Abstract
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system that is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta as well as motor impairment. Aggregation of α-synuclein in neuronal cells plays a key role in this disease. At present, therapeutics for PD provides moderate symptomatic benefits, but it is not able to delay the development of the disease. Current efforts toward the treatment of PD are to identify new drugs that slow or arrest the progressive course of PD by interfering with a disease-specific pathogenetic process in PD patients. Irisflorentin derived from the roots of Belamcanda chinensis (L.) DC. is an herb which has been used for the treatment of inflammatory disorders in traditional Chinese medicine. The purpose of the present study was to assess the potential for irisflorentin to ameliorate PD in Caenorhabditis elegans models. Our data reveal that irisflorentin prevents α-synuclein accumulation in the transgenic Caenorhabditis elegans model and also improves dopaminergic neuron degeneration, food-sensing behavior, and life-span in a 6-hydroxydopamine-induced Caenorhabditis elegans model, thus indicating its potential as a anti-parkinsonian drug candidate. Irisflorentin may exert its effects by promoting rpn-3 expression to enhance the activity of proteasomes and down-regulating egl-1 expression to block apoptosis pathways. These findings encourage further investigation on irisflorentin as a possible potent agent for PD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders and is becoming increasingly prevalent among elderly populations. PD is seen not only as a major health problem, but since it is care-intensive, it also carries a significant economic challenge. PD impairs the motor control of the body and is the result of the selective death of dopaminergic (DA) neurons in the substantia nigra of the midbrain [1]. The disease is characterized by the aggregation of α-synuclein to form Lewy bodies in the neuron [2]. The cause of most cases of PD (sporadic) remains unclear and likely results from a complex interplay of genetic and environmental factors [3]. Only 5-10% of PD cases (familial) occur because of the mutation of several specific genes. These genes include α-synuclein (SNCA), parkin (PRKN), leucine-rich repeat kinase 2 (LRRK2), PTEN-induced putative kinase 1 (PINK1), DJ-1 and ATP13A2 [4].
α-synuclein is a small acidic protein and is found mainly at the presynaptic terminals of neurons. It can be divided into the N-terminal α-helical domain, the central hydrophobic domain, and the acidic carboxyl-terminal domain [2]. α-synuclein may maintain the supply of synaptic vesicles in neuronal terminals and regulate dopamine release to control voluntary and involuntary movements [5]. Aggregation of α-synuclein has been observed in both sporadic and familial forms of PD. Three mutations in the α-helical domain (A53T, A30P, E46K) were indicated to promote oligomer/fibril formation and were associated with autosomal dominant early onset PD [6].
Many reports have shown a link between toxin exposure and raised risk of PD [7]. The 6-hydroxydopamine (6-OHDA) is a specific neurotoxin which targets catecholamine neurons through the dopamine active transporter (DAT). When 6-OHDA is injected into the median forebrain bundle or into the neostriatum of brain, it causes an irreversible loss of DA neurons in the ventral midbrain. The consistent loss of dopamine innervation in target areas is linked with a range of long-term, behavioural deficits.
Thus, a 6-OHDA –induced lesion is the most widely used animal model of PD [8].
Presently there is no effective therapy for PD. Current drugs, including levodopa, dopamine agonists, and monoamine oxidase B inhibitors, only relieve and delay the symptoms [9]. The most hopeful therapeutic direction for PD involves the discovery of small compounds that can prevent the disease as well as improve its symptoms. A number of Chinese herbs have been confirmed to be effective neuroprotective agents. Belamcanda chinensis (L.) DC. (synonym: Iris domestica) is a flowering perennial herb distributed over the region of East Asia, and is valuable in Chinese villages for its medicinal uses. The dried rhizome (Shegan in Chinese) has been listed for treatments such as pharyngitis, coughing, swollen spleen, asthma and liver, gonorrhea, malaria, and arrow poisoning [10]. Isoflavones were the main bioactive constituents of Belamcandae Rhizoma [11], and have physiological benefits including anti-oxidant, anti-inflammatory, anti-cancer, and hypoglycemic properties [12]. Irisflorentin (Fig. 1) is the major isoflavone component derived from Belamcandae Rhizoma [13]. Here, we consider that irisflorentin could be tested as a prophylactic as well as an adjuvant agent for its advantageous effects on PD using C. elegans model system. We also address the possible mechanisms of irisflorentin action.
