Abstract
It is commonly known—and previous studies have indicated—that time appears to last longer during unpleasant situations. This study examined whether a reciprocal statement can be made—that is, whether changes in the perception of time can influence our judgment (or rating) of a negative event. We used a temporal illusion method (Pomares et al. Pain 152, 230–234, 2011) to induce distortions in the perception of time. Two stimuli were presented for a constant time: a full clock, which stayed on the screen until its clock hand completed a full rotation (360°); and a short clock, in which the clock hand moved just three-quarters of the way (270°), thus suggesting a reduced interval duration. However, both stimuli were shown for the same amount of time. We specifically investigated (a) whether we could induce a temporal illusion with this simple visual manipulation, and (b) whether this illusion could change participants’ ratings of a painful stimulus. In Experiment I (n = 22), to answer (a) above, participants were asked to reproduce the duration in which the different clocks were presented. In Experiment II (n = 30), a painful thermal stimulation was applied on participants’ hands while the clocks were shown. Participants were asked to rate the perceived intensity of their pain, and to reproduce its duration. Results showed that, for both experiments, participants reproduced a longer interval after watching the full clock compared with the short clock, confirming that the clock manipulation was able to induce a temporal illusion. Furthermore, the second experiment showed that participants rated the thermal stimuli as less painful when delivered with the short clock than with the full clock. These findings suggest that temporal distortions can modulate the experience of pain.
Similar content being viewed by others
Humans, like other animals, can accurately measure the passage of time by several orders of magnitude. The sense of temporal duration guides behavior and is required for predicting future events. Despite the importance of timing for survival (Matthews & Meck, 2014), no time-sensing organ or specific biological structures devoted to keeping track of time has yet been identified. Over the last decades, many models have been proposed to determine how time works in the brain. The scalar expectancy theory (SET; Gibbon et al., 1984; Wearden, 1992), arguably the most popular timing model, proposes that time is processed by an internal clock that includes a pacemaker (which emits pulses like a clock), a switch, and an accumulator. A time interval is represented by the quantity of pulses in the accumulator after the interval elapses (i.e., the greater the number of pulses, the longer the interval). A decision rule comparing the number of pulses in the accumulator with a random sample from the reference memory of a previously experienced interval determines when responses are emitted. This model has been able to accurately describe temporal performance in a wide range of timing procedures (e.g., Buhusi & Meck, 2005; Gibbon et al., 1984; Grondin, 2010). However, SET has been criticized for having no biological plausibility, as no evidence for its components has so far been identified in the brain (Gorea, 2011; Matell & Meck, 2000, 2004).
There have been attempts to provide more biologically plausible timing models, such as the state-dependent network models (Karmarkar & Buonomano, 2007) and the striatal beat frequency model (SBF; Matell & Meck, 2004). The SBF, for example, proposes that activity in the cortico-striatal-thalamic loop is a plausible process with which an organism can time short intervals. Specifically, it suggests that striatal neurons receiving massive cortical projections could act as coincidence detectors, identifying unique activity patterns from oscillations generated by the cortical cells.
A more recent neurobiological model, which did not intend to directly address how we perceive the passage of time but ended up revealing a probable link between interoception and the ability to perceive and estimate time, has also been proposed (Craig, 2009). In this model, interoception is defined as the process of perceiving, interpreting, and integrating information about the state of inner body systems (Berntson & Khalsa, 2021; Craig, 2002), which could provide a mechanism for encoding time. According to the model, interoceptive inputs fill an interoceptive buffer, which analyzes and compares data with previous body states. Due to the limited capacity of this buffer, a high rate of salient stimuli may saturate it, slowing time perception. However, time appears to fly when the interoceptive buffer is empty (Craig, 2009; Di Lernia et al., 2018). Therefore, the subjective experience of time, which is influenced by arousal and bodily signals (Gil & Droit-Volet, 2012; Ogden et al., 2015; Pollatos et al., 2014), is susceptible to distortions (i.e., when physical time [the absolute duration of an interval] and its perceived duration differ).
Many studies show that distortions in time perception can be induced by factors such as emotion (Angrilli et al., 1997; Droit-Volet et al., 2004; Droit-Volet & Gil, 2009; Noulhiane et al., 2007; Tipples, 2008), age (Lamotte & Droit-Volet, 2017; Wittmann, 2005), neurological disorders (Teixeira et al., 2013; Thönes & Oberfeld, 2015), pain (Ogden et al., 2015; Rey et al., 2017), and the type of task or stimulus used (Baccarani et al., 2021; Gil & Droit-Volet, 2011; Gros et al., 2015; Xuan et al., 2007; Zhang et al., 2019). These studies generally support the idea that context and experience influence the perception of time. However, an intriguing possibility is that the modulation of perceived duration may influence the individual’s behavior and physiological responses (Lake et al., 2016). It has been proposed that distortions in time perception can influence memory processes (Dirnberger et al., 2012), pain (Pomares et al., 2011), and emotions. There is some evidence that time perception and emotion have a bidirectional relationship. Zhao et al. (2017) demonstrated that manipulating the duration of the loading time of smartphone applications influenced user satisfaction. Specifically, as loading time increased, user satisfaction decreased. Ryan et al. (2015) indicated that various internet waiting situations, such as password recovery, download time, software installation, and time spent searching, were associated with different emotional reactions. Furthermore, longer wait times in ambulatory clinics were negatively correlated with various aspects of the patient experience, such as trust in the care provider and perceived quality of care (Bleustein et al., 2014). According to these findings, time perception may impact emotion and personal evaluations of an event.
