Abstract
Studies of object-recognition memory in lab rats began in the late 1980s, using variants of the trial-unique delayed nonmatching-to-sample (DNMS) task. By the end of the 20th century, most investigators who wanted to study object-recognition in rodents had abandoned the DNMS task in favor of the novel-object-preference (NOP) test, mainly because the latter test is relatively easy to employ, whereas conventional DNMS tasks are not. Some concerns have been raised, however, about the internal validity of the NOP test as a method of measuring object-recognition abilities. We describe two experiments using a new DNMS procedure which requires considerably less training than the DNMS tasks of the 1980s and 1990s, and which cannot be subject to the same criticisms that have been leveled at the NOP test. In Experiment 1, rats were trained on the new modified-DNMS (mDNMS) task using short delays. Rats successfully learned the nonmatching rule in fewer than 25 trials, and they made accurate choices with retention intervals of up to 10 min. Experiment 2 examined a different group of rats’ performance on the mDNMS task following long retention intervals (72 h, 3 weeks, and ~45 weeks). Rats made accurate choices on all retention intervals, even the longest retention interval of ~45 weeks. Overall, the findings demonstrate some benefits of an alternative approach to assess object-recognition memory in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Object-recognition memory is the ability to discriminate the familiarity of previously encountered objects. It is a fundamental memory ability that most people engage hundreds of times each day – often with little or no conscious awareness, but sometimes accompanied by explicit recollection of one or more specific episodes on which a previous encounter occurred (Aggleton & Brown, 1999).
Laboratory rodents also distinguish between objects they have previously encountered and ones they have not. The extent to which object-recognition memory involves similar cognitive processes in rodents and humans is not entirely clear. Still, numerous studies in rats and mice have examined how drugs, brain lesions, or other treatments affect performance on tests that are presumed to be effective for discriminating between animals with different object-recognition capabilities (Brown, Warburton, & Aggleton, 2010; Warburton & Brown, 2015; Winters, Saksida, & Bussey, 2008).
Two behavioral paradigms have been used to assess object recognition in rats: delayed non-matching-to-sample (DNMS), using trial-unique or pseudo-trial-unique stimuli, and novel-object preference (NOP). Variants of the NOP test are the most widely used, by far. Concerns have been raised, however, about the internal validity of the NOP test as a method of measuring object-recognition abilities (Gaskin et al., 2010; Gervais, Brake, & Mumby, 2013; Gervais, Hamel, Brake, & Mumby, 2016); these concerns are outlined, below. Although DNMS tasks have not been subjected to the same criticisms, they possess different shortcomings that limit their usefulness; namely, compared to the NOP test, conventional DNMS tasks are considerably more difficult and time-consuming to employ.
The limitations inherent in the NOP test and conventional DNMS tasks motivated us to develop a new method for assessing object-recognition memory in rats, which we describe in this report, along with normative data. In order to appreciate the merits of the new procedure, it is important to first examine the existing methodologies for assessing object-recognition memory in rats, and examine their respective advantages and limitations.
Various DNMS procedures were developed in the late 1980s (e.g., Aggleton, 1985; Mumby, Pinel, & Wood, 1990; Rothblat & Hayes, 1987). Each variant uses a somewhat different apparatus, but all use three-dimensional objects for test stimuli, and follow the same general procedure: On each DNMS trial, a sample object is briefly presented (usually for only a few seconds or less), and after a retention interval, the sample is presented again along with a novel object (i.e., one the rat has not previously encountered during the current session). The rat receives a reward if it selects the novel object. Different sample and novel objects are used on each trial, so reliably accurate performance requires that rats can recognize the sample objects. Memory demands are manipulated by varying the retention interval or the number of objects to remember on each trial. There are several trials per session, and a well-trained rat may be able to complete 20 or 25 trials in less than half an hour (if the retention interval on each trial is only a few seconds, and the inter-trial interval is similarly brief). If rats that receive different treatments consistently perform at similar levels of accuracy when memory demands are minimal, then it can be inferred with some confidence that different levels of accuracy under more challenging conditions reflect real differences in object-recognition abilities.
Although a DNMS task can provide a fairly precise estimate of a rat's object-recognition abilities, the DNMS procedures developed in the 1980s share some drawbacks in common: They are difficult for inexperienced investigators to employ effectively, primarily because the experimenter is in the room with the rat and plays an active and ongoing role in administering the trials (Herremans, Hijzen, & Slangen, 1995; Mumby, 1995; Mumby, Kornecook, Wood, and Pinel, 1995). Even in the hands of a capable experimenter, most rats require hundreds of trials before they reach peak performance, which can require weeks of daily training. Many investigators would consider this time-requirement to be prohibitively long. Moreover, even after extensive training, rats can perform accurately only if the retention delay is no more than a few minutes, and for this reason, most previous studies have used maximum retention delays of 120–300 s (see Supplemental Table 1). Thus, conventional DNMS tasks cannot be used to study long-term memory with retention intervals of several minutes, hours, or days.
