Abstract
Bradyrhizobium sp. strain WSM1417 is an aerobic, motile, Gram-negative, non-spore-forming rod that was isolated from an effective nitrogen (N2) fixing root nodule of Lupinus sp. collected in Papudo, Chile, in 1995. However, this microsymbiont is a poorly effective N2 fixer with the legume host Lupinus angustifolius L.; a lupin species of considerable economic importance in both Chile and Australia. The symbiosis formed with L. angustifolius produces less than half of the dry matter achieved by the symbioses with commercial inoculant strains such as Bradyrhizobium sp. strain WSM471. Therefore, WSM1417 is an important candidate strain with which to investigate the genetics of effective N2 fixation in the lupin-bradyrhizobia symbioses. Here we describe the features of Bradyrhizobium sp. strain WSM1417, together with genome sequence information and annotation. The 8,048,963 bp high-quality-draft genome is arranged in a single scaffold of 2 contigs, contains 7,695 protein-coding genes and 77 RNA-only encoding genes, and is one of 20 rhizobial genomes sequenced as part of the DOE Joint Genome Institute 2010 Community Sequencing Program.
Similar content being viewed by others
Introduction
The Fabaceae plant family is the third largest family of flowering plants with a unique ecological role in nitrogen (N2) fixation. This family encompasses the three subfamilies Caesalpinioideae, Mimosoideae, and Faboideae (or Papilionoideae). The legume genus Lupinus (commonly known as lupin) consists of around 280 species classified within the Genisteae tribe of the subfamily Faboideae with major centers of diversity in South and Western North America, the Andes, the Mediterranean regions, and Africa. This legume has been grown in rotations with cereals for at least 2000 years [1] and is widely distributed within the old and new worlds [2]. The grain may be easily harvested and contains the full range of essential amino acids, and because of its high concentration of sulfur containing amino acids has high feed value for stock [2].
The lupin root nodule bacteria have all been classified within the genus Bradyrhizobium [3,4] with the exception of Microvirga lupini that was found to nodulate with Lupinus texensis [5]. Bradyrhizobium spp. are commonly associated with the nodulation of sub-tropical and tropical legumes such as soybean [6,7]. In contrast, lupins are the only agricultural grain legume nodulated by this genus in Mediterranean-type climatic zones. Strains of lupin-nodulating Bradyrhizobium are also able to nodulate the herbaceous Mediterranean legume Ornithopus (seradella) spp. In this context, lupin Bradyrhizobium strains are rare microsymbionts of herbaceous and crop legumes endemic to the cool climatic regions of the world.
The cultivation of lupin in these regions provides a cash crop alternative to soy. Lupinus angustifolius in particular has been extensively used to extend grain production into poor quality soils without fertilizer supplementation since fixed nitrogen can be obtained from the symbiosis with Bradyrhizobium [8]. Considerable variation exists in the amount of N2 fixed in the lupin-Bradyrhizobium association [8]. This is significant in agricultural ecosystems, as the benefits derived from growing lupins accrue both to the grain produced and the N2 fixed [9]. A well-grown lupin crop may fix up to 300 kg of N per ha. It is therefore important to understand the genetic constraints to optimal N2 fixation in this symbiosis. Bradyrhizobium sp. strain WSM1417 represents the lower end of the scale in strain N2 fixation capacity on L. angustifolius, and hence its genome sequence presents an opportunity to understand the genetic elements responsible for this trait. Here we present a summary classification and a set of general features for Bradyrhizobium sp. WSM1417 together with the description of the complete genome sequence and its annotation.
