Abstract
Rhizobium leguminosarum bv. trifolii SRDI565 (syn. N8-J) is an aerobic, motile, Gram-negative, non-spore-forming rod. SRDI565 was isolated from a nodule recovered from the roots of the annual clover Trifolium subterraneum subsp. subterraneum grown in the greenhouse and inoculated with soil collected from New South Wales, Australia. SRDI565 has a broad host range for nodulation within the clover genus, however N2-fixation is sub-optimal with some Trifolium species and ineffective with others. Here we describe the features of R. leguminosarum bv. trifolii strain SRDI565, together with genome sequence information and annotation. The 6,905,599 bp high-quality-draft genome is arranged into 7 scaffolds of 7 contigs, contains 6,750 protein-coding genes and 86 RNA-only encoding genes, and is one of 100 rhizobial genomes sequenced as part of the DOE Joint Genome Institute 2010 Genomic Encyclopedia for Bacteria and Archaea-Root Nodule Bacteria (GEBA-RNB) project.
Similar content being viewed by others
Introduction
Plant available nitrogen is a precious commodity in many agricultural soils and the most commonly limiting nutrient in plant growth. The supply of plant available nitrogen to nitrogen (N)-deficient farming systems is thus vital to productivity [1]. The application of industrially fixed nitrogenous fertilizer can meet the demand for N. However, this is a costly option as the price of nitrogenous fertilizer is connected to the cost of fossil fuels required for its production. Furthermore, the use of nitrogenous fertilizer contributes to greenhouse gas emissions and pollution of the environment. A more environmentally sustainable option is to exploit the process of biological nitrogen fixation that occurs in the symbiosis between legumes and rhizobia [2].
In this symbiotic association, rhizobia reduce atmospheric dinitrogen (N2) into bioavailable N that can be used by the plant for growth. Pasture legumes, including the clovers that comprise the Trifolium genus, are major contributors of biologically fixed N2 to mixed farming systems throughout the world [3,4]. In Australia, soils with a history of growing Trifolium spp. have developed large and symbiotically diverse populations of Rhizobium leguminosarum bv. trifolii (R. l. trifolii) that are able to infect and form nodules on a range of clover species. The N2-fixation capacity of the symbioses established by different combinations of clover hosts (Trifolium spp.) and strains of R. l. trifolii can vary from 10 to 130% when compared to an effective host-strain combination [3–9].
R. l. trifolii strain SRDI565 (syn. N8-J [10]) was isolated from a nodule recovered from the roots of the annual clover Trifolium subterraneum subsp. subterraneum that had been inoculated with soil collected from under a mixed pasture stand from Tumet, New South Wales, Australia and grown in N deficient media for four weeks after inoculation, in the greenhouse. SRDI565 was first noted for its sub-optimal N2-fixation capacity on T. subterraneum cv. Campeda (<60% of that with strain WSM1325) and formation of white (Fix-) pseudo-nodules on T. subterraneum cv. Clare [10,11]. Here we present a preliminary description of the general features for R. leguminosarum bv. trifolii strain SRDI565 together with its genome sequence and annotation.
Classification and general features
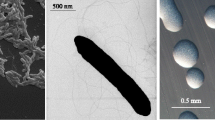
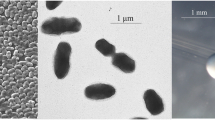
R. l. trifolii strain SRDI565 is a motile, Gram-negative rod (Figure 1 Left and Center) in the order Rhizobiales of the class Alphaproteobacteria. It is fast growing, forming colonies within 3–4 days when grown on half strength Lupin Agar (½LA) [12] at 28°C. Colonies on ½LA are white-opaque, slightly domed and moderately mucoid with smooth margins (Figure 1 Right).