In the current study, we investigated the role of time perception in pain intensity ratings by employing a method to induce a temporal illusion. This procedure builds on a previous study (Pomares et al., 2011), which used two clocks to simulate different temporal conditions. Pomares et al. (2011) informed participants that there were two temporal conditions, during which a painful stimulus was presented. In one temporal condition, the interval during which the painful stimulus was delivered was represented by a full clock, in which the clock hand completed a full rotation. In the other temporal condition, the interval was represented by a short clock, in which the clock hand rotated only three-quarters of the way (Fig. 1). Even though the absolute duration of both temporal conditions was identical, participants reported less pain in short clock trials.
Stimuli used to induce the temporal illusion. Note. (A) The full clock is composed of a gray circle and a clock hand which completes an eight-step clockwise rotation around its origin. (B) The short clock is composed of a gray circle cut off at the bottom-left quarter, with a clock hand that runs six-steps around its origin. The time taken by the full clock to complete its eight steps was identical to the time taken by the short clock to complete its six steps
Although Pomares et al. (2011), suggested that the temporal illusion induced by the different clocks affected the perception of pain, their study did not employ any direct measures of the temporal illusions per se. In order to confirm that the different clocks could induce a temporal distortion in the interval estimated, we partially replicated the original study. Importantly, we asked participants to perform an additional time reproduction task. In Experiment I, we assessed whether a temporal interval represented by the different clocks (full vs. short) would lead to different interval reproductions. If the clocks can indeed induce such temporal distortions, as assumed by Pomares et al. (2011), temporal reproductions should be longer when the interval is represented by the full clock than by the short clock. We tested the effects of the clocks on two different intervals. After establishing the parameters for the clock-induced illusion in Experiment I, we introduced a painful stimulus similar to Pomares et al. (2011) in Experiment II to determine whether subjective time perception affects the experience of pain.
Experiment I
Method
Participants
Twenty-two (11 males) students from Universidade Federal do ABC (UFABC; Mage = 21.5 years; SDage = 3.7 years) participated in this experiment. They were randomly assigned to one of two groups. One group performed a 15-s time reproduction task (n = 11), while the other performed a 24-s reproduction task. All participants had a normal or corrected-to-normal vision; did not report any neurological and/or psychiatric illness at the time of the experiment; had no chronic pain; and were not under any treatment involving analgesics or antidepressants. The Human Research Ethics Committee at UFABC authorized all experimental protocols (Approval 98000618.7.0000.5594), and all subjects provided informed consent to participate.
Temporal illusion
In order to induce a temporal illusion, two different clocks (full and short) were used. The clocks (Pomares et al., 2011) consisted of a gray circle and a black line connecting its center to the left horizontal margin, comprising half of its diameter (Fig. 1). The angular displacement of the clock hand was kept constant between the full and short clocks (i.e., the distance traveled by the clock hand in each step was the same for both clocks). However, in the full clock (Fig. 1A), the hand made a complete clockwise rotation around its origin (360°) in eight steps. In the short clock (Fig. 1B), the gray circle was discontinued at its bottom-left quarter. Therefore, the hand made a clockwise rotation of 270° in six steps. To keep the time constant between clocks, the interval between steps in each clock were different. For the 15-s condition, each hand step was set to move every 1,667 ms and every 2,143 ms for the full and short clocks, respectively. Therefore, they both equally took 15 seconds to rotate 360° or 270°, respectively. For the 24-s condition, the hands moved every 2,667 ms and 3,429 ms for the full and short clocks, respectively.
Time reproduction task
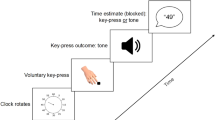
In each trial, participants had to reproduce the duration of the clock after its presentation. To begin the reproduction, participants had to press the left arrow key. The screen went blank immediately, indicating the beginning of the reproduced interval. When the participants estimated that the time elapsed during the reproduction equaled the duration in which the clock was just presented, the right directional key should be pressed, which ended the reproduction.
Participants were asked to “try to focus on the duration of the clock and try not to count (physically or mentally) or use any rhythmic method” to estimate its duration.
Procedure
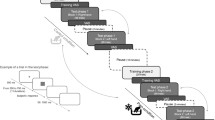
Stimuli were presented on a 17-inch CRT monitor with a refresh rate of 60 Hz using the PsychoPy (Version 3.0; Peirce et al., 2019). The monitor was placed 50 cm in front of the participant. A private room with low ambient light and isolated from noise was used for experimental sessions, during which: (a) Participants were briefly informed that the task consisted in reproducing the duration in which a clock would appear on the screen. They were told that each clock lasted for a different duration (even though they did not), such that the short clock lasted 75% of the duration of the full clock. (b) Participants performed two practice trials, one for each clock type, and moved on to the main task. Each trial (Fig. 2A) started with the presentation of a fixation cross in the center of the screen for a variable duration between 0.4 and 0.7 seconds. (c) A full or short clock was shown, and the clock hand began to move clockwise until it reached the end of its path, at which point the clock disappeared from the screen.
