Abstract
Sleep has long been thought of and promoted to be beneficial for memory. Some claims that sleep aids memory have been made in the absence of a critical interaction. This condition is necessary when using a commonly-used experimental design (a type of AM-PM PM-AM design). We propose that a sleep effect exists only if there is an interaction between groups (experimental and time-of-day controls) and the time of test or study (morning and evening). We show different patterns of results that would and would not support a sleep effect with empirical and model-generated data from recognition memory experiments and hypothetical data. While we use these data to make our point, our suggestions apply to any memory and non-memory-related investigation (e.g., emotional memory, false memory susceptibility, language learning, problem-solving). Testing for and finding the proper interaction will add to the evidence that sleep boosts performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Papers on the topic of sleep and cognition, especially memory, often include emphatic claims about the dramatic benefits of sleep (e.g., Ashton et al., 2019; Feld et al., 2013; Huguet et al., 2019; Inostroza & Born, 2013; Morgan et al., 2019; Rasch & Born, 2013; but see Ackermann et al., 2015; Cordi & Rasch, 2021). In a review with 1.6 k citations, including 385 recent ones, according to the journal’s article metrics (August 2022), the authors wrote, “Over more than a century of research has established the fact that sleep benefits the retention of memory” (Rasch & Born, 2013, p. 681). These conclusions are made based on results from studies that use one of many experimental designs. We suggest that if a certain design (AM-PM PM-AM) is used, to support the claim that sleep aids memory or other cognitions, there must be a significant interaction between experimental and control groups and AM and PM test (or study) times. It is rare to report this interaction in the literature, and it needs to be to show that sleep does indeed benefit memory, or whatever the phenomenon under investigation is.
While we focus on recognition memory in this paper, our recommendations apply to any investigation of sleep’s impact, including recall memory, autobiographical memory, associative memory, emotional memory, false memory susceptibility, language learning, motor learning, creativity, and problem-solving. We show the patterns of results that would support the claim that sleep benefits memory with experimental, simulated, and hypothetical data. As our point is a methodological one, we stay atheoretical when referring to a sleep benefit, which, according to various accounts, could be due to boosted consolidation, protection from interference, or contributions of both (for reviews on prominent theories, see Berres & Erdfelder, 2021; Cordi & Rasch, 2021; Squire et al., 2015; Wixted, 2005).
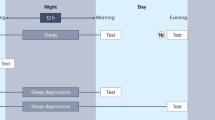
Several different experimental designs aim to understand sleep’s effects, including pharmacological manipulations (e.g., Feld & Diekelmann, 2015; Pinggal et al., 2022), stimulation methods (e.g., Cellini & Mednick, 2019; Grimaldi et al., 2020), and targeted memory reactivation (e.g., Cellini & Capuozzo, 2018; Hu et al., 2020). In many naturalistic sleep experiments, there is a daytime wake group (AM-PM) and a night-time sleep group (PM-AM). In investigations of sleep and recognition memory, participants study a list of stimuli and test on the same “old” stimuli (targets) and “new” items (lures). When the study and test phases occur differ for the different groups. Participants in the wake group take part in the study phase in the morning (AM) and, about 12 h later, are tested in the evening (PM) after going about their days. Participants in the sleep group take part in the study phase in the evening (PM) and, about 12 h later, are tested in the morning (AM) after sleeping. If memory is better for the sleep group, then sleep is thought to be beneficial for memory (e.g., Jones et al., 2018; Wilhelm et al., 2011).
Time-of-day effects can masquerade as sleep benefits because performance, including memory performance, fluctuates throughout the day (e.g., Barrett & Ekstrand, 1972; Folkard & Monk, 1980; Keisler et al., 2007; Nesca & Koulack, 1994; Pan & Rickard, 2015; Schmidt et al., 2007). Most articles involving naturalistic sleep experiments include concerns about time-of-day, or circadian, effects and at least one of many ways to mitigate the potential impact. Some designs, about 20% in a recent large metanalytic review paper (Berres & Erdfelder, 2021), are within-subject designs where participants take part in both sleep and wake groups (e.g., Gais et al., 2006; Maski et al., 2015; Prehn-Kristensen et al., 2009; Schönauer et al., 2015).
The other 80% are between-subjects studies. Many include analyses comparing groups' difference scores (memory performance on the later testing is subtracted from performance immediately after learning the material; e.g., Nissen et al., 2011; Sonni & Spencer, 2015; van Rijn et al., 2017). Other approaches include comparing outcomes on various scales, including sleepiness scores (e.g., Diekelmann et al., 2010; van Rijn et al., 2017) and mood scales (e.g., Prehn-Kristensen et al., 2015), and other cognitive tests (e.g., working memory tests; Potkin & Bunney, 2012). Still, others use data from nap studies as a control (e.g., Baran et al., 2010; Payne et al., 2009). Some researchers collect data from participants with 24-h retention intervals (Ellenbogen et al., 2006). Some combine multiple approaches (e.g., Diekelmann et al., 2010; Himmer et al., 2017; Jurewicz et al., 2016; Kurdziel & Spencer, 2016). And some suggest follow-up research is needed that controls for time-of-day effects (e.g., Hupbach, 2018; Takeuchi et al., 2014). Any methodological concerns about these approaches to cope with the challenging time-of-day problem are outside the scope of this paper (see Nemeth et al., 2021). The ever-present concern for time-of-day confounds has led to varied attempts to control them. We present an improvement to one approach (that could be applied more widely).