Classification and general features
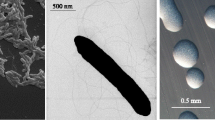
Bradyrhizobium sp. WSM1417 is a motile, Gram-negative, non-spore-forming rod (Figure 1 Left and Center) in the order Rhizobiales of the class Alphaproteobacteria. It is slow growing in laboratory culture, forming 1–2mm colonies within 7–10 days when grown on half Lupin Agar (½LA) [10] at 28°C. Colonies on ½LA are white-opaque, slightly domed, moderately mucoid with smooth margins (Figure 1C). Minimum Information about the Genome Sequence (MIGS) is provided in Table 1. Figure 2 shows the phylogenetic neighborhood of Bradyrhizobium sp. strain WSM1417 in a 16S rRNA sequence based tree. This strain clusters closest to Bradyrhizobium canariense LMG 22265T and Bradyrhizobium japonicum LMG 6138T with 99.85% and 99.48% sequence identity, respectively.
Phylogenetic tree showing the relationships of Bradyrhizobium sp. strain WSM1417 (shown in blue print) with some of the root nodule bacteria in the order Rhizobiales based on aligned sequences of the 16S rRNA gene (1,334 bp internal region). All sites were informative and there were no gap-containing sites. Phylogenetic analyses were performed using MEGA, version 5.05 [20]. The tree was built using the maximum likelihood method with the General Time Reversible model. Bootstrap analysis [21] with 500 replicates was performed to assess the support of the clusters. Type strains are indicated with a superscript T. Strains with a genome sequencing project registered in GOLD [22] are in bold print and the GOLD ID is mentioned after the accession number. Published genomes are designated with an asterisk.
Symbiotaxonomy
Bradyrhizobium sp. WSM1417 is poorly effective on L. angustifolius, producing only 45% of the dry matter compared to that achieved by the commercial inoculant strain Bradyrhizobium sp. WSM471 on this species. In contrast on L. mutabilis, WSM1417 performs much better, yielding 83% of the dry matter produced by WSM471 on this same host.
Genome sequencing and annotation information
Genome project history
This organism was selected for sequencing on the basis of its environmental and agricultural relevance to issues in global carbon cycling, alternative energy production, and biogeochemical importance, and is part of the Community Sequencing Program at the U.S. Department of Energy, Joint Genome Institute (JGI) for projects of relevance to agency missions. The genome project is deposited in the Genomes OnLine Database [22] and an improved-high-quality-draft genome sequence in IMG. Sequencing, finishing and annotation were performed by the JGI. A summary of the project information is shown in Table 2.
Growth conditions and DNA isolation
Bradyrhizobium sp. strain WSM1417 was grown to mid logarithmic phase in TY rich medium [23] on a gyratory shaker at 28°C. DNA was isolated from 60 mL of cells using a CTAB (Cetyltrimethylammonium bromide) bacterial genomic DNA isolation method [24].
Genome sequencing and assembly
The genome of Bradyrhizobium sp. strain WSM1417 was sequenced at the Joint Genome Institute (JGI) using a combination of Illumina [25] and 454 technologies [26]. An Illumina GAii shotgun library which generated 82,690,654 reads totaling 6,284.5 Mb, and a paired end 454 library with an average insert size of 10 kb which generated 770,255 reads totaling 144.4 Mb of 454 data were generated for this genome. All general aspects of library construction and sequencing performed at the JGI can be found at the JGI website [24]. The initial draft assembly contained 2 contigs in 1 scaffold. The 454 paired end data was assembled with Newbler, version 2.3. The Newbler consensus sequences were computationally shredded into 2 kb overlapping fake reads (shreds). Illumina sequencing data were assembled with Velvet, version 1.0.13 [27], and the consensus sequences were computationally shredded into 1.5 kb overlapping fake reads (shreds). We integrated the 454 Newbler consensus shreds, the Illumina Velvet consensus shreds and the read pairs in the 454 paired end library using parallel phrap, version SPS - 4.24 (High Performance Software, LLC). The software Consed (Ewing and Green 1998; Ewing et al. 1998; Gordon et al. 1998) was used in the following finishing process. Illumina data was used to correct potential base errors and increase consensus quality using the software Polisher developed at JGI (Alla Lapidus, unpublished). Possible mis-assemblies were corrected using gapResolution (Cliff Han, unpublished), Dupfinisher (Han, 2006), or sequencing cloned bridging PCR fragments with subcloning. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR (J-F Cheng, unpublished) primer walks. A total of 126 additional reactions were necessary to close gaps and to raise the quality of the finished sequence. The estimated genome size is 8.1 Mb and the final assembly is based on 65.8 Mb of 454 draft data, which provides an average 8.1× coverage of the genome.