Experimental procedure for Experiments I and II. Note. (A) Experiment I: after the fixation point, a full or short clock was presented. Participants had to press the start and stop keys to reproduce the interval, then press the spacebar to move on to the next trial. (B) Experiment II: the procedure was similar to Experiment I, except that during clock presentation, participants received a heat-induced painful stimulation and rated their pain level before the time reproduction task
Participants then had to reproduce the interval in which the clock was displayed (see details in Time Reproduction Task section). No feedback was provided. Each experimental session consisted of a single block of 60 randomly shuffled trials, half with the full and half with the short clock.
Data analysis
All data analyses were performed using R (Version 4.1.0; R Foundation for Statistical Computing). Interval reproductions for the different groups (15 and 24 seconds) and during the different clock type (full and short) were the main dependent variables for the analyses. Individual reproductions for either clock type deviating more than 3 standard deviations from their mean were discarded, assuming they did not represent a valid estimate of the interval. In total, 13 trials (1% of the data) were excluded from the analyses under this criterion. The accuracy of the temporal reproductions was measured by the ratio between time reproduced (Tr) and target duration (Td; i.e., true time interval), Tr/Td. A ratio close to one represents perfect reproductions; ratios larger than one indicate interval overestimations, and ratios smaller than one indicate underestimations. To assess temporal variability, the coefficient of variation (CV) for each group and clock type was calculated. The Shapiro–Wilk test was used to test for normality in the data. Repeated-measures analyses of variance (ANOVAs), paired-sample t tests, and Wilcoxon tests were used to compare groups and clock types, where appropriate.
Results
The group-averaged reproductions for the different clocks are shown in Fig. 3A and B. For both groups, reproduced intervals for the short clocks (M = 13.5 s, SD = 1.15; M = 22.9 s, SD = 2.78 for the 15- and 24-s groups, respectively) were lower than for the full clocks (M = 14.63 s, SD = 1.21; M = 24.27 s, SD = 2.6). Pairwise comparisons within each group revealed significant clock effects, t(10) = 5.20, p < .001, d = 1.57; and t(10) = 6.15, p < .001, d = 1.85; for the 15- and 24-s groups, respectively. The ratio between interval reproduced (Tr) and target duration (Td), averaged across participants for each clock type are reported in (Fig. 3C). A mixed repeated-measures ANOVA, with clock type (full and short) as the within-subject factor and group (15- vs. 24-s) as the between-subject factor, revealed a significant effect of clock type on temporal accuracy, F(1, 20) = 59.42, p < .001, ηp2= 0.75, with better accuracy in the full-clock trials. Interval duration had no effect on temporal accuracy, F(1, 20) = 1.24, p = .279, ηp2 = 0.06, and there was no statistically significant interaction between clock type and group, F(1, 20) = 1.01, p = .327, ηp2 = 0.05. To assess a possible effect of clock type on the variability of intervals reproduced, data from the 15- and 24-s conditions were aggregated and CVs for each clock type were calculated. A Wilcoxon paired test revealed that the difference between short clocks (Mdn = 0.17) and full clocks (Mdn = 0.15) was not significant (z = 1.73, p = .080, r = .92).
Experiment I: Interval reproductions. Note. (A) Interval reproductions for the 15-s group by clock type. (B) Interval reproductions for the 24-s group by clock type. Dashed lines represent target durations. (C) Reproduction accuracy (Tr/Td) as a function of clock type for the 15- and 24-s groups. Error bars indicate standard errors of the mean (SEM). *** indicate p < .001
Discussion
The goal of Experiment I was to reproduce and confirm the temporal illusion induced by different clock representations, as previously suggested (Pomares et al., 2011). Our results showed that, when presented with the short clocks, participants underestimated the target interval, which was illustrated by a significant reduction in interval reproductions in this clock type. It is important to note that in the original study by Pomares et al. (2011) the effectiveness of the illusion was assessed by asking participants if they noticed any problems with the clocks in the experiment. By employing a time reproduction task, this study adopted a more objective method to confirm and quantify the temporal illusion.
Interestingly, although our results confirmed the temporal illusion induced by different clock representations, the magnitude of the illusion was smaller than that expected from the configuration of the clocks. Since the short clock had 75% of the area of the full clock, it would be plausible that the subjective duration of the short clock could also be close to 75% of the subjective duration of the full clock, which was not observed. In summary, Experiment I was critical to confirm that the clock manipulation can induce a temporal illusion. Moreover, it showed which intervals and clock parameters can induce such an illusion. Next, we examined whether the clock-induced temporal illusion can affect the experience of pain.
Experiment II
The goal of Experiment II was to verify if a temporal illusion can modulate the perceived intensity of a painful stimulus.
Method
Participants
Thirty participants (six males) from the University of São Paulo-USP were recruited (Mage = 25.3 years; SDage = 4.8 years). All participants had a normal or corrected-to-normal vision; did not report any neurological and/or psychiatric illness at the time of the experiment; had no chronic pain; and were not under any treatment involving analgesics or antidepressants. All experimental protocols were approved by the Human Research Ethics Committee at UFABC (Approval 98000618.7.0000.5594), and all subjects provided informed consent to participate.
Heat pain thresholds (HPT)
The heat pain thresholds (HPT) were determined using a TSA 2001-II (MEDOC, Israel). The participant’s right hand was stimulated using a 9 cm2 thermode. The baseline temperature was set at 32°C and increased by 1°C/s until the participant reported thermal pain by pressing a button with their left hand. The instruction for the HPT phase was as follows: “Press the button when you feel the warmth is a little uncomfortable for you.” Individual HPTs were defined as the mean threshold temperature from four consecutive measurements.