Symbiotaxonomy
R. l. trifolii SRDI565 forms nodules on (Nod+), and fixes N2 (Fix+) with, a range of annual and perennial clover species of Mediterranean origin (Table 2). SRDI565 forms white, ineffective (Fix−) nodules with annual clovers T. glanduliferum and T. subterraneum cv. Clare, and with the perennial clovers T. pratense and T. polymorphum. SRDI565 does not form nodules on T. vesiculosum.
Genome sequencing and annotation information
Genome project history
This organism was selected for sequencing on the basis of its environmental and agricultural relevance to issues in global carbon cycling, alternative energy production, and biogeochemical importance, and is part of the Community Sequencing Program at the U.S. Department of Energy, Joint Genome Institute (JGI) for projects of relevance to agency missions. The genome project is deposited in the Genomes OnLine Database [30] and an improved-high-quality-draft genome sequence in IMG. Sequencing, finishing and annotation were performed by the JGI. A summary of the project information is shown in Table 3.
Minimum Information about the Genome Sequence (MIGS) is provided in Table 1. Figure 2 shows the phylogenetic neighborhood of R. l. trifolii strain SRDI565 in a 16S rRNA sequence based tree. This strain clusters closest to R. l. trifolii T24 and Rhizobium leguminosarum bv. phaseoli RRE6 with 99.8% and 99.6% sequence identity, respectively.
Phylogenetic tree showing the relationship of Rhizobium leguminosarum bv. trifolii SRDI565 (shown in blue print) with some of the root nodule bacteria in the order Rhizobiales based on aligned sequences of the 16S rRNA gene (1,307 bp internal region). All sites were informative and there were no gap-containing sites. Phylogenetic analyses were performed using MEGA, version 5.05 [28]. The tree was built using the maximum likelihood method with the General Time Reversible model. Bootstrap analysis [29] with 500 replicates was performed to assess the support of the clusters. Type strains are indicated with a superscript T. Strains with a genome sequencing project registered in GOLD [30] are in bold print and the GOLD ID is shown after the accession number. Published genomes are indicated with an asterisk.
Growth conditions and DNA isolation
Rhizobium leguminosarum bv. trifolii strain SRDI565 was cultured to mid logarithmic phase in 60 ml of TY rich media [31] on a gyratory shaker at 28°C. DNA was isolated from the cells using a CTAB (Cetyl trimethyl ammonium bromide) bacterial genomic DNA isolation method [32].
Genome sequencing and assembly
The genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 was sequenced at the Joint Genome Institute (JGI) using Illumina [33] data. An Illumina short-insert paired-end library with an average insert size of 243 ± 58 bp was used to generate 18,700,764 reads and an Illumina long-insert paired-end library with an average insert size of 8,446 ± 2,550 bp was used to generate 21,538,802 reads totalling 6,036 Mbp of Illumina data (unpublished, Feng Chen).
All general aspects of library construction and sequencing performed at the JGI can be found at the JGI user homepage [34]. The initial draft assembly contained 22 contigs in 16 scaffolds. The initial draft data was assembled with Allpaths, version 39750, and the consensus was computationally shredded into 10 Kb overlapping fake reads (shreds). The Illumina draft data was also assembled with Velvet, version 1.1.05 [35], and the consensus sequences were computationally shredded into 1.5 Kb overlapping fake reads (shreds). The Illumina draft data was assembled again with Velvet using the shreds from the first Velvet assembly to guide the next assembly. The consensus from the second VELVET assembly was shredded into 1.5 Kb overlapping fake reads. The fake reads from the Allpaths assembly and both Velvet assemblies and a subset of the Illumina CLIP paired-end reads were assembled using parallel phrap, version 4.24 (High Performance Software, LLC). Possible mis-assemblies were corrected with manual editing in Consed [36–38]. Gap closure was accomplished using repeat resolution software (Wei Gu, unpublished), and sequencing of bridging PCR fragments with PacBio (unpublished, Cliff Han) technology. For improved high quality draft, 4 PCR PacBio consensus sequences were completed to close gaps and to raise the quality of the final sequence. The estimated total size of the genome is 7 Mb and the final assembly is based on 6,036 Mb of Illumina draft data, which provides an average 862× coverage of the genome.