Genome annotation
Genes were identified using Prodigal [28] as part of the DOE-JGI Annotation pipeline [29], followed by a round of manual curation using the JGI GenePRIMP pipeline [30]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) non-redundant database, UniProt, TIGRFam, Pfam, PRIAM, KEGG, COG, and InterPro databases. These data sources were combined to assert a product description for each predicted protein. Non-coding genes and miscellaneous features were predicted using tRNAscan-SE [31], RNAMMer [32], Rfam [33], TMHMM [34], and SignalP [35]. Additional gene prediction analyses and functional annotation were performed within the Integrated Microbial Genomes (IMG-ER) platform [24,36].
Genome properties
The genome is 8,048,963 nucleotides with 63.16% GC content (Table 3) and comprised of a single scaffold of two contigs. From a total of 7,772 genes, 7,695were protein encoding and 77 RNA only encoding genes. Within the genome, 272 pseudogenes were also identified. The majority of genes (74.03%) were assigned a putative function whilst the remaining genes were annotated as hypothetical. The distribution of genes into COGs functional categories is presented in Table 4 and Figure 3.
Graphical circular map of the chromosome of Bradyrhizobium sp. strain WSM1417. From outside to the center: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
References
Hamblin J. Preface to “Lupins: production and utilisation”. In: Gladstones J, Hamblin J, Atkins C, editors. Lupins: production and utilisation: CAB International, U.K.; 1998. p xi–xiii.
Gladstones JS. Distribution, Origin, Taxonomy, History and Importance. In: Gladstones J, Hamblin J, Atkins C, editors. Lupins: production and utilisation: CAB International, U.K.; 1998. p 1–40.
Stepkowski T, Moulin L, Krzyzanska A, McInnes A, Law IJ, Howieson J. European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl Environ Microbiol 2005; 71:7041–7052. PubMed http://dx.doi.org/10.1128/AEM.71.11.7041-7052.2005
Garrity GM, Bell JA, Lilburn T. Class I. Alphaproteobacteria class. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Second ed: New York: Springer-Verlag; 2005.
Ardley JK. Symbiotic specificity and nodulation in the southern African legume clade Lotononis s. l. and description of novel rhizobial species within the Alphaproteobacterial genus Microvirga: Murdoch University, Murdoch, WA, Australia; 2012.
Sprent J. Nodulation in Legumes. London: Kew Publishing 2001. 156 p.
Sprent JI. Legume Nodulation: A Global Perspective. Oxford: Wiley-Blackwell; 2009. 183 p.
Howieson JG, Fillery I, Legocki AB, Sikorski MM, Stepkowski T, Minchin FR, Dilworth MD. Nodulation, nitrogen fixation and nitrogen balance. In: Gladstones J, Hamblin J, Atkins C, editors. Lupins: production and utilisation: CAB International, U.K.; 1998. p 149–180.
Pannell DJ. Economic assessment of the role and value of lupins in the farming system. In: Gladstones J, Hamblin J, Atkins C, editors. Lupins: production and utilisation: CAB International, UK; 1998. p 339–352.
Howieson JG, Ewing MA, D’antuono MF. Selection for acid tolerance in Rhizobium meliloti. Plant Soil 1988; 105:179–188. http://dx.doi.org/10.1007/BF02376781
Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen M, Angiuoli SV, et al. Towards a richer description of our complete collection of genomes and metagenomes “Minimum Information about a Genome Sequence” (MIGS) specification. Nat Biotechnol 2008; 26:541–547. PubMed http://dx.doi.org/10.1038/nbt1360
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576–4579. PubMed http://dx.doi.org/10.1073/pnas.87.12.4576
Garrity GM, Bell JA, Lilburn T. Phylum XIV. Proteobacteria phyl. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT (eds), Bergey’s Manual of Systematic Bacteriology, Second Edition, Volume 2, Part B, Springer, New York, 2005, p. 1.
