.cmu.edu.tw (C.-H. Lai), d6355@mail.cmuh.org.tw (C.-D. Lin).
Open Access This article is distributed under terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided original author(s) and source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, CJ., Lai, CK., Kao, MC. et al. Impact of cholesterol on disease progression. BioMed 5, 7 (2015). https://doi.org/10.7603/s40681-015-0007-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40681-015-0007-8