Using time-of-day control participants is another way researchers deal with the potential confound (e.g., Abel & Bäuml, 2012, 2013; Bäuml et al., 2014 Experiments 1 and 3; Baran et al., 2012; Bennion et al., 2015; Bennion et al., 2017; Chambers & Payne, 2014; Ekstrand, 1967; Fenn et al., 2009; Lutz et al., 2017; Monaghan et al., 2015; Morgan et al., 2019; Payne et al., 2008; Racsmány et al., 2010, Experiment 2; Scullin & McDaniel, 2010; Sheth et al., 2009). The inclusion of the control groups is a prerequisite for the analysis we recommend. Unlike the experimental groups with approximately 12-h long wake- or sleep-filled retention intervals, the control groups participate in one session, with considerably shorter retention intervals. Participants in the AM control group take part in the study and test phases in the morning, and participants in the PM control group take part in the study and test phases in the evening. If performance is better for the AM control group than the PM control group, and if performance is better for the sleep group than the wake group, this could reflect that testing in the AM benefits memory, not that sleep, per se, benefits memory. That is, performance could be better because the sleep group and AM control group take the test in the morning.Footnote 1 This may be the case even if pairwise comparisons are made and the difference in accuracy is significant for the experimental groups but not for the control groups. Assuming this pattern of results supports a sleep benefit is, in effect, turning the null hypothesis of no difference between the control groups into a finding (Gelman & Stern, 2006). If there is no interaction of performance between the groups and the test time, then it is a mistake to claim a sleep benefit. The results need to show that having a period of sleep between the study and test phases improves memory over a period of wake, beyond being tested in the morning. These analyses, however, are not reported in the literature, with one exception (Scullin & McDaniel, 2010).
Instead of conducting the analysis that we suggest, it is most common to analyze the data from the experimental and control groups separately (e.g., Baran et al., 2012; Bäuml et al., 2014 Experiments 1 and 3; Bennion et al., 2015; Bennion et al., 2017; Chambers & Payne, 2014; Ekstrand, 1967; Fenn et al., 2009; Monaghan et al., 2015). These experiments set a precedent for research that follows, including ours. We published a registered report in which the first stage included our analysis plans (Morgan et al., 2019). We proposed to use the standard AM-PM PM-AM design with time-of-day controls. The planned analyses included separately analyzing the experimental and control data. In the Stage 1 manuscript, we wrote that we would rule out a time of day confound by simply comparing the AM control group to the PM control group. Our implicit assumption was that there would be no difference between the AM and PM control groups. Only after the results were published did we realize this is the wrong approach to support a true sleep benefit.Footnote 2
The right approach is to test for the right interaction. There is not enough information reported in the literature to test for the interaction with published data. We contacted the corresponding authors of the experiments that were set up in a way allowing us to conduct the recommended analysis. We received data from approximately 30% of our requests, resulting in data from multiple experiments collected by one lab and three experiments from two other labs (including the current paper’s corresponding author’s lab). Even in the limited number of datasets, we found half of the recall data yielded a significant interaction, thus providing existence proof that the method here has utility. We offer empirical and simulated data (and hypothetical data in the discussion section) as examples of interactions that would and would not support that memory is better when sleep occurs after learning.
Empirical data
The empirical data are from a standard list-learning recognition memory experiment in which we used the AM-PM PM-AM design with time-of-day control groups. There were two lists of English (List 1) and Japanese (List 2) words for participants to try to remember. As we collected the data for illustrative purposes to make our methodological point, not to test a hypothesis, we did not choose a sample size in advance.Footnote 3 We also use these data to generate simulated data to provide more examples.
Method
Participants
Participants (N = 178) were first-year students enrolled in the psychology undergraduate program at the University of Bristol. All participants consented to take part. The University of Bristol Ethics Board gave ethical approval, #030,321,116,988. Due to a computer issue, data from one participant were excluded from the analyses. Another participant gave the same response to all items, so those data were also excluded from all analyses.
Group assignment depended on whether participants were born on an odd or even day and month. This assignment yielded 46 participants in the AM control group, 58 participants in the PM control group, 39 participants in the wake group, and 33 participants in the sleep group (138 female, 38 male; µage = 19.82 years, SD = 2.58). Ages were not significantly different by group, F(3, 172) = 0.631, p = 0.596. Because nine participants indicated knowing some Japanese, their data were excluded from List 2 analyses. Without those participants, there were 45 participants in the AM control group, 53 participants in the PM control group, 39 participants in the wake group, and 31 participants in the sleep group (132 female, 36 male, µage = 19.79 years, SD = 2.53). Ages were not significantly different by group, F(3, 164) = 0.686, p = 0.562.
Materials
Stimuli
List 1 was made of 50 English monosyllabic concrete nouns (e.g., girl, tent), half animate and half inanimate and List 2 was made of 50 Japanese nouns (e.g., tori, nezumi), half bisyllabic and half trisyllabic. For both lists, we randomly selected 25 words as targets and 25 words as lures, so that there was an approximately equal proportion of animate versus inanimate words (List 1) and two- versus three-syllable words (List 2) for targets and lures. Eight practice words (four words per list) were not on List 1 or List 2.