Procedure
All testing procedures were done in a single, uninterrupted session for each participant. The task was conducted in a private room with low ambient light and noise isolation in the Laboratory of Pain Neuromodulation at the University of São Paulo (USP). The experimental session consisted of a block of eight trials, half with the full clock and half with the short clock, randomly ordered. The session started with the TSA device set to the participant’s heat pain threshold or 45.9°C (the maximum temperature set to avoid possible skin burns), whichever was lower. Participants were told, as in Experiment I, that two clocks would be presented, and the duration of the short clock would be 25% shorter than the full clock. However, both clocks were always displayed for 15 seconds. Each trial (Fig. 2A) began with the fixation cross in the center of the screen for a variable duration between 0.4 and 0.7 seconds. Then, the full or short clock appeared, and, simultaneously, the painful stimulation at the individual threshold was delivered (Fig. 2B). This temperature was maintained for 15 seconds, until the clock hand reached the end of its path, at which point the clock disappeared from the screen, and the painful stimulation was turned off. Then, a computerized version of the Visual Analog Scale (VAS), with a line anchored by the words “no pain” on the far left and “worst pain” on the far right, was shown on the screen. Participants rated the intensity of the pain felt during the trial by clicking on a point along the line. Finally, participants had to reproduce the interval in which the clock was presented following the same procedure described previously (see Time Reproduction Task section). Before the main task, participants did two practice trials, one for each clock type.
Data analysis
Two participants reported no pain during the experiment, so they were excluded from the analyses. Therefore, data from 28 participants were analyzed. The primary dependent variables for the analyses were the interval reproductions for the different clock types (full and short), and the reported experience of pain (ratings on the VAS). No individual reproductions, regardless of clock type, deviated more than 3 standard deviations from their overall individual mean, so no trials were excluded from the analyses in Experiment II. The accuracy of the temporal reproductions was again measured by the ratio between time reproduced (Tr) and target duration (Td; i.e., true time interval), Tr/Td. The coefficient of variation (CV) for each clock type was also calculated to assess temporal variability. The Shapiro–Wilk test was used to test for normality in the data. Paired t tests and Wilcoxon tests were used to compare the two experimental conditions. To further characterize the effect of the temporal illusion on the experience of pain, we calculated the Spearman correlation coefficient between the difference in temporal reproduction (∆time = Trfull − Trshort) and the difference in pain ratings (∆pain = Painfull − Painshort) between the two clock types.
Results
Interval reproduction
As in Experiment I, the paired t test showed that the interval reproductions for the short clocks (M = 13.8 s, SD = 2.84) were significantly shorter than for the full clocks (M = 14.6 s, SD = 3.21),; t(27) = 2.35, p = .030, d = 0.44 (Fig. 4A). In terms of accuracy (Tr/Td; Fig. 4B) temporal reproductions were more accurate in trials with full clocks than with short clocks (M = 0.97, SD = 0.2; and M = 0.92, SD = 0.2, respectively). Finally, there was no difference in the variability of temporal reproductions, as measured by the CVs, between full (Mdn = 0.14) and short (Mdn = 0.13) clocks (Wilcoxon-paired test, z = 0.98, p = .330, r = −.03).
Experiment II: Interval reproductions and pain ratings. Note. (A) Time reproduction task: Interval reproductions by clock type. The dashed line represents the target duration. (B) Reproduction accuracy (ratio; Tr/Td) as a function of clock type. Bars indicate standard errors of the mean (SEM). (C) Pain ratings: VAS scale by clock type. (D) Illusion effects: pain ratings (ΔPain = Painfull - Painshort) as a function of time perception (ΔTime = Trfull - Trshort; Spearman’s ρ = .44*). *p < .05, ** p < .01
Pain ratings
Eleven participants had heat pain thresholds (HPTs) higher than 45.9°C, the set limit for safety (MHPT = 47.79 °C, SDHPT = 1.20), so their stimulations were adjusted to 45.9°C There were no statistically significant differences in pain ratings between these participants (Mdn = 4.01, n = 11) and those stimulated at their HPTs (Mdn = 2.59, n = 17; Mann–Whitney U test: z = −1.25, p = .213, r = −.24). Pain ratings are shown in (Fig. 4C). Participants reported feeling more pain during full clock trials (Mdn = 3.17) than during short clock trials (Mdn = 2.63; Wilcoxon-paired test, z = 2.60, p = .009, r = .34). To assess whether a difference in temporal perception corresponds to a difference in the experience of pain, we used a Spearman’s rank correlation test to assess the relationship between the illusion effect (i.e., the difference in interval reproductions between the two clock types; Trfull − Trshort) and the pain effect (i.e., the difference in pain ratings between the two clock types; Painfull − Painshort). Results showed a significant positive correlation between them (Fig. 4D), Spearman’s 𝜌 = .44, p = .020), suggesting that temporal underestimations induced by the temporal illusion were directly associated with a perception of diminished intensity of pain.