2. Materials and methods
2.1. Strains, maintenance, and synchronization
C. elegans of wild-type Bristol N2, transgenic BZ555 (Pdat-1::GFP; green fluorescent protein [GFP] visible in dopaminergic neurons) and transgenic OW13 (Punc-54::α-synuclein::YFP+unc-119; human α-synuclein protein with yellow fluorescent protein [YFP] observable in the muscles) were gained from the Caenorhabditis Genetics Center (University of Minnesota). On the basis of previous standard procedures [14], we maintained the animals on NGM plates seeded with the E. coli strain OP50 as food sources at 22°C. Fertilized eggs were collected by hypochlorite treatment of gravid adults. After 20 h incubation at 22°C in M9 buffer to acquire synchronized L1 larvae, the animals were spread on OP50/NGM plates and then incubated for 24 h at 22°C to obtain L3 larvae.
2.2. Food clearance assay
Synthesized irisflorentin (mol. wt. 386.35, 98% purity) was purchased from Fusol Material Co., Ltd. (Tainan, Taiwan), and then dissolved in dimethyl sulfoxide (DMSO) to 1M as a master stock solution. A food clearance assay was used to determine the effect of irisflorentin on C. elegans physiology [15]. E. coli were cultured overnight and then re-suspended at a final OD of 6.6 in nematode S-medium. Irisflorentin was diluted into the E. coli suspension to the desired concentrations. Fifty microliters of the final mixture was added per well in a 96-well plate. Approximately 20-30 synchronized L1 animals in 10 μl of S-medium were added to an E. coli suspension containing a series of concentrations of irisflorentin and incubated in a 96-well microtiter plate at 25°C. The absorbance (OD 595 nm) of the culture was measured every day for 6 days using a SpectraMax M2 Microplate Reader (Molecular Devices, Silicon Valley, CA).
2.3. Quantitative analysis of α-synuclein aggregation
Aggregation of α-synuclein protein was assessed in control and irisflorentin- treated OW13 animals. Synchronized OW13 L3 larvae were cultured on OP50/NGM plates containing 0.04 mg/mL FUDR (Sigma, St. Louis, MI) and irisflorentin for 72 h at 22°C, then washed three times with M9 buffer and transferred to 2% agarose pads on glass slides, mounted with 100 mM sodium azide and enclosed with a coverslip. Immobilized animals were imaged on an Axio Observer inverted fluorescence microscope (Carl Zeiss, Germany) to monitor the aggregation of α-synuclein protein, and the aggregation was quantified using AxioVision software by measuring fluorescence intensity.
2.4. Exposure to 6-OHDA and treatment with irisflorentin
In brief, 50 mM 6-OHDA and 10 mM ascorbic acid were added to OP50/S-medium mix with irisflorentin. Synchronized L3 larvae were then transferred onto the treated cultures, incubated for 1 h at 22°C and mixed gently every 10 min. After 1 h of treatment, animals were washed three times with M9 buffer and then incubated in OP50/NGM plates with irisflorentin. After 24 h, animals were transferred to OP50/NGM plates containing irisflorentin and 0.04 mg/mL 5-fluoro-2′-deoxyuridine (FUDR, Sigma, St. Louis, MI) to lessen the production of progeny. Animals were scored with various assays 72 h after treatment.
2.5. Quantitative analysis of dopaminergic neurodegeneration
Analysis of dopaminergic neurodegeneration was performed in animals treated with 6-OHDA or irisflorentin/6-OHDA, as described previously. After 72 h of treatment at 22°C, BZ555 animals were washed three times using M9 buffer, and then mounted onto a 2% agar pad on a glass slide using 100 mM sodium azide (Sigma, St. Louis, MI) and enclosed with a coverslip. Imaging of immobilized animals was carried out with an Axio Observer inverted fluorescence microscope (Carl Zeiss MicroImaging GmbH, Göttingen Germany). Fluorescence intensity was quantified using AxioVision software (Carl Zeiss, Göttingen, Germany).