Sleep-related information
We administered the Epworth Sleepiness Scale, which measures general daytime sleepiness (Johns, 1991). We also asked participants how many hours they slept the night prior to the day they took part in the experiment. Participants in the sleep group reported hours they slept for 2 nights.
Individual information
Participants provided their age and sex, and whether they speak Japanese, to what extent, and if they knew the meaning of any of the words on List 2.
Procedure
All participation took part online using the platform, Gorilla (Anwyl-Irvine et al., 2020). Participants in the control groups completed the experiment in one session. Participants in the AM control group completed the experiment between 8 a.m. and 11 a.m., and participants in the PM control group completed the experiment between 8 p.m. and 11 p.m. Participants in the experimental groups completed the experiment in two sessions. The first session was the study phase, and the second session was the test phase. Participants in the wake group completed the first session between 8 a.m. and 11 a.m. and completed the second session on the same day between 8 p.m. and 11 p.m. Participants in the sleep group completed the first session between 8 p.m. and 11 p.m. and completed the second session the following morning between 8 a.m. and 11 a.m. (Himmer et al., 2017; Morgan et al., 2019). Whether they participated in one or two sessions, the experiment took the same overall time.
After consenting, the participants proceeded with the study phase for List 1 (English words) followed by List 2 (Japanese words). They were instructed that they would see English and Japanese words and that their memory for the words would be tested later. For List 1, participants had 3 s to indicate with key presses whether each word corresponded to an animate or inanimate object. For List 2, participants had 3 s to indicate with key presses whether each word contained two or three syllables. Between items, a fixation cross appeared for 250 ms. Each list was preceded by a 4-item practice session which was identical to the task except overall feedback was provided (e.g., “You were correct on 3 out of 4 trials.”).
During the test phase, participants indicated whether each item was presented during the study phase on a 6-point scale (100% certain not on the list, probably not on the list, maybe not on the list, maybe on the list, probably on the list, 100% certain on the list). Responses were self-paced. All items presented during the practice, study phase, and test phase were randomly presented.
At the end of the experiment, participants answered the Epworth Sleepiness Scale questions (Johns, 1991), reported how many hours they slept the night before, and answered the demographic questions. They were debriefed later during class.
Results
Empirical data
Here we focus on the results that directly bear on our point about interactions, and therefore provide the Epworth Sleepiness Scale scores and sleep time comparisons across groups in Supplementary Information. The data and word lists are available at the Open Science Framework (https://osf.io/vmjry/?view_only=8f68a6f60fd44ec4817f983e9d3a8e1e).
To measure discriminability (i.e., the ability to discern targets from lures), da scores were computed for each participant and then averaged across participants in each group. da is like d′, but it does not assume that the target and lure distributions have equal variances and is often more appropriate for data from list-learning experiments (e.g., Mickes et al., 2007; Rotello et al., 2008). The dependent variable da is given by
where z(H) and z(F) are the z-scores of hit and false alarm rates, respectively, and s is the z-ROC slope (Macmillan & Creelman, 2005). The slope gives an estimate of σlure/σtarget. We used 0.80 for all z-ROC slopes.
We used JASP for conducting inferential statistical tests (JASP team, 2022). We performed a 2 (group: experimental vs. control) × (time of test: morning vs. evening) ANOVA on the da values. Figure 1a and b shows the average da scores by group as a function of test time for List 1 and List 2, respectively.
Table 1 shows the ANOVA results. There was not a significant main effect of time of test (morning vs. evening), there was a significant main effect of group (experimental: wake/sleep vs. control: AM/PM), and the interaction was not significant for List 1 or List 2. The main effect of group is expected given that the control groups’ retention interval is considerably shorter than the experimental groups’ retention interval.
Model fits and simulated data
In the empirical data, the interactions were not significant. There may not have been enough power in our opportunity sample to detect a small interaction effect. Our goal with data collection was to provide examples and to use them for generating data. We generated data using the unequal variance signal detection model (UVSD; Egan, 1958; Ratcliff et al., 1992; Wixted, 2007) fitted to the empirical data. The details of the fits and data generation are presented in the Supplementary Information. The average da values of the simulated data are shown in Fig. 2.
Between-subjects 2 × 2 ANOVA. Average da by group as a function of time of test for the simulated data from List 1 (a) and List 2 (b). The different colors and shapes represent the different groups. The error bars are 95% confidence intervals. The interaction is significant in a but not in b. (Colour figure online)
As with the empirical data, the da values from the generated data were subjected to a 2 (group: experimental vs. control) × 2 (time of test: morning vs. evening) ANOVA test. The results are shown in Table 2. There was a significant, small interaction in the List 1 simulated data. This interaction is needed to claim that sleep benefits memory. The interaction was not significant in the List 2 simulated data. List 2 data provide an opportunity to show the pattern of results that can lure researchers into erroneously concluding a sleep benefit. That is, the sleep group significantly outperforms the wake group but the difference between the control groups is not significantly different.
To show how this pattern can be misleading, we conducted t-tests on the List 2 da scores from the experimental groups (sleep vs. wake) and control groups (AM vs. PM), as has been done in the sleep literature. Because the difference in da scores between the control groups was not significant, t(378) = 1.22, p = 0.223, but the difference between the experimental groups was significant, t(378) = 2.27, p = 0.024, one may be tempted to conclude a sleep benefit. However, the lack of the interaction from the ANOVA, p = 0.482, does not support a sleep benefit interpretation. Moreover, if time-of-day controls were not included, then the significant difference in scores between the sleep group and wake group would be erroneously interpreted as a sleep benefit.