Discussion
In Experiment II, the two clocks were again used to induce a temporal illusion while painful stimuli were delivered. We found that, as in Experiment I, the clocks were able to modulate the participants’ perception of time. Furthermore, we observed an effect of the temporal illusion on the experience of pain. Pain ratings indicated that, despite the constant duration of the painful stimuli in all trials, participants felt that the pain was less intense in short-clock trials compared with full-clock trials. These findings are consistent with previous reports suggesting a link between time perception and pain (Pomares et al., 2011).
When individuals are in pain, they tend to experience time as being longer than it is (i.e., pain seems to dilate the perception of time; Ogden et al., 2015; Rey et al., 2017; Somov, 2000). Conversely, as in the original work by Pomares et al. (2011), we showed that time perception can modulate one’s experience of pain.
Different from what was reported by Pomares et al. (2011) we were able to identify differences in pain ratings with heat stimuli at or below the participant’s threshold, delivered for a shorter duration (15 seconds) than originally reported. These results suggest that the illusion effects may vary across participants and/or experimental conditions. We return to this point in the general discussion.
General discussion
The present study investigated the effects of a simple clock manipulation on the perception of time and pain. On each trial, the full and short clocks were presented for the same amount of time, and participants were asked to reproduce their duration by generating an equivalent temporal interval. Importantly, to confirm the temporal illusion effect, the original procedure (Pomares et al., 2011) asked whether the participant noticed anything wrong with the clocks. In our study, a temporal task was added, which allowed us to objectively confirm and assess the magnitude of the illusion.
In Experiment I, two intervals were tested—15 and 24 seconds. We found that, for both intervals, the reproductions of short-clock trials were underestimated compared with full clocks. We also observed that the magnitude of illusion occurred in a smaller proportion than that expected by the difference between clocks. That is, the short-clock reproductions were not 25% shorter than reproductions on full-clock trials. Reproductions for the short clock were about 7.7% (15-s) and 5.7% (24-s) shorter than for the full clock. It would be interesting to explore ways to increase the magnitude of the illusion effect. Since attention to the clock may be a powerful modulator of this illusion, perhaps changing the position, size, and/or transparency of the clock could result in a larger effect.
According to the scalar expectancy theory (SET; Gibbon et al., 1984; Wearden, 1992), a time interval is represented by the quantity of pulses in the accumulator after the interval elapses. When this quantity reaches a certain threshold, a decision is made that the interval has elapsed (or that it is close enough, so responding starts). Our results could be explained by the SET by adjusting several possibly different parameters in the model, such as the pacemaker rate or the threshold. For example, the different attributes of the clocks could influence the work of the pacemaker-accumulator component. In the full clock, the clock hand makes a complete rotation around its axis. The clock hand for the full clock makes more steps (i.e., “clock ticks”) to complete its rotation than does the clock hand for the short clock, which runs only three-quarters of the way. Thus, short-clock trials may collect fewer pulses resulting in underestimations compared with full-clock trials.
It is also possible that the different times between hand steps for the full and short clocks, which negatively correlates with the number of steps, may play an important role in the temporal illusion. Future studies may investigate which aspects of the topography of the clock contribute to the temporal illusion herein reported.
In Experiment II, we tested the effect of the illusion on the experience of pain. Previous studies demonstrated a relation between negative experiences and the subjective time (i.e., time perception dilates when the individuals are in an unpleasant situation; Fayolle et al., 2015; Ogden et al., 2015; Piovesan et al., 2022; Rey et al., 2017). With this in mind, we expected that pain levels would be modified by their duration (i.e., if unpleasant stimuli appeared to last less time, their negative evaluation would be reduced. In accordance with our predictions and coherently with previous study; Pomares et al., 2011) participants reported experiencing less pain in short-clock trials in comparison to full-clock trials, even though the duration of stimulations was the same in all trials.
Craig (2009) suggests that time perception and emotional/visceral processes share an anatomical structure, namely the insular cortex. This region progressively integrates salient factors such as the current homeostatic information, environmental context, and motivational and social state, producing a global emotional moment, which represents the sentient self at one moment of time. Craig’s model suggests that the accumulation of global emotional moments in buffers could play an important role in time perception. In events of high emotional salience and arousal, emotional buffers are rapidly "filled up" by global emotional moments, resulting in an expanded subjective experience of time. According to this model, one possible way to explain why participants reproduced a shorter interval when presented with the short clock is that, by thinking that the interval is passing faster, arousal levels change the rate at which the global emotional moments accumulate in the buffer. Thus, time perception could influence the perceived body state and the progression of emotional moments, affecting the comparisons of past feelings with the present feelings.
As for the pain, previous studies have suggested an effect of arousal on the perception of pain (Kyle & McNeil, 2014; Rey et al., 2017). Therefore, differences in arousal produced by the temporal illusion could also explain why participants reported feeling less pain during the short clock.
Another interesting possibility is that, since the clock stimulus is isomorphic to the pain rating, participants might have simply used the clock stimulus as a proxy, in which the final position of the clock hand in each trial suggests to the participant how much pain it must be feeling. To test this hypothesis, different stimuli could be used to induce the temporal illusion. For example, previous studies have demonstrated that static images (Nather & Bueno, 2012) or pendular motion (Giorjiani et al., 2021; Karşılar et al., 2018) can induce temporal distortions. In this case, the position of the stimulus (e.g., a human silhouette running in place) at the end of the interval is not likely to bias responses on a rating scale.