Genome annotation
Genes were identified using Prodigal [39] as part of the DOE-JGI annotation pipeline [40], followed by a round of manual curation using the JGI GenePRIMP pipeline [41]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) non-redundant database, UniProt, TIGRFam, Pfam, PRIAM, KEGG, COG, and InterPro databases. These data sources were combined to assert a product description for each predicted protein. Non-coding genes and miscellaneous features were predicted using tRNAscan-SE [42], RNAMMer [43], Rfam [44], TMHMM [45], and SignalP [46]. Additional gene prediction analyses and functional annotation were performed within the Integrated Microbial Genomes (IMG-ER) platform [47,48].
Genome properties
The genome is 6,905,599 nucleotides with 60.67% GC content (Table 4) and comprised of 7 scaffolds (Figures 3,4,5,6,7,8, and 9) of 7 contigs. From a total of 6,836 genes, 6,750 were protein encoding and 86 RNA-only encoding genes. The majority of genes (77.98%) were assigned a putative function whilst the remaining genes were annotated as hypothetical. The distribution of genes into COGs functional categories is presented in Table 5.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (scaffold 1.1). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (scaffold 2.2). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (scaffold 3.3). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (scaffold 4.4). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (scaffold 5.5). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (6.6). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
Graphical map of the genome of Rhizobium leguminosarum bv. trifolii strain SRDI565 (7.7). From bottom to the top of each scaffold: Genes on forward strand (color by COG categories as denoted by the IMG platform), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, sRNAs red, other RNAs black), GC content, GC skew.
References
O’Hara GW. The role of nitrogen fixation in crop production. J Crop Prod 1998; 1:115–138. http://dx.doi.org/10.1300/J144v01n02_06
Howieson JG, Yates RJ, Foster K, Real D, Besier B. Prospects for the future use of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE, editors. Leguminous Nitrogen-Fixing Symbioses. London, UK: Elsevier; 2008. p 363–394.
Herridge DF, Peoples MB, Boddey RM. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008; 311:1–18. http://dx.doi.org/10.1007/s11104-008-9668-3
Unkovich MJ, Baldock J, Peoples MB. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant Soil 2010; 329:75–89. http://dx.doi.org/10.1007/s11104-009-0136-5
Ballard RA, Shepherd BR, Charman N. Nodulation and growth of pasture legumes with naturalised soil rhizobia. 3. Lucerne (Medicago sativa L.). Aust J Exp Agric 2003; 43:135–140. http://dx.doi.org/10.1071/EA02047
Denton MD, Coventry DR, Bellotti WD, Howieson JG. Distribution, abundance and symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii from alkaline pasture soils in South Australia. Anim Prod Sci 2000; 40:25–35. http://dx.doi.org/10.1071/EA99035
Drew EA, Charman N, Dingemanse R, Hall E, Ballard RA. Symbiotic performance of Mediterranean Trifolium spp. with naturalised soil rhizobia. Crop Pasture Sci 2011; 62:903–913. http://dx.doi.org/10.1071/CP11047
Rys GJ, Bonish PM. Effectiveness of Rhizobium trifolii populations associated with Trifolium species in Taranaki, New Zealand. New Zealand Journal of Experimental Agriculture 1981; 9:329–335. http://dx.doi.org/10.1080/03015521.1981.10425430
Slattery JF, Coventry DR. Acid-tolerance and symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii isolated from subterranean clover growing in permanent pastures. Soil Biol Biochem 1995; 27:111–115. http://dx.doi.org/10.1016/0038-0717(94)00143-O