Discussion
We propose that interactions are needed to strengthen claims about sleep’s benefits on memory when using time-of-day control groups with experimental groups using the specific design described here. We presented empirical and simulated data, showing one acceptable interaction with the List 1 simulated data. However, not all interactions are sufficient (e.g., Pashler et al., 2008; Redick, 2015).
Figure 3 shows hypothetical data plotted in different patterns, including acceptable and unacceptable interactions, to demonstrate that the interaction must take a specific form to support a sleep benefit. Consider that these hypothetical data are from the same type of recognition memory experiment presented earlier. Figure 3 illustrates eight possible outcomes of this hypothetical experiment. As in Figs. 1 and 2, in each panel, da is plotted by group (experimental and control) as a function of test time (morning or evening).
Between-subjects 2 × 2 ANOVA for eight hypothetical datasets. Average da by group as a function of time of test. The different colors and shapes represent the different groups. There are no acceptable patterns in the top panels, a-d, and there are acceptable interactions in the bottom panels, e–h. (Colour figure online)
In the top panels, Fig. 3a–d, no patterns would support a sleep benefit claim. In Fig. 3a, the sleep group outperformed the wake group, and the AM control group outperformed the PM control group. Thus, the groups tested in the morning performed better than those tested in the evening. In Fig. 3b, the AM control group performed better than the PM control group, as did the sleep group over the wake group. Yet neither difference is enough for a significant interaction. The pattern of the experimental group data in Fig. 3a and b may be confused for a sleep benefit without considering the AM and PM control group data. However, the lack of the interaction shows that would be the wrong conclusion.
Figure 3c shows the PM control group outperformed the AM control group, but the experimental groups performed similarly. The pattern, therefore, supports a time-of-day effect, not a sleep one. Figure 3d shows an interaction in the opposite direction where the wake group outperforms the sleep group. The lack of significant interactions in Fig. 3a and b and the interactions in Fig. 3c and d in the wrong direction precludes interpretations of a sleep benefit.
In the bottom panels, Fig. 3e–h, the interactions allow for interpretations of a sleep benefit. In Fig. 3e and f, the cross-over interactions are evident. In Fig. 3e, the sleep group performed better than the wake group, and the AM control group performed worse than the PM control group. This pattern clearly shows the interaction and that both sleep and time of day contributed. Similarly, in Fig. 3f, the sleep group outperformed the wake group, and the am and pm groups performed alike. Thus, the interaction is driven by the experiment groups’ performance. The patterns in these two figures (Fig. 3e and f) are unlikely given that the control groups have a substantially shorter retention interval than the experimental groups. While unlikely, that pattern is not unheard of (e.g., Abel & Bäuml, 2013), and it is a type of acceptable interaction.
In Fig. 3g, the sleep group outperformed the wake group, and the control groups’ performance is similar. Therefore, the experimental groups drove the interaction. The patterns in Fig. 3f and g clearly show that the time the test was taken did not influence the interaction. Figure 3h is similar, where the sleep group outperformed the wake group, but the PM control group outperformed the AM control group. This pattern indicates sleep and time-of-day influenced performance, both driving the interaction. All the interactions in Fig. 3e–h allow for interpretations of a sleep benefit. Note that the data can be plotted the other way with the group on the x-axis and the test time as separate lines and it may be easier to see a crossover interaction this way.
Interpreting interactions is challenging (e.g., Redick, 2015; Rohrer & Arslan, 2021). Because da (and d') theoretically lies on an interval measurement scale, given the assumptions of signal detection theory, interactions can be meaningfully interpreted (Embretson, 1996). A signal detection-based theory is a theory of underlying memory signals, with a firm grounding in measurement methodology dating back to Fechner (Fechner 1860/1966; see Wixted, 2019, for a historical review). According to Wickelgren (1974),
Using the methods of statistical decision theory, an interval-scale measurement of the memory strength is possible for recognition memory, making only rather weak and plausible assumptions concerning the decision process that translates strength into yes-no decisions (p. 776).
Thus, if the theory embraced underlying da (or d') is signal detection-based, there is further credence for interpreting any interactions.
This brings us to an important caveat regarding other kinds of dependent measures. Non-crossover interactions can be changed by transformations of the data such that there is no longer an interaction and if there is no noncrossover interaction, transformations of the data can change that outcome into an interaction (Loftus, 1978; Wagenmakers et al., 2012). This feature applies when memory is tested with a recall test yielding proportion correct scores subjected to an interaction test. Unlike when da (or d') is considered, it is highly unlikely that there is a linear relationship, or direct mapping, between the dependent measure (i.e., the proportion of items correctly recalled) and the underlying memory processes (i.e., recall; Wixted, 1990). Therefore, any interpretation of a noncrossover interaction test of proportion correct scores, even if statistically significant, should be made with caution.
The methodological limitations include unequal sample sizes. Unequal group sizes may decrease the ability to detect an interaction. However, reducing the sample size to match the group with the smallest n yielded the same results—no significant interactions—as with the full sample size. Another limitation is that our opportunity sample of university students may not reflect a wider demographic (e.g., Schlarb et al., 2017). Yet another limitation is the varied and large window of time for participation. Presenting the stimuli closer to sleep would reduce interference in the sleep group.