Importantly, we also provided verbal instructions to emphasize that there were two temporal conditions. As a result, one could speculate that these instructions contributed to the effects of the illusion. This information could induce a bias in the participants to reproduce the intervals differently depending on the clock type, resulting in underestimations for short clocks compared with the full clocks. Although plausible, we believe this is not the case and that the effects of the illusion might be more complex. In the original study (Pomares et al., 2011), the authors also used a similar instruction. However, the difference in pain ratings between full- and short-clock trials was observed only when high intensities of thermal stimulations were used (pain threshold +1°C) and presented for long durations (over 24 seconds). They failed to observe any effects of the clock manipulation in all other conditions, even though the same instructions were provided at the beginning of the experiment.
Regardless of the source of the illusion (the clocks with or without the contribution of the initial instructions), it is essential to notice that establishing the illusion was only the means to evaluate what was the goal of the study to assess whether a temporal illusion could be induced and then investigate if it could affect one’s experience of pain. Our results suggest that the answer is yes: that the relationship between time perception and pain is bidirectional (Lake et al., 2016). Nevertheless, it would be interesting to investigate the source of the temporal illusion—whether the verbal instructions, the number of clock steps, or the interval between steps in each clock can drive the temporal distortions.
Furthermore, based on the anatomical overlap shared by time perception and emotional/visceral processes in the insula, it would also be fascinating to explore the effects of the illusion on the evaluation of appetitive or emotionally charged stimuli, such as images or sounds. Previous studies have already described that interval reproductions are overestimated when bitter tasting stimuli and sour foods are used (Zhang et al., 2019) or when disturbing images (Gil & Droit-Volet, 2012; Ogden et al., 2019) or aversive sounds (Droit-Volet et al., 2010; Mella et al., 2011) have to be estimated. Conversely, based on our findings, it is possible that a temporal distortion can modify our evaluation of such stimuli. In this sense, an induced temporal illusion can be used to amplify or attenuate the negative experience under those appetitive or emotionally-charged stimuli.
Limitations and future directions
One limitation of our study was the use of a “minimum pain” threshold (i.e., the minimum intensity needed for the participants to report discomfort). As a result, we do not know whether higher pain intensities are prone to the illusion effects. Another limitation is the lack of previous studies on the topic. Even though the association between pain and time perception has been studied over the years, few studies have examined how time perception may influence the experience of pain. Time is an important dimension of pain given that, when in pain, people tend to overestimate an event’s duration (Somov, 2000). Inducing a temporal distortion, particularly promoting time underestimations, could be useful for managing the intensity of perceived pain, especially in clinical populations. Various questions related to the temporal distortions and subsequent behaviors should be directed in future research with techniques measuring cognitive and physiological processes such as skin conductance, heart rate, and pupil dilation.
Conclusion
To summarize, the primary goal of this study was to confirm and quantify the effects of a simple clock manipulation on time perception using a time reproduction task, and test whether this induced temporal illusion could alter one’s experience of pain. Our findings showed that participants reproduced a longer interval after watching the full clock compared with the short clock, confirming that the clock manipulation was able to induce a temporal illusion. Furthermore, the second experiment showed that participants rated the thermal stimuli as less painful when delivered with the short clock than with the full clock. These findings suggest that temporal distortions can modulate the experience of pain.
Data availability
Data and materials generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Angrilli, A., Cherubini, P., Pavese, A., & Manfredini, S. (1997). The influence of affective factors on time perception. Perception & Psychophysics, 59(6), 972–982. https://doi.org/10.3758/BF03205512
Baccarani, A., Grondin, S., Laflamme, V., & Brochard, R. (2021). Relaxing and stimulating effects of odors on time perception and their modulation by expectancy. Attention, Perception, & Psychophysics, 83(1), 448–462. https://doi.org/10.3758/s13414-020-02182-0
Berntson, G. G., & Khalsa, S. S. (2021). Neural circuits of interoception. Trends in Neurosciences, 44(1), 17–28. https://doi.org/10.1016/j.tins.2020.09.011
Bleustein, C., Rothschild, D. B., Valen, A., Valatis, E., Schweitzer, L., & Jones, R. (2014). Wait times, patient satisfaction scores, and the perception of care. The American Journal of Managed Care, 20(5), 393–400.