2.6. Analysis of food-sensing behavior
Briefly, test plates were prepared by spreading E. coli overnight at 37°C in a ring with an inner diameter of 1 cm and an outer diameter of 8 cm on 9-cm diameter NGM agar plates to prevent the animals from reaching the edge of the plate during the test. Well-fed 6-OHDA-treated or irisflorentin/6-OHDA-treated adult animals were washed with M9 buffer and then transferred to the center of a test plate with or without a bacterial lawn in a drop of M9 buffer. Five minutes after the transfer, the locomotory rate of each animal was counted in 20-s intervals. The slowing rate was calculated as the percentage of the locomotory rate in the bacteria lawn compared with that in the no-bacteria lawn. The average slowing rate among 10 animals was defined as the result of each analysis. In all tests, plates were numbered so that the experimenter was blind to the treatment of the animal.
2.7. RNA isolation and quantitative RT–PCR
Total RNA was extracted from synchronized control or experimental adult animals using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. cDNA was generated using the SuperScript One-Step RT-PCR system (Invitrogen, Carlsbad, CA). SYBR Green real-time qPCR analyses were carried out with a 1:20 dilution of cDNA using an ABI StepOnePlus system (Applied Biosystems). Data were calculated with the comparative 2ΔΔCt method using the geometric mean of cdc-42, pmp-3 and Y45F10D.4 as the endogenous control [16]. Table 1 shows details of the primers used for this study.
2.8. 26S proteasome activity assays
Briefly, using a Precellys 24 homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France), animals were lysed in a proteasome activity assay buffer containing 50 mM Tris-HCl (pH 7.5), 250 mM sucrose, 5 mM MgCl2, 2 mM ATP, 1 mM dithiothreitol and 0.5 mM EDTA. The lysate was centrifuged at 10,000 g for 15 min at 4°C. For each experiment, 25 μg of total lysate was loaded into each well of a 96-well microtiter plate, after which a fluorogenic substrate was added. For testing the chymotrypsinlike activity of the proteasome, Z-Gly-Gly-Leu-AMC (Enzo Life Sciences, Farmingdale, NY) was used as a substrate. After incubation for 1 h at 25°C, fluorescence (an excitation wavelength of 380 nm and an emission wavelength of 460 nm) was detected with a SpectraMax M2 Microplate Reader (Molecular Devices, Silicon Valley, CA).
2.9. Statistical analysis
Statistical analyses are expressed as mean ± standard deviation (SD) from independent tests. Three replicates were done of each test. The differences between two means were determined by a student’s t-test. Values of P < 0.05 were determined to be statistically significant.
3. Results
3.1. Determining the irisflorentin concentration range for C. elegans treatment by food clearance assay
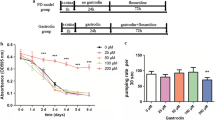
To assess the effects of irisflorentin on DA neuron degeneration and α-synuclein accumulation, we first determined the optimal concentrations of irisflorentin to evaluate in our C. elegans PD models via food clearance assay. Given the advantage of the short life cycle and the ability of C. elegans to grow in a liquid culture of E. coli, irisflorentin was tested at the rate at which the E. coli suspension was consumed. Each adult animal can produce hundreds of offspring that quickly consume the limited E. coli supply. Consequently, the OD of the wells without irisflorentin was significantly reduced in 3 days in N2, BZ555 and OW13 strains (Fig. 2). The addition of 0.1 mM, 0.5 mM or 2.5 mM irisflorentin to the cultures containing N2, BZ555 or OW13 strains revealed no effect on food clearance compared to that in the control animals, whereas animals exposed to 12.5 mM irisflorentin had considerably delayed food clearance (Fig. 2). Additionally, animals exposed to 12.5 mM irisflorentin did not produce offspring over the time course of the experiment (data not shown), which was linked to the lack of clearance of the E. coli source. In the experiments following this assay, animals were treated with irisflorentin at concentrations of up to 2.5 mM.