Going forward, new experiments should be designed to allow for the test of the interaction. If future empirical results turn out such that there is a critical interaction, as there was in the List 1 simulated data, then an interpretation of a sleep benefit can be made. If, on the other hand, there is no interaction, despite there being significantly better performance in the sleep group than the wake group but no significant difference between the control group's performance, as there was in the List 2 simulated data, no sleep benefit claims should be made. Ideally, the robustness of the memory benefit when memory is probed by recall and recognition tests is established before drilling down into smaller components (emotional vs. nonemotional, feedback vs. no feedback; recollection vs. familiarity, etc.). Additionally, the interaction in the behavioral data must exist before making any links to the physiological or neural data (Krakauer et al., 2017).
Our focus on one type of design does not minimize our point as researchers use the results from these studies to support the idea that there is a sleep benefit. The data from these studies are also included in meta-analyses that provide overviews of the state of the field (e.g., Berres & Erdfelder, 2021; Lipinska et al., 2019; Schäfer et al., 2020). There are a host of designs to understand the impacts of sleep. This between-subjects design is a convenient way to measure sleep’s effects, necessary for some investigations, and when used, interactions are key. Implementing this analysis and meaningfully interpreting the results would mean making some changes to research practices (see also Nemeth et al., 2021). Unambiguously finding the critical interaction is one way to minimize concerns about a time-of-day confound and help determine if memory is better when we sleep after learning compared to a retention interval filled with daytime wakefulness.
Notes
We are agnostic about whether study or test time is used for the purposes here. We opted for the time of test over time of study because of the evidence that time of test, not sleep or time of study, boosts motor learning outcomes (Pan & Rickard, 2015). It may not be the case for declarative memory, and these are interesting future considerations.
Fortunately, the ideas proposed in the current paper do not change the conclusions reported by Morgan et al. (2019).
Our sample size of 176 is larger than most of the studies, where, according to a recent meta-analysis, the median sample size was 16 participants per group (Berres & Erdfelder, 2021).
References
Abel, M., & Bäuml, K.-H.T. (2012). Retrieval-induced forgetting, delay, and sleep. Memory, 20(5), 420–428. https://doi.org/10.1080/09658211.2012.671832
Abel, M., & Bäuml, K.-H.T. (2013). Sleep can eliminate list-method directed forgetting. Journal of Experimental Psychology: Learning, Memory, and Cognition, 39(3), 946–952. https://doi.org/10.1037/a0030529
Ackermann, S., Hartmann, F., Papassotiropoulos, A., de Quervain, D.J.-F., & Rasch, B. (2015). No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep, 38(6), 951–959. https://doi.org/10.5665/sleep.4748
Anwyl-Irvine, A. L., Massonnié, J., Flitton, A., Kirkham, N., & Evershed, J. K. (2020). Gorilla in our midst: An online behavioral experiment builder. Behavior Research Methods, 52, 388–407. https://doi.org/10.3758/s13428-019-01237-x
Ashton, J. E., Harrington, M. O., Guttesen, A. Á., Smith, A. K., & Cairney, S. A. (2019). Sleep preserves physiological arousal in emotional memory. Scientific Reports, 9(1), 1–10.
Baran, B., Wilson, J., & Spencer, R. M. C. (2010). Rem-dependent repair of competitive memory suppression. Experimental Brain Research, 203(2), 471–477. https://doi.org/10.1007/s00221-010-2242-2
Baran, B., Pace-Schott, E. F., Ericson, C., & Spencer, R. M. (2012). Processing of emotional reactivity and emotional memory over sleep. Journal of Neuroscience, 32(3), 1035–1042.
Barrett, T. R., & Ekstrand, B. R. (1972). Effects of sleep on memory: Controlling for time of day effects. Journal of Experimental Psychology, 96(2), 321–327.
Bäuml, K. T., Holterman, C., & Abel, M. (2014). Sleep can reduce the testing effect: It enhances recall of restudied items but can leave recall of retrieved items unaffected. Journal of Experimental Psychology: Learning, Memory, and Cognition, 40(6), 1568–1581. https://doi.org/10.1037/xlm0000025
Bennion, K. A., Mickley Steinmetz, K. R., Kensinger, E. A., & Payne, J. D. (2015). Sleep and cortisol interact to support memory consolidation. Cerebral Cortex (New York, N.Y. : 1991), 25(3), 646–657. https://doi.org/10.1093/cercor/bht255
Bennion, K. A., Payne, J. D., & Kensinger, E. A. (2017). Residual effects of emotion are reflected in enhanced visual activity after sleep. Cognitive, Affective, & Behavioral Neuroscience, 17(2), 290–304. https://doi.org/10.3758/s13415-016-0479-3
Berres, S., & Erdfelder, E. (2021). The sleep benefit in episodic memory: An integrative review and a meta-analysis. Psychological Bulletin, 147(12), 1309–1353. https://doi.org/10.1037/bul0000350
Cellini, N., & Capuozzo, A. (2018). Shaping memory consolidation via targeted memory reactivation during sleep. Annals of the New York Academy of Sciences, 1426(1), 52–71. https://doi.org/10.1111/nyas.13855
Cellini, N., & Mednick, S. C. (2019). Stimulating the sleeping brain: Current approaches to modulating memory-related sleep physiology. Journal of Neuroscience Methods, 316, 125–136. https://doi.org/10.1016/j.jneumeth.2018.11.011
Chambers, A. M., & Payne, J. D. (2014). Laugh yourself to sleep: Memory consolidation for humorous information. Experimental Brain Research, 232(5), 1415–1427. https://doi.org/10.1007/s00221-013-3779-7
Cordi, M. J., & Rasch, B. (2021). How robust are sleep-mediated memory benefits? Current Opinion in Neurobiology, 67, 1–7. https://doi.org/10.1016/j.conb.2020.06.002
Diekelmann, S., Born, J., & Wagner, U. (2010). Sleep enhances false memories depending on general memory performance. Behavioural Brain Research, 208(2), 425–429. https://doi.org/10.1016/j.bbr.2009.12.021
Egan, J. P. (1958). Recognition memory and the operating characteristic (Tech. Note AFCRC-TN-58–51). Bloomington: Indiana University, Hearing and Communication Laboratory.