Conventional NOP procedures vary slightly from one laboratory to another, but all are generally similar to those described by Ennaceur and Delacour (1988). A rat is placed in an arena, where it is allowed to explore and investigate two identical objects for a few minutes. The rat is then removed for a retention delay, after which it is returned to the arena, where there are now two new objects – one is identical to the sample and the other is novel. Rats tend to spend more time investigating the novel object during the test, indicating that they recognize the sample object. With conventional procedures, rats may show a novel-object preference after retention intervals of up to 24 h, and with modified procedures, after intervals of up to several weeks (Gaskin, Tremblay, & Mumby, 2003; Mumby, Glenn, Nesbitt, & Kyriazis, 2002; Mumby, Piterkin, Lecluse, & Lehmann, 2007; Mumby, Tremblay, Lecluse, & Lehmann, 2005). Thus, the NOP test has the potential to assess long-term object-recognition memory after retention intervals of several days.
The NOP test exploits rats' tendency to investigate novel objects more than familiar objects, when those objects are encountered in a familiar environment (Berlyne, 1950; Besheer & Bevins, 2000). Because the novelty preference is displayed spontaneously under appropriate conditions, no extensive training is required for either experimenters or rats, which makes the NOP test a widely accessible and time-efficient procedure for generating data with the potential to provide insight into rats' object-recognition abilities.
Despite the practical advantages of the NOP test, however, some recent observations have raised concerns about the internal validity of the NOP test as a gauge for object-recognition abilities. For instance, the amount of time rats spend investigating objects during the familiarization phase does not predict the magnitude of their novel-object preference, nor does providing rats with prolonged or repeated exposure to a sample object affect preference magnitude (Gaskin et al., 2010; Gervais et al., 2013, 2016). These findings are contrary to two major assumptions that underlie the manner in which NOP data are usually interpreted: (1) that rats are encoding information about the sample object's features when investigating it during the familiarization phase, and (2) the magnitude of the novel-object preference is a reflection of the persistence or accuracy of a rat's memory for the sample object. One implication is that differences in the magnitude of a novelty-preference should not be uncritically taken to reflect differences in memory ability.
The interpretational problems associated with the NOP test would likely apply to any test of incidental learning that lacks an unambiguous instrumental behavior. Accordingly, we decided early in our plan to develop a new object-recognition test that should incorporate some advantageous features of conventional DNMS tasks: (1) the involvement of an instrumental response with which the rat makes an explicit choice between familiar and unfamiliar objects, (2) a reward for accurate choices, and (3) the possibility of testing individual rats on several trials per session, with each trial consisting of an independent test of recognition memory. A new object-recognition task would be appealing only if it were easier for rats to learn than the DNMS tasks developed in the 1980s. We reasoned that the latter objective could be achieved by diminishing the presence and role of the experimenter in administering individual trials. What we came up with is essentially a modified-DNMS (mDNMS) task. There were three objectives when we developed this task, namely, we wanted a DNMS task that rats could master quicker than conventional DNMS tasks, required little human intervention when administering trials, and could successfully be used to assess long-term object-recognition memory in rats.
This paper describes two experiments that assessed rats’ performance on the new task. The goal of Experiment 1 was to assess rats’ ability to acquire and perform the mDNMS task using retention intervals ranging from 30 s to 4 h. The aim of Experiment 2 was to assess rats’ accuracy on the mDNMS task following much longer retention intervals: 72 h, 3 weeks, and ~45 weeks.
Experiment 1
Materials and method
Subjects
The subjects were ten experimentally naïve male Long-Evans rats (Charles River, St. Constant, QC), weighing 225–275 g (~8 weeks old) at the start of the experiment. The rats were pair-housed in polypropylene cages (48 × 25 × 20 cm) in a colony room maintained under a reverse 12:12 light-dark cycle, with light onset at 8:00 p.m. The rats had continuous access to water and each received a daily ration of ~25 g of rat chow (Charles River Rodent Animal Diet, no. 5075) in the late afternoon, after behavioral testing was finished for the day. All procedures were approved by the Concordia University Animal Care and Use Committee, and were in accordance with the guidelines of the Canadian Council on Animal Care.
Apparatus
A large multi-level environment (152 × 145 × 86 cm) was used to test the rats (Fig. 1). The apparatus was a modified, freestanding steel cage rack, enclosed on three sides by wire mesh, with a removable, clear acrylic front panel. The apparatus had five levels, each covered with woodchip. The top four levels were divided into two equal halves by a plastic barrier wall, and the bottom level remained undivided. A loading cage (58 × 37 × 20 cm) was placed on the top left side of the apparatus. A rat entered the apparatus via a hole in the bottom of the loading cage that was placed over a passageway leading to the top level of the apparatus. Rats traversed the different levels via wire mesh passageways located on both sides of the apparatus. The design of the apparatus was such that a rat had to climb down the passageways on the left side of the apparatus in order to