3.2. Irisflorentin decreased accumulation of α-synuclein protein
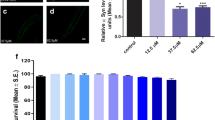
C. elegans lacks orthologous genes of α-synuclein. However, the genetic flexibility of the animal allows transgenic expression of human α-synuclein genes and the study of α-synuclein aggregation. OW13 animals of untreated and irisflorentin- treated groups were observed for their α-synuclein protein accumulation range. Treatment of animals with irisflorentin displayed significantly diminished fluorescence intensity of accumulation compared to that of untreated animals (Fig. 3A). Irisflorentin reduced the YFP expression of OW13 animals in a dose-dependent manner. At 2.5 mM irisflorentin, the fluorescence intensity of YFP expression related to α-synuclein protein accumulation in OW13 animals decreased by about 45% (P < 0.01) compared to that in untreated animals (Fig. 3B).
The concentration of irisflorentin for experiments was determined with a food clearance assay. Between 20 and 30 newly hatched L1 synchronized animals of N2, BZ555 or OW13 were incubated in E. coli (OD A595 = 0.6) in a 96-well plate at 25°C containing different irisflorentin concentrations to a total volume of 60 μL. The OD of the plate was measured daily for 6 days. Note the lower OD (~0.6) in a well due to the decreased path length of the 60 μL final suspension compared to the 1 cm path length in a spectrophotometer. The OD of E. coli was recorded daily for each concentration of irisflorentin.
Irisflorentin hinders α-synuclein accumulation in the OW13 strain of C. elegans. (A) YFP expression patterns in muscles of transgenic C. elegans strain OW13. The figures show fluorescence images. Scale bar, 50 μm. (B) Graphical representation for fluorescence intensity of YFP expression pattern in muscles of transgenic C. elegans strain OW13 as quantified using AxioVision software. The data represent the mean ± SD (n = 10). An asterisk (*) indicates significant differences between the control samples and the irisflorentintreated samples (* P < 0.05, ** P < 0.01).
3.3. 6-OHDA-induced degeneration of DA neurons was diminished by treatment of irisflorentin
C. elegans contains eight DA neurons, including one pair of anterior deirid (ADE) neurons and two pairs of cephalic (CEP) neurons in the head position, and one pair of posterior deirid (PDE) neurons in the posterior lateral region. Selective degeneration of these DA neurons was observed through exposure to 6-OHDA. To examine irisflorentin efficacy, we assessed neuronal viability by testing the loss of expression of a GFP reporter construct in DA neurons of 6-OHDA-treated BZ555 animals. Data showed that ADE and CEP neurons lost a partial GFP with a slight reduction in GFP expression in PDE neurons after 6-OHDA treatment (Fig. 4A). When animals were treated with irisflorentin, remarkable protection was found in DA neurons with ADE, and CEP neurons were presenting an augmented expression of GFP (Fig 4A). We further calculated the fluorescence intensity in DA neurons using AxioVision software. In 6-OHDA-treated animals, the mean fluorescence (GFP) intensity lessened by about 56% (P < 0.01) compared to that of untreated animals (Fig. 4B). Irisflorentin increased the GFP expression in a dose-dependent manner. At 2.5 mM irisflorentin, the fluorescence intensity of GFP expression in DA neurons of 6-OHDA-treated animals increased by about 1.9-fold (P < 0.01) compared to that in animals treated only with 6-OHDA (Fig. 4B).
Irisflorentin protects dopaminergic (DA) neurons of C. elegans from degeneration resulting from 6-OHDA treatment. (A) GFP expression pattern in DA neurons of transgenic C. elegans strain BZ555. The figures show the fluorescence images. Scale bar, 50 μm. (B) Graphical representation for fluorescence intensity of GFP expression pattern in DA neurons of a transgenic C. elegans strain as quantified using AxioVision software. The data represent the mean ± SD (n = 10). A hash (#) indicates significant differences between 6-OHDA-treated and untreated animals (P < 0.001); an asterisk (*) indicates significant differences between the 6-OHDA-treated control samples and the Irisflorentin/6-OHDA-treated samples (* P < 0.05, ** P < 0.01).