Ekstrand, B. R. (1967). Effect of sleep on memory. Journal of Experimental Psychology, 75(1), 64–72. https://doi.org/10.1037/h0024907
Ellenbogen, J. M., Hulbert, J. C., Stickgold, R., Dinges, D. F., & Thompson-Schill, S. L. (2006). Interfering with theories of sleep and memory: Sleep, declarative memory, and associative interference. Current Biology, 16(13), 1290–1294. https://doi.org/10.1016/j.cub.2006.05.024
Embretson, S. E. (1996). Item response theory models and spurious interaction effects in factorial ANOVA designs. Applied Psychological Measurement, 20(3), 201–212.
Fechner. G. T. (1966). Elements of psychophysics (Vol. I) (E. G. Boring & D. H. Howes, Eds.; H. E. Adler, Trans.). Holt. Rinehart & Winston. (Original work published 1860.)
Feld, G. B., & Diekelmann, S. (2015). Sleep smart—Optimizing sleep for declarative learning and memory. Frontiers in Psychology, 6. https://doi.org/10.3389/fpsyg.2015.00622
Feld, G. B., Lange, T., Gais, S., & Born, J. (2013). Sleep-dependent declarative memory consolidation–unaffected after blocking NMDA or AMPA receptors but enhanced by NMDA coagonist d-Cycloserine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38(13), 2688–2697. https://doi.org/10.1038/npp.2013.179
Fenn, K. M., Gallo, D. A., Margoliash, D., Roediger, H. L., & Nusbaum, H. C. (2009). Reduced false memory after sleep. Learning & Memory, 16(9), 509–513.
Folkard, S., & Monk, T. H. (1980). Circadian rhythms in human memory. British Journal of Psychology, 71(2), 295–307. https://doi.org/10.1111/j.2044-8295.1980.tb01746.x
Gais, S., Lucas, B., & Born, J. (2006). Sleep after learning aids memory recall. Learning & Memory, 13(3), 259–262.
Gelman, A., & Stern, H. (2006). The difference between “significant” and “not significant” is not itself statistically significant. American Statistician, 60(4), 328–331.
Grimaldi, D., Papalambros, N. A., Zee, P. C., & Malkani, R. G. (2020). Neurostimulation techniques to enhance sleep and improve cognition in aging. Neurobiology of Disease, 141. https://doi.org/10.1016/j.nbd.2020.104865
Himmer, L., Müller, E., Gais, S., & Schönauer, M. (2017). Sleep-mediated memory consolidation depends on the level of integration at encoding. Neurobiology of Learning and Memory, 137, 101–106. https://doi.org/10.1016/j.nlm.2016.11.019
Hu, X., Cheng, L. Y., Chiu, M. H., & Paller, K. A. (2020). Promoting memory consolidation during sleep: A meta-analysis of targeted memory reactivation. Psychological Bulletin, 146(3), 218–244. https://doi.org/10.1037/bul0000223
Huguet, M., Payne, J. D., Kim, S. Y., & Alger, S. E. (2019). Overnight sleep benefits both neutral and negative direct associative and relational memory. Cognitive, Affective, & Behavioral Neuroscience, 19(6), 1391–1403. https://doi.org/10.3758/s13415-019-00746-8
Hupbach, A. (2018). Long-term effects of directed forgetting. Memory, 26(3), 321–329. https://doi.org/10.1080/09658211.2017.1358748
Inostroza, M., & Born, J. (2013). Sleep for preserving and transforming episodic memory. Annual Review of Neuroscience, 36(1), 79–102. https://doi.org/10.1146/annurev-neuro-062012-170429
JASP Team. (2022). JASP (Version 0.16.1) [Computer software]. https://jasp-stats.org/
Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14(6), 540–545.
Jones, B. J., Mackay, A., Mantua, J., Schultz, K. S., & Spencer, R. M. (2018). The role of sleep in emotional memory processing in middle age. Neurobiology of Learning and Memory, 155, 208–215.
Jurewicz, K., Cordi, M. J., Staudigl, T., & Rasch, B. (2016). No evidence for memory decontextualization across one night of sleep. Frontiers in Human Neuroscience, 10, 7–7. https://doi.org/10.3389/fnhum.2016.00007
Keisler, A., Ashe, J., & Willingham, D. T. (2007). Time of day accounts for overnight improvement in sequence learning. Learning & Memory, 14, 669–672.
Krakauer, J. W., Ghazanfar, A. A., Gomez-Marin, A., MacIver, M. A., & Poeppel, D. (2017). Neuroscience needs behavior: Correcting a reductionist bias. Neuron, 93(3), 480–490.