Buhusi, C. V., & Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience, 6(10), 755–765. https://doi.org/10.1038/nrn1764
Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. https://doi.org/10.1038/nrn894
Craig, A. D. (2009). Emotional moments across time: A possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1933–1942. https://doi.org/10.1098/rstb.2009.0008
Di Lernia, D., Serino, S., Pezzulo, G., Pedroli, E., Cipresso, P., & Riva, G. (2018). Feel the time: Time perception as a function of interoceptive processing. Frontiers in Human Neuroscience, 12, Article 74. https://doi.org/10.3389/fnhum.2018.00074
Dirnberger, G., Hesselmann, G., Roiser, J. P., Preminger, S., Jahanshahi, M., & Paz, R. (2012). Give it time: Neural evidence for distorted time perception and enhanced memory encoding in emotional situations. NeuroImage, 63(1), 591–599. https://doi.org/10.1016/j.neuroimage.2012.06.041
Droit-Volet, S., & Gil, S. (2009). The time–emotion paradox. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1943–1953. https://doi.org/10.1098/rstb.2009.0013
Droit-Volet, S., Brunot, S., & Niedenthal, P. (2004). Perception of the duration of emotional events. Cognition & Emotion, 18(6), 849–858. https://doi.org/10.1080/02699930341000194
Droit-Volet, S., Mermillod, M., Cocenas-Silva, R., & Gil, S. (2010). The effect of expectancy of a threatening event on time perception in human adults. Emotion, 10(6), 908–914. https://doi.org/10.1037/a0020258
Fayolle, S., Gil, S., & Droit-Volet, S. (2015). Fear and time: Fear speeds up the internal clock. Behavioural Processes, 120, 135–140. https://doi.org/10.1016/j.beproc.2015.09.014
Gibbon, J., Church, R. M., & Meck, W. H. (1984). Scalar timing in memory. Annals of the New York Academy of Sciences, 423(1), 52–77. https://doi.org/10.1111/j.1749-6632.1984.tb23417.x
Gil, S., & Droit-Volet, S. (2011). “Time flies in the presence of angry faces”… depending on the temporal task used! Acta Psychologica, 136(3), 354–362. https://doi.org/10.1016/j.actpsy.2010.12.010
Gil, S., & Droit-Volet, S. (2012). Emotional time distortions: The fundamental role of arousal. Cognition & Emotion, 26(5), 847–862. https://doi.org/10.1080/02699931.2011.625401
Giorjiani, G. M., Biazoli, C. E., & Caetano, M. S. (2021). Differences in perceived durations between plausible biological and non-biological stimuli. Experimental Brain Research, 239(1), 161–173. https://doi.org/10.1007/s00221-020-05904-w
Gorea, A. (2011). Ticks per thought or thoughts per tick? A selective review of time perception with hints on future research. Journal of Physiology–Paris, 105(4/6), 153–163. https://doi.org/10.1016/j.jphysparis.2011.09.008
Grondin, S. (2010). Timing and time perception: A review of recent behavioral and neuroscience findings and theoretical directions. Attention, Perception, & Psychophysics, 72(3), 561–582. https://doi.org/10.3758/APP.72.3.561
Gros, A., Giroud, M., Bejot, Y., Rouaud, O., Guillemin, S., Aboa Eboulé, C., Manera, V., Daumas, A., & Lemesle Martin, M. (2015). A time estimation task as a possible measure of emotions: Difference depending on the nature of the stimulus used. Frontiers in Behavioral Neuroscience, 9, 143. https://doi.org/10.3389/fnbeh.2015.00143
Karmarkar, U. R., & Buonomano, D. V. (2007). Timing in the absence of clocks: Encoding time in neural network states. Neuron, 53(3), 427–438. https://doi.org/10.1016/j.neuron.2007.01.006
Karşılar, H., Kısa, Y. D., & Balcı, F. (2018). Dilation and constriction of subjective time based on observed walking speed. Frontiers in Psychology, 9, Article 2565. https://doi.org/10.3389/fpsyg.2018.02565
Kyle, B. N., & McNeil, D. W. (2014). Autonomic arousal and experimentally induced pain: A critical review of the literature. Pain Research and Management, 19(3), 159–167. https://doi.org/10.1155/2014/536859
Lake, J. I., LaBar, K. S., & Meck, W. H. (2016). Emotional modulation of interval timing and time perception. Neuroscience and Biobehavioral Reviews, 64, 403–420. https://doi.org/10.1016/j.neubiorev.2016.03.003
Lamotte, M., & Droit-Volet, S. (2017). Aging and time perception for short and long durations: A question of attention? Timing & Time Perception, 5(2), 149–167. https://doi.org/10.1163/22134468-00002086
Matell, M. S., & Meck, W. H. (2000). Neuropsychological mechanisms of interval timing behavior. BioEssays, 22(1), 94–103. https://doi.org/10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E
Matell, M. S., & Meck, W. H. (2004). Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cognitive Brain Research, 21(2), 139–170. https://doi.org/10.1016/j.cogbrainres.2004.06.012
Matthews, W. J., & Meck, W. H. (2014). Time perception: The bad news and the good. Wiley Interdisciplinary Reviews: Cognitive Science, 5(4), 429–446. https://doi.org/10.1002/wcs.1298
Mella, N., Conty, L., & Pouthas, V. (2011). The role of physiological arousal in time perception: Psychophysiological evidence from an emotion regulation paradigm. Brain and Cognition, 75(2), 182–187. https://doi.org/10.1016/j.bandc.2010.11.012
Nather, F. C., & Bueno, J. L. O. (2012). Timing perception in paintings and sculptures of Edgar Degas. Kronoscope, 12(1), 16–30. https://doi.org/10.1163/156852412X631628
Noulhiane, M., Mella, N., Samson, S., Ragot, R., & Pouthas, V. (2007). How emotional auditory stimuli modulate time perception. Emotion, 7(4), 697–704. https://doi.org/10.1037/1528-3542.7.4.697
Ogden, R. S., Moore, D., Redfern, L., & McGlone, F. (2015). The effect of pain and the anticipation of pain on temporal perception: A role for attention and arousal. Cognition and Emotion, 29(5), 910–922. https://doi.org/10.1080/02699931.2014.954529
Ogden, R. S., Henderson, J., McGlone, F., & Richter, M. (2019). Time distortion under threat: Sympathetic arousal predicts time distortion only in the context of negative, highly arousing stimuli. PLOS ONE, 14(5), Article e0216704. https://doi.org/10.1371/journal.pone.0216704
Peirce, J., Gray, J. R., Simpson, S., MacAskill, M., Höchenberger, R., Sogo, H., Kastman, E., & Lindeløv, J. K. (2019). PsychoPy2: Experiments in behavior made easy. Behavior Research Methods, 51(1), 195–203. https://doi.org/10.3758/s13428-018-01193-y
Piovesan, A., Mirams, L., Poole, H., & Ogden, R. (2022). The effect of pain on reference memory for duration. Psychological Research, 86(2), 531–543. https://doi.org/10.1007/s00426-021-01508-3
Pollatos, O., Laubrock, J., & Wittmann, M. (2014). Interoceptive focus shapes the experience of time. PLOS ONE, 9(1), e86934–e86934. https://doi.org/10.1371/journal.pone.0086934
Pomares, F. B., Creac’h, C., Faillenot, I., Convers, P., & Peyron, R. (2011). How a clock can change your pain? The illusion of duration and pain perception. Pain, 152(1), 230–234. https://doi.org/10.1016/j.pain.2010.10.047
Rey, A. E., Michael, G. A., Dondas, C., Thar, M., Garcia-Larrea, L., & Mazza, S. (2017). Pain dilates time perception. Scientific Reports, 7(1), 15682–15682. https://doi.org/10.1038/s41598-017-15982-6
Ryan, G., Pámies, M. M., & Valverde, M. (2015). WWW= wait, wait, wait: Emotional reactions to waiting on the internet. Journal of Electronic Commerce Research, 16, 261–275.