Irisflorentin improved food-sensing behavior in 6- OHDA-treated N2 C. elegans. The locomotory rate (frequency of bending) of 6-OHDA-untreated animals, 6-OHDA-treated animals, or irisflorentin/6-OHDA-treated animals with or without bacteria lawns was tested. Shown are the slowing rates calculated as the percentage reduction of the locomotory rate in the bacteria lawn as compared with that in no bacteria lawn. The data represent the mean ± SD (n = 20). A hash (#) indicates significant differences between 6-OHDA-treated and untreated animals (P < 0.01); an asterisk (*) indicates significant differences between the 6-OHDA-treated control samples and the irisflorentin/6-OHDA-treated samples (* P < 0.05, ** P < 0.01).
3.4. Irisflorentin improved food-sensing behavior of 6-OHDA-treated C. elegans
It has been revealed that 6-OHDA-treated animals show a phenotype of DA neuron degeneration that should affect food-sensing behavior [17, 18]. C. elegans bend their bodies to move themselves for migration, and the rate of movement is determined by the bending frequency. Once animals come across food, they lessen the bending frequency to feed themselves more effectively. 6-OHDA-treated animals, however, fail to display a decremental bending frequency in response to food sensing. Therefore, the function of DA neurotransmission in C. elegans is linked with this food-sensing behavior. We examined whether 6-OHDA treatment in C. elegans induces a defect in this function in 3-day-old animals that were synchronized for age. Wild-type N2 animals presented a 40% reduction in bending frequency upon contact with bacteria (Fig. 5). In contrast, 6-OHDA-treated animals revealed a significant lessening in this decremental response compared with wild-type N2 animals (20%, P < 0.01). Irisflorentin improved the decremental response of 6-OHDA-treated animals in a dosedependent manner. At 2.5 mM irisflorentin treatment, 6-OHDAtreated animals presented decreased bending movements upon contact with bacteria by about 1.9-fold (P < 0.01) compared with that in untreated animals (Fig. 5).
3.5. Irisflorentin raised somatic proteasome activity by enhancing proteasome regulatory subunit rpn-3 expressionin an α-synuclein-expressing model of C. elegans
We hypothesized that the ubiquitin proteasome system might be changed in irisflorentin-treated OW13 animals. To evaluate whether the observed decrease in total α-synuclein in the muscle of OW13 animals was the result of augmented proteasomal activity, we analyzed 26S proteasome activity upon treatment with irisflorentin by using a proteasome activity assay with a fluorescent substrate. As shown in Fig. 6A, the basal level of chymotrypsin-like proteasome activity was about 35% lower in OW13 animals compared to that in N2 animals (P < 0.01). Irisflorentin treatment significantly augmented the chymotrypsin-like proteasome activity in OW13 animals in a dose-dependent manner. Chymotrypsin-like proteasome activity following 2.5 mM irisflorentin treatment was augmented by about 1.8-fold in the OW13 animals (P < 0.01) (Fig. 6A). These results indicate that enhanced proteasome activity results in a reduction in α-synuclein and that irisflorentin treatment can improve proteasome activity in the animal model of PD. We therefore next assessed whether the proteasome activity of irisflorentin-treated OW13 animals correlated with an augmented expression level of the regulatory particles of the 19S proteasome or the catalytically active subunits of the 20S proteasome. The basal level of all subunits was not different in OW13 animals compared to that in N2 animals. Irisflorentin enhanced the expression level of the rpn-3 of the regulatory subunit. The expression level of rpn-3 following 2.5 mM irisflorentin treatment was augmented by about 67% in the OW13 animals (P < 0.01) (Fig. 6B).
Irisflorentin increases proteasome activity by enhancing rpn-3 expression in the OW13 strain of C. elegans. (A) Chymotrypsin-like activity of the proteasome was monitored by Z-Gly-Gly-Leu-AMC digestion in a day 3 extract of adult animal containing equal amounts of total protein. The data represent the mean ± SD (n = 3). A hash (#) indicates significant differences between N2 and OW13 animals (P < 0.01); an asterisk (*) indicates significant differences between the OW13 control samples and the irisflorentin-treated OW13 samples (* P < 0.05, ** P < 0.01). (B) Irisflorentin raises the expression of rpn3 of the regulatory subunit of proteasome in the OW13 strain of C. elegans. Quantitative real-time RT-PCR experiments show the expression level of the Rpn subunit of the 26S proteasome using cDNAs isolated from OW13 control or irisflorentin-treated animals. The data represent the mean ± SD (n = 3). An asterisk (*) indicates significant differences between the OW13 control samples and the irisflorentintreated samples (** P < 0.01).