Validation List No. 107. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol 2006; 56:1–6. PubMed http://dx.doi.org/10.1099/ijs.0.64188-0
Kuykendall LD. Order VI. Rhizobiales ord. nov. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Second ed: New York: Springer-Verlag; 2005. p 324.
Garrity GM, Bell JA, Lilburn TG. Family VII. Bradyrhizobiaceae. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Volume 2. New York: Springer-Verlag; 2005. p 438.
Jordan DC. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a genus of slow-growing, root nodule bacteria from leguminous plants. Int J Syst Bacteriol 1982; 32:136–139. http://dx.doi.org/10.1099/00207713-32-1-136
Agents B. Technical rules for biological agents. TRBA (http://www.baua.de):466.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25–29. PubMed http://dx.doi.org/10.1038/75556
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28:2731–2739. PubMed http://dx.doi.org/10.1093/molbev/msr121
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985; 39:783–791. http://dx.doi.org/10.2307/2408678
Liolios K, Mavromatis K, Tavernarakis N, Kyrpides NC. The Genomes On Line Database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2008; 36:D475–D479. PubMed http://dx.doi.org/10.1093/nar/gkm884
Reeve WG, Tiwari RP, Worsley PS, Dilworth MJ, Glenn AR, Howieson JG. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiology 1999; 145:1307–1316. PubMed http://dx.doi.org/10.1099/13500872-145-6-1307
DOE Joint Genome Institute. http://my.jgi.doe.gov/general/index.html
Bennett S. Solexa Ltd. Pharmacogenomics 2004; 5:433–438. PubMed http://dx.doi.org/10.1517/14622416.5.4.433
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature 2005; 437:376–380. PubMed
Zerbino DR. Using the Velvet de novo assembler for short-read sequencing technologies. Current Protocols in Bioinformatics 2010; Chapter 11:Unit 11 5.
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119. PubMed http://dx.doi.org/10.1186/1471-2105-11-119
Mavromatis K, Ivanova NN, Chen IM, Szeto E, Markowitz VM, Kyrpides NC. The DOE-JGI Standard operating procedure for the annotations of microbial genomes. Stand Genomic Sci 2009; 1:63–67. PubMed http://dx.doi.org/10.4056/sigs.632
Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455–457. PubMed http://dx.doi.org/10.1038/nmeth.1457
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955–964. PubMed
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100–3108. PubMed http://dx.doi.org/10.1093/nar/gkm160
Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res 2003; 31:439–441. PubMed http://dx.doi.org/10.1093/nar/gkg006
Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567–580. PubMed http://dx.doi.org/10.1006/jmbi.2000.4315
Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783–795. PubMed http://dx.doi.org/10.1016/j.jmb.2004.05.028
Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271–2278. PubMed http://dx.doi.org/10.1093/bioinformatics/btp393
Acknowledgements
This work was performed under the auspices of the US Department of Energy’s Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396. We gratefully acknowledge the funding received from the Murdoch University Strategic Research Fund through the Crop and Plant Research Institute (CaPRI) and the Centre for Rhizobium Studies (CRS) at Murdoch University. The authors would like to thank the Australia-China Joint Research Centre for Wheat Improvement (ACCWI) and SuperSeed Technologies (SST) for financially supporting Mohamed Ninawi’s PhD project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Reeve, W., Terpolilli, J., Melino, V. et al. Genome sequence of the lupin-nodulating Bradyrhizobium sp. strain WSM1417. Stand in Genomic Sci 9, 273–282 (2013). https://doi.org/10.4056/sigs.4518260
Published:
Issue Date:
DOI: https://doi.org/10.4056/sigs.4518260