Drew EA, Ballard RA. Improving N2 fixation from the plant down: Compatibility of Trifolium subterraneum L. cultivars with soil rhizobia can influence symbiotic performance. Plant Soil 2010; 327:261–277. http://dx.doi.org/10.1007/s11104-009-0052-8
Melino VJ, Drew EA, Ballard RA, Reeve WG, Thomson G, White RG, O’Hara GW. Identifying abnormalities in symbiotic development between Trifolium spp. and Rhizobium leguminosarum bv. trifolii leading to sub-optimal and ineffective nodule phenotypes. Ann Bot (Lond) 2012; 110:1559–1572. PubMed http://dx.doi.org/10.1093/aob/mcs206
Howieson JG, Ewing MA, D’antuono MF. Selection for acid tolerance in Rhizobium meliloti. Plant Soil 1988; 105:179–188. http://dx.doi.org/10.1007/BF02376781
Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen M, Angiuoli SV, et al. Towards a richer description of our complete collection of genomes and metagenomes “Minimum Information about a Genome Sequence” (MIGS) specification. Nat Biotechnol 2008; 26:541–547. PubMed http://dx.doi.org/10.1038/nbt1360
Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576–4579. PubMed http://dx.doi.org/10.1073/pnas.87.12.4576
Garrity GM, Bell JA, Lilburn T. Phylum XIV. Proteobacteria phyl. nov. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT (eds), Bergey’s Manual of Systematic Bacteriology, Second Edition, Volume 2, Part B, Springer, New York, 2005, p. 1.
Garrity GM, Bell JA, Lilburn T. Class I. Alphaproteobacteria class. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Second ed: New York: Springer-Verlag; 2005.
Kuykendall LD. Order VI. Rhizobiales ord. nov. In: Garrity GM, Brenner DJ, Kreig NR, Staley JT, editors. Bergey’s Manual of Systematic Bacteriology. Second ed: New York: Springer-Verlag; 2005. p 324.
Validation List No. 107. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol 2006; 56:1–6. PubMed http://dx.doi.org/10.1099/ijs.0.64188-0
Skerman VBD, McGowan V, Sneath PHA. Approved Lists of Bacterial Names. Int J Syst Bacteriol 1980; 30:225–420. http://dx.doi.org/10.1099/00207713-30-1-225
Conn HJ. Taxonomic relationships of certain non-sporeforming rods in soil. J Bacteriol 1938; 36:320–321.
Frank B. Über die Pilzsymbiose der Leguminosen. Ber Dtsch Bot Ges 1889; 7:332–346.
Jordan DC, Allen ON. Genus I. Rhizobium Frank 1889, 338; Nom. gen. cons. Opin. 34, Jud. Comm. 1970, 11. In: Buchanan RE, Gibbons NE (eds), Bergey’s Manual of Determinative Bacteriology, Eighth Edition, The Williams and Wilkins Co., Baltimore, 1974, p. 262–264.
Young JM, Kuykendall LD, Martínez-Romero E, Kerr A, Sawada H. A revision of Rhizobium Frank 1889, with an emended description of the genus, and the inclusion of all species of Agrobacterium Conn 1942 and Allorhizobium undicola de Lajudie et al. 1998 as new combinations: Rhizobium radiobacter, R. rhizogenes, R. rubi, R. undicola and R. vitis. Int J Syst Evol Microbiol 2001; 51:89–103. PubMed
Editorial Secretary (for the Judicial Commission of the International Committee on Nomenclature of Bacteria). OPINION 34: Conservation of the Generic Name Rhizobium Frank 1889. Int J Syst Bacteriol 1970; 20:11–12. http://dx.doi.org/10.1099/00207713-20-1-11
Ramírez-Bahena MH, García-Fraile P, Peix A, Valverde A, Rivas R, Igual JM, Mateos PF, Martínez-Molina E, Velázquez E. Revision of the taxonomic status of the species Rhizobium leguminosarum (Frank 1879) Frank 1889AL, Rhizobium phaseoli Dangeard 1926AL and Rhizobium trifolii Dangeard 1926AL. R. trifolii is a later synonym ofR. leguminosarum. Reclassification of the strain R. leguminosarum DSM 30132 (=NCIMB 11478) as Rhizobium pisi sp. nov. Int J Syst Evol Microbiol 2008; 58:2484–2490. PubMed http://dx.doi.org/10.1099/ijs.0.65621-0