Irisflorentin reduces egl-1 expression in apoptosis modulation in 6-OHDA-treated C. elegans. Quantitative real-time RT-PCR experiments show the expression levels of egl-1, ced-3, ced-4 and ced-9 using cDNAs isolated from N2, 6- OHDA-treated, or irisflorentin/6-OHDA-treated animals. The data represent the mean ± SD (n = 3). An asterisk (*) indicates significant differences between the 6-OHDA-treated control samples and the irisflorentin/6-OHDA-treated samples (** P < 0.01).
3.6. Irisflorentin diminished apoptosis modulator egl-1 expression in 6-OHDA- treated C. elegans
We hypothesized that an important aspect of the apoptosis pathway might be changed in the 6-OHDA-treated animals by irisflorentin. To evaluate whether the observed decrease in DA neuron degeneration of C. elegans was the result of reduced apoptosis activity, after treating the animals with irisflorentin, we used a real-time PCR method to analyze the mRNA levels of egl-1, ced-3, ced-4 and ced-9, which are related to apoptosis of C. elegans. As shown in Fig. 7, the expression level of egl-1, ced-3, ced-4 and ced-9 was not augmented in 6-OHDA-treated animals compared to that in untreated animals. At 2.5 mM irisflorentin, the expression level of egl-1 in 6-OHDA-treated animals lessened by about 64% (P < 0.01) compared to that in animals treated only with 6-OHDA (Fig. 7).
4. Discussion
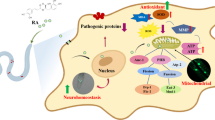
In this study, our methods used the benefits of the C. elegans model for drug testing, including convenient and exact visualization of live DA neurons and α-synuclein accumulation. These tests could be powerful for inexpensive, rapid examination and screening of drugs for PD. Our data demonstrate that irisflorentin attenuates α-synuclein accumulation; reduces DA neuron degeneration; and recovers food-sensing behavior in a pharmacological or transgenic C. elegans model. To the best of our knowledge, this is the first report of the anti-parkinsonian role of irisflorentin in an animal model.
The SNCA gene (PARK1) encodes human α-synuclein. In PD, the accumulation of α-synuclein is a particular challenge for DA neurons, especially as they aggregate in inclusions and aggresomes and overwhelm the cellular machinery for their degradation. These effects are possibly involved in the PD-related dysregulation of chaperones, a down-regulation of the degradation system itself, and an accelerating loss in overall cellular homeostasis [19]. Irisflorentin diminished the accumulation of α-synuclein, thus arresting its toxic effect in the cells. We identified that the preventive effect of irisflorentin was due to its regulation of the proteosome activity. It is well known that the ubiquitin-proteosome system salvages the cells from harm under stressful conditions [20].
The 26S proteasome complex of C. elegans is composed of a 20S “catalytic core” and 19S “regulatory caps”. 19S can be further separated into two distinct substructures containing a ring of six homologous ATPase subunits of the AAA family (Rpt-1-6), two α-helical solenoid structures (Rpn-1 and Rpn-2), eight essential RP non-ATPase subunits (Rpn-3, Rpn-5-9 and Rpn-11-12), and two Ub receptors (Rpn-10) [21]. The effect of irisflorentin may be linked with augmented expression of rpn3, a subunit of the 19S proteasome. Rpn-3 expression is tightly controlled; both overexpression and deletion of Rpn-3 impair proteasome functions. The exact mechanisms underlying these results require further study [22].