Kurdziel, L. B. F., & Spencer, R. M. C. (2016). Consolidation of novel word learning in native English-speaking adults. Memory, 24(4), 471–481. https://doi.org/10.1080/09658211.2015.1019889
Lipinska, G., Stuart, B., Thomas, K. G. F., Baldwin, D. S., & Bolinger, E. (2019). Preferential consolidation of emotional memory during sleep: A meta-analysis. Frontiers in Psychology, 10(MAY). https://doi.org/10.3389/fpsyg.2019.01014
Loftus, G. R. (1978). On interpretation of interactions. Memory & Cognition, 6, 312–319.
Lutz, N. D., Diekelmann, S., Hinse-Stern, P., Born, J., & Rauss, K. (2017). Sleep supports the slow abstraction of gist from visual perceptual memories. Scientific Reports, 7(1). https://doi.org/10.1038/srep42950
Macmillan, N. A., & Creelman, C. D. (2005). Detection theory: A user’s guide (2nd ed.). Mahwah, NJ: Erlbaum.
Maski, K., Holbrook, H., Manoach, D., Hanson, E., Kapur, K., & Stickgold, R. (2015). Sleep dependent memory consolidation in children with autism spectrum disorder. Sleep, 38(12), 1955–1963. https://doi.org/10.5665/sleep.5248
Mickes, L., Wixted, J. T., & Wais, P. E. (2007). A direct test of the unequal-variance signal detection model of recognition memory. Psychonomic Bulletin & Review, 14(5), 858–865.
Monaghan, P., Sio, U. N., Lau, S. W., Woo, H. K., Linkenauger, S. A., & Ormerod, T. C. (2015). Sleep promotes analogical transfer in problem solving. Cognition, 143, 25–30.
Morgan, D. P., Tamminen, J., Seale-Carlisle, T. M., & Mickes, L. (2019). The impact of sleep on eyewitness identifications. Royal Society Open Science, 6(12). https://doi.org/10.1098/rsos.170501
Nemeth, D., Gerbier, E., Born, J., Rickard, T., Albouy, G., Diekelman, S., Fogel, S., Genzel, L.; Prehn-Kristensen, A., Payne, J., Dresler, M., Simor, P., Mazza, S., Hoedlmoser, Ruby, P, Spencer, R., Schabus, M., Janacsek, K. (2021). Pitfalls in sleep and memory research and how to avoid them: A consensus paper. Preprints, 2019080208. https://doi.org/10.20944/preprints201908.0208.v2
Nesca, M., & Koulack, D. (1994). Recognition memory, sleep and circadian rhythms. Canadian Journal of Experimental Psychology, 48(3), 359–359.
Nissen, C., Kloepfer, C., Feige, B., Piosczyk, H., Spiegelhalder, K., Voderholzer, U., & Riemann, D. (2011). Sleep-related memory consolidation in primary insomnia. Journal of Sleep Research, 20(1, Pt. 2), 129–36. https://doi.org/10.1111/j.1365-2869.2010.00872.x
Pan, S., & Rickard, T. (2015). Sleep and motor learning: Is there room for consolidation? Psychological Bulletin, 141, 812–834.
Pashler, H., McDaniel, M., Rohrer, D., & Bjork, R. (2008). Learning styles: Concepts and evidence. Psychological Science in the Public Interest, 9(3), 105–119.
Payne, J. D., Stickgold, R., Swanberg, K., & Kensinger, E. A. (2008). Sleep preferentially enhances memory for emotional components of scenes. Psychological Science, 19(8), 781–788.
Payne, J. D., Schacter, D. L., Propper, R. E., Huang, L.-W., Wamsley, E. J., Tucker, M. A., Walker, M. P., & Stickgold, R. (2009). The role of sleep in false memory formation. Neurobiology of Learning and Memory, 92(3), 327–334. https://doi.org/10.1016/j.nlm.2009.03.007
Pinggal, E., Dockree, P. M., O’Connell, R. G., Bellgrove, M. A., & Andrillon, T. (2022). Pharmacological manipulations of physiological arousal and sleep-like slow waves modulate sustained attention. The Journal of Neuroscience, 42(43), 8113–8124. https://doi.org/10.1523/JNEUROSCI.0836-22.2022
Potkin, K. T., & Bunney, W. E. (2012). Sleep improves memory: The effect of sleep on long term memory in early adolescence. PLoS ONE, 7(8), 8–11.
Prehn-Kristensen, A., Göder, R., Chirobeja, S., Breßmann, I., Ferstl, R., & Baving, L. (2009). Sleep in children enhances preferentially emotional declarative but not procedural memories. Journal of Experimental Child Psychology, 104(1), 132–139. https://doi.org/10.1016/j.jecp.2009.01.005
Prehn-Kristensen, A., Lotzkat, K., Bauhofer, E., Wiesner, C. D., & Baving, L. (2015). Sleep supports memory of odors in adults but not in children. PLOS ONE, 10(9), Article 0139069. https://doi.org/10.1371/journal.pone.0139069
Racsmány, M., Conway, M. A., & Demeter, G. (2010). Consolidation of episodic memories during sleep: Long-term effects of retrieval practice. Psychological Science, 21(1), 80–85.
Rasch, B., & Born, J. (2013). About sleep’s role in memory. Physiological Reviews, 93(2), 681–766.