Agents B. Technical rules for biological agents. TRBA (http://www.baua.de):466.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 2000; 25:25–29. PubMed http://dx.doi.org/10.1038/75556
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 2011; 28:2731–2739. PubMed http://dx.doi.org/10.1093/molbev/msr121
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 1985; 39:783–791. http://dx.doi.org/10.2307/2408678
Liolios K, Mavromatis K, Tavernarakis N, Kyrpides NC. The Genomes On Line Database (GOLD) in 2007: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2008; 36:D475–D479. PubMed http://dx.doi.org/10.1093/nar/gkm884
Reeve WG, Tiwari RP, Worsley PS, Dilworth MJ, Glenn AR, Howieson JG. Constructs for insertional mutagenesis, transcriptional signal localization and gene regulation studies in root nodule and other bacteria. Microbiology 1999; 145:1307–1316. PubMed http://dx.doi.org/10.1099/13500872-145-6-1307
General Information for Collaborators. http://my.jgi.doe.gov/general/index.html
Bennett S. Solexa Ltd. Pharmacogenomics 2004; 5:433–438. PubMed http://dx.doi.org/10.1517/14622416.5.4.433
Zerbino DR. Using the Velvet de novo assembler for short-read sequencing technologies. Current Protocols in Bioinformatics 2010; Chapter 11:Unit 11 5.
Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 1998; 8:175–185. PubMed http://dx.doi.org/10.1101/gr.8.3.175
Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 1998; 8:175–185. PubMed http://dx.doi.org/10.1101/gr.8.3.175
Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res 1998; 8:195–202. PubMed http://dx.doi.org/10.1101/gr.8.3.195
Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119. PubMed http://dx.doi.org/10.1186/1471-2105-11-119
Mavromatis K, Ivanova NN, Chen IM, Szeto E, Markowitz VM, Kyrpides NC. The DOE-JGI Standard operating procedure for the annotations of microbial genomes. Stand Genomic Sci 2009; 1:63–67. PubMed http://dx.doi.org/10.4056/sigs.632
Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455–457. PubMed http://dx.doi.org/10.1038/nmeth.1457
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 1997; 25:955–964. PubMed
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 2007; 35:3100–3108. PubMed http://dx.doi.org/10.1093/nar/gkm160
Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR. Rfam: an RNA family database. Nucleic Acids Res 2003; 31:439–441. PubMed http://dx.doi.org/10.1093/nar/gkg006
Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567–580. PubMed http://dx.doi.org/10.1006/jmbi.2000.4315
Dyrløv Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783–795. PubMed http://dx.doi.org/10.1016/j.jmb.2004.05.028
Markowitz VM, Mavromatis K, Ivanova NN, Chen IM, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271–2278. PubMed http://dx.doi.org/10.1093/bioinformatics/btp393
DOE Joint Genome Institute. http://img.jgi.doe.gov/er
Acknowledgements
This work was performed under the auspices of the US Department of Energy’s Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396. We gratefully acknowledge the funding received from the Murdoch University Strategic Research Fund through the Crop and Plant Research Institute (CaPRI) and the Centre for Rhizobium Studies (CRS) at Murdoch University and the GRDC National Rhizobium Program (UMU00032). The authors would like to thank the Australia-China Joint Research Centre for Wheat Improvement (ACCWI) and SuperSeed Technologies (SST) for financially supporting Mohamed Ninawi’s PhD project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Reeve, W., Drew, E., Ballard, R. et al. Genome sequence of the clover-nodulating Rhizobium leguminosarum bv. trifolii strain SRDI565. Stand in Genomic Sci 9, 220–231 (2013). https://doi.org/10.4056/sigs.4468250
Published:
Issue Date:
DOI: https://doi.org/10.4056/sigs.4468250