Given that DA neurons are more susceptible to oxidative stress, reactive oxygen species are important regulators in neuron apoptosis and death. 6-OHDA destroys neuronal cells by generating reactive oxygen species such as the superoxide radical [23]. The neuroprotective role of irisflorentin in 6-OHDA-induced DA neuron degeneration and food-sensing behavior defects is possibly related to its antioxidant and antiapoptotic activity. Additionally, it has also been demonstrated that mitochondrial dysfunction induced by 6-OHDA triggers the release of cytochrome c and the activation of caspase-3 [24]. Caspase-3 is a crucial effector in apoptosis that is triggered through different pathways in various mammalian cell types, especially in the cytochrome c-dependent apoptosis pathways. In C. elegans, the BH3-only domain protein EGL-1, the Apaf-1 homolog CED-4, and the CED-3 caspase are involved in apoptosis triggering, whereas the Bcl-2 homolog CED-9 inhibits apoptosis. CED-9 is obligatory to block CED-4 by abrogating CED-4 accumulation in the perinuclear space in response to proapoptotic stimuli. EGL-1 antagonizes CED-9, causing CED-4 oligomerization and triggering apoptosis through CED-3 caspase activation [25]. In this study, we showed that irisflorentin lessened the expression of egl-1, which, in turn, antagonized 6-OHDA-induced DA neuron apoptosis. The protective effects of irisflorentin on DA neurons could possibly be connected with these events; but the mechanistic aspects need to be studied in more detail.
Evidence implies that chronic neuroinflammation is linked with the pathophysiology of PD [26]. Activation of microglia and augmented levels of pro-inflammatory mediators such as TNF-α, IL-1β and IL-6 have been described in the substantia nigra of PD patients and in animal models of PD [27]. It is hypothesized that activated microglia secretes high levels of proinflammatory cytokines that impair neurons and further activate microglia, resulting in further inducing of inflammation and neurodegeneration. Moreover, DA neurons are more susceptible to proinflammatory mediators than other cell types. In previous research, irisflorentin was found to suppress LPS-induced inflammatory responses in RAW 264.7 macrophages [28]. It markedly decreases the transcriptional and translational levels of inducible nitric oxide synthase as well as the production of NO, and downregulates expression levels of TNF-α, IL-1β and IL-6. These effects act via ERK1/2 - and p38-mediated activator protein-1. Hence, irisflorentin may also be able to improve inflammation in the brains of patients with PD and lessen DA neuron damage.
The present study shows that irisflorentin is a new member on the list of isoflavones with anti-parkinsonian properties and provides traditional Chinese medicine practitioners with information about the utilization of herbs containing irisflorentin in the treatment of neuron-related diseases. This readily obtainable compound provides a convenient, low-cost, and highly effective means of regulating the survival and function of DA neurons. In the future, we plan to investigate the precise mechanism by which irisflorentin maintains DA neuron activity in other animal PD models and evaluate the suitability of irisflorentin for disease control in clinical trials.
Acknowledgements
This work was supported by the Ministry of Science and Technology (Taiwan) (MOST 103-2314-B-039-025), the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002) and China Medical University (DMR-104-052).
Declaration of interest
The authors declare no conflicts of interest for this work.
References
Sharma P, Pienaar IS. Pharmacogenetic and optical dissection for mechanistic understanding of Parkinson’s disease: Potential utilities revealed through behavioural assessment. Neurosci Biobehav Rev 2014; 47C: 87–100.
Lee HJ, Bae EJ, Lee SJ. Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 2014; 10: 92–8.
Fujita KA, Ostaszewski M, Matsuoka Y, Ghosh S, Glaab E, Trefois C, et al. Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol Neurobiol 2014; 49: 88–102.
Trinh J, Farrer M. Advances in the genetics of Parkinson disease. Nat Rev Neurol 2013; 9: 445–54.
Schapira AH, Olanow CW, Greenamyre JT, Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet 2014; 384: 545–55.
Lemkau LR, Comellas G, Lee SW, Rikardsen LK, Woods WS, George JM, et al. Site-specific perturbations of alpha-synuclein fibril structure by the Parkinson’s disease associated mutations A53T and E46K. PLoS One 2013; 8: e49750.
Ramsey CP, Tansey MG. A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson’s disease and potential molecular targets. Exp Neurol 2014; 256: 126–32.
Padovan-Neto FE, Cavalcanti-Kiwiatkoviski R, Carolino RO, Anselmo- Franci J, Del Bel E. Effects of prolonged neuronal nitric oxide synthase inhibition on the development and expression of l-DOPAinduced dyskinesia in 6-OHDA-lesioned rats. Neuropharmacology 2015; 89: 87–99.
Jankovic J, Poewe W. Therapies in Parkinson’s disease. Curr Opin Neurol 2012; 25: 433–47.