Ratcliff, R., Sheu, C. F., & Gronlund, S. D. (1992). Testing global memory models using ROC curves. Psychological Review, 99(3), 518–535.
Redick, T. S. (2015). Working memory training and interpreting interactions in intelligence interventions. Intelligence, 50, 14–20. https://doi.org/10.1016/j.intell.2015.01.014
Rohrer, J. M., & Arslan, R. C. (2021). Precise answers to vague questions: Issues with interactions. Advances in Methods and Practices in Psychological Science. https://doi.org/10.1177/25152459211007368
Rotello, C. M., Masson, M. E., & Verde, M. F. (2008). Type I error rates and power analyses for single-point sensitivity measures. Perception & Psychophysics, 70(2), 389–401.
Schäfer, S. K., Wirth, B. E., Staginnus, M., Becker, N., Michael, T., & Sopp, M. R. (2020). Sleep’s impact on emotional recognition memory: A meta-analysis of whole-night, nap, and REM sleep effects. Sleep Medicine Reviews, 51. https://doi.org/10.1016/j.smrv.2020.101280
Schlarb, A. A., Friedrich, A., & Claßen, M. (2017). Sleep problems in university students—An intervention. Neuropsychiatric Disease and Treatment, 13, 1989–2001. https://doi.org/10.2147/NDT.S142067
Schönauer, M., Grätsch, M., & Gais, S. (2015). Evidence for two distinct sleep-related long-term memory consolidation processes. Cortex, 63, 68–78. https://doi.org/10.1016/j.cortex.2014.08.005
Schmidt, C., Collette, F., Cajochen, C., & Peigneux, P. (2007). A time to think: Circadian rhythms in human cognition. Cognitive Neuropsychology, 24(7), 755–789.
Scullin, M. K., & McDaniel, M. A. (2010). Remembering to execute a goal: Sleep on it! Psychological Science, 21(7), 1028–1035.
Sheth, B. R., Nguyen, N., & Janvelyan, D. (2009). Does sleep really influence face recognition memory? PLoS ONE, 4(5), 1–11.
Sonni, A., & Spencer, R. M. C. (2015). Sleep protects memories from interference in older adults. Neurobiology of Aging, 36(7), 2272–2281. https://doi.org/10.1016/j.neurobiolaging.2015.03.010
Squire, L. R., Genzel, L., Wixted, J. T., & Morris, R. G. (2015). Memory consolidation. Cold Spring Harbor Perspectives in Biology, 7(8), Article a021766. https://doi.org/10.1101/cshperspect.a021766
Takeuchi, M., Suzuki, M., Kurachi, M., Furuta, H., Ochiai, Y., Hosokawa, M., Sumiyoshi, T., & Matsui, M. (2014). Does sleep improve memory organization? Frontiers in Behavioral Neuroscience, 8(Apr). https://doi.org/10.3389/fnbeh.2014.00065
van Rijn, E., Carter, N., McMurtrie, H., Willner, P., & Blagrove, M. T. (2017). Sleep does not cause false memories on a story-based test of suggestibility. Consciousness and Cognition, 52, 39–46. https://doi.org/10.1016/j.concog.2017.04.010
Wagenmakers, E. J., Krypotos, A. M., Criss, A. H., & Iverson, G. (2012). On the interpretation of removable interactions: A survey of the field 33 years after Loftus. Memory & Cognition, 40(2), 145–160.
Wickelgren, W. (1974). Single-trace fragility theory of memory dynamics. Memory & Cognition, 2, 775–780.
Wilhelm, I., Diekelmann, S., Molzow, I., Ayoub, A., Mölle, M., & Born, J. (2011). Sleep selectively enhances memory expected to be of future relevance. The Journal of Neuroscience, 31(5), 1563–1569. https://doi.org/10.1523/JNEUROSCI.3575-10.2011
Wixted, J. T. (1990). Analyzing the empirical course of forgetting. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16(5), 927–935.
Wixted, J. T. (2005). A theory about why we forget what we once knew. Current Directions in Psychological Science, 14(1), 6–9.
Wixted, J. T. (2007). Dual-process theory and signal-detection theory of recognition memory. Psychological Review, 114(1), 152–176.
Wixted, J. T. (2019). The forgotten history of signal detection theory. Journal of Experimental Psychology: Learning Memory and Cognition, 46(2), 201–233.
Acknowledgements
We thank the following people for discussions on this topic and manuscript versions: Yonatan Goshen-Gottstein, D. Stephen Lindsay, Colleen M. Parks, David R. Shanks, and John T. Wixted. We thank the authors for providing their data for reanalysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open practices statement
The data are available on the Open Science Framework repository, https://osf.io/vmjry/?view_only=8f68a6f60fd44ec4817f983e9d3a8e1e. The experiment was not preregistered as we used empirical data (1) for examples of what patterns of results constitute a sleep benefit, not to test a hypothesis, (2) to generate simulated data, and (3) there are no publicly available data in previous studies to use as examples and, when we requested data, we did not receive enough data to provide an unbiased account.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mickes, L., Morgan, D.P., Fuentes Grandón, D.A. et al. Illustrations of interactions needed when investigating sleep using a type of AM-PM PM-AM design. Psychon Bull Rev 30, 2106–2115 (2023). https://doi.org/10.3758/s13423-023-02248-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-023-02248-8