Somov, P. G. (2000). Time perception as a measure of pain intensity and pain type. Journal of Back and Musculoskeletal Rehabilitation, 14(3), 111–121. https://doi.org/10.3233/BMR-2000-14306
Teixeira, S., Machado, S., Paes, F., Velasques, B., Silva, J., Sanfim, A., Minc, D., Anghinah, R., Menegaldo, L., Salama, M., Cagy, M., Nardi, A., Poppel, E., Bao, Y., Szelag, E., Ribeiro, P., & Arias-Carrion, O. (2013). Time perception distortion in neuropsychiatric and neurological disorders. CNS & Neurological Disorders–Drug Targets, 12(5), 567–582. https://doi.org/10.2174/18715273113129990080
Thönes, S., & Oberfeld, D. (2015). Time perception in depression: A meta-analysis. Journal of Affective Disorders, 175, 359–372. https://doi.org/10.1016/j.jad.2014.12.057
Tipples, J. (2008). Negative emotionality influences the effects of emotion on time perception. Emotion, 8(1), 127–131. https://doi.org/10.1037/1528-3542.8.1.127
Wearden, J. H. (1992). Temporal generalization in humans. Journal of Experimental Psychology: Animal Behavior Processes, 18(2), 134–144. https://doi.org/10.1037/0097-7403.18.2.134
Wittmann, M. (2005). Age effects in perception of time. Psychological Reports, 97(7), 921–921. https://doi.org/10.2466/PR0.97.7.921-935
Xuan, B., Zhang, D., He, S., & Chen, X. (2007). Larger stimuli are judged to last longer. Journal of Vision, 7(10), 2–2. https://doi.org/10.1167/7.10.2
Zhang, M., Lu, A., & Hodges, B. H. (2019). Lifting, tasting, and carrying: The interaction of magnitude and valence effects in time perception. Acta Psychologica, 193, 1–10. https://doi.org/10.1016/j.actpsy.2018.11.010
Zhao, W., Ge, Y., Qu, W., Zhang, K., & Sun, X. (2017). The duration perception of loading applications in smartphone: Effects of different loading types. Applied Ergonomics, 65, 223–232. https://doi.org/10.1016/j.apergo.2017.06.015
Funding
This work was supported by grants from the São Paulo Research Foundation—FAPESP (2018/18483-1 to V.R.S.O.; 2018/14560-1 to C.S.D.; 2014/50909-8 and 2019/25795-2 to M.S.C.); from the Brazilian National Research Council—CNPq (165068/2018-3 to I.O.; 465686/2014-1 to M.S.C.; a CNPq Productivity Scholarship Level 2 to A.B.F.); and from the Coordination of Superior Level Staff Improvement—CAPES (88887.136407/2017-00 to M.S.C.). A research assistantship was awarded to C.M.S. from UFABC. V.S.Z.M. is an employee at UFABC and was granted a sabbatical leave to conduct this work.
Author information
Authors and Affiliations
Contributions
V.S.Z.M. and M.S.C. conceived the experiments; V.S.Z.M. and C.M.S. performed Experiment I; V.S.Z.M., C.M.S., V.R.S.O., and I.O. performed Experiment II; V.S.Z.M. and M.S.C. analyzed the data. V.S.Z.M. wrote the first draft of the manuscript. All authors revised the final version of the manuscript; C.S.D. and A.B.F. provided technical and material support.
Corresponding author
Ethics declarations
Ethics approval
All experimental protocols were approved and authorized by The Human Research Ethics Committee at UFABC (Approval 98000618.7.0000.5594). All subjects provided informed consent to participate.
Conflicts of interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
S. Z. Maia, V., Silva, C.M., de Paula Oliveira, I. et al. Time perception and pain: Can a temporal illusion reduce the intensity of pain?. Learn Behav 51, 321–331 (2023). https://doi.org/10.3758/s13420-023-00575-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-023-00575-3