Yamaki M, Kato T, Kashihara M, Takagi S. Isoflavones of Belamcanda chinensis. Planta Med 1990; 56: 33–5.
Lee YS, Kim SH, Kim JK, Lee S, Jung SH, Lim SS. Preparative isolation and purification of seven isoflavones from Belamcanda chinensis. Phytochem Anal 2011; 22: 468–73.
Jun HJ, Hoang MH, Lee JW, Yaoyao J, Lee JH, Lee DH, et al. Iristectorigenin B isolated from Belamcanda chinensis is a liver X receptor modulator that increases ABCA1 and ABCG1 expression in macrophage RAW 264.7 cells. Biotechnol Lett 2012; 34: 2213–21.
Liu M, Yang S, Jin L, Hu D, Wu Z. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012; 17: 6156–69.
Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77: 71–94.
Fu RH, Harn HJ, Liu SP, Chen CS, Chang WL, Chen YM, et al. n-butylidenephthalide protects against dopaminergic neuron degeneration and alpha-synuclein accumulation in Caenorhabditis elegans models of Parkinson’s disease. PLoS One 2014; 9: e85305.
Fu RH, Wang YC, Chen CS, Tsai RT, Liu SP, Chang WL, et al. Acetylcorynoline attenuates dopaminergic neuron degeneration and alpha-synuclein aggregation in animal models of Parkinson’s disease. Neuropharmacology 2014; 82: 108–20.
Nass R, Hall DH, Miller DM, 3rd, Blakely RD. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci USA 2002; 99: 3264–9.
Tucci ML, Harrington AJ, Caldwell GA, Caldwell KA. Modeling dopamine neuron degeneration in Caenorhabditis elegans. Methods Mol Biol 2011; 793: 129–48.
Burre J, Sharma M, Sudhof TC. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA 2014; 111: E4274–83.
Alvarez-Castelao B, Goethals M, Vandekerckhove J, Castano JG. Mechanism of cleavage of alpha-synuclein by the 20S proteasome and modulation of its degradation by the RedOx state of the Nterminal methionines. Biochim Biophys Acta 2014; 1843: 352–65.
Baptista MS, Duarte CB, Maciel P. Role of the ubiquitin-proteasome system in nervous system function and disease: using C. elegans as a dissecting tool. Cell Mol Life Sci 2012; 69: 2691–715.
Joshi KK, Chen L, Torres N, Tournier V, Madura K. A proteasome assembly defect in rpn3 mutants is associated with Rpn11 instability and increased sensitivity to stress. J Mol Biol 2011; 410: 383–99.
Choi DH, Kim JH, Seo JH, Lee J, Choi WS, Kim YS. Matrix Metalloproteinase- 3 Causes Dopaminergic Neuronal Death through Nox1- Regenerated Oxidative Stress. PLoS One 2014; 9: e115954.
Hu LW, Yen JH, Shen YT, Wu KY, Wu MJ. Luteolin modulates 6-hydroxydopamine-induced transcriptional changes of stress response pathways in PC12 cells. PLoS One 2014; 9: e97880.
Arvanitis M, Li DD, Lee K, Mylonakis E. Apoptosis in C. elegans: lessons for cancer and immunity. Front Cell Infect Microbiol 2013; 3: 6–7.
Russo I, Bubacco L, Greggio E. LRRK2 and neuroinflammation: partners in crime in Parkinson’s disease? J Neuroinflammation 2014; 11: 5–2.
Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C. Depression and cognitive impairment in Parkinson’s disease: a role for inflammation and immunomodulation? Neuroimmunomodulation 2014; 21: 88–94.
Gao Y, Fang L, Liu F, Zong C, Cai R, Chen X, et al. Suppressive effects of irisflorentin on LPS-induced inflammatory responses in RAW 264.7 macrophages. Exp Biol Med (Maywood) 2014; 239: 1018–24.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open Access This article is distributed under terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided original author(s) and source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, YM., Liu, SP., Lin, HL. et al. Irisflorentin improves α-synuclein accumulation and attenuates 6-OHDA-induced dopaminergic neuron degeneration, implication for Parkinson’s disease therapy. BioMed 5, 4 (2015). https://doi.org/10.7603/s40681-015-0004-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40681-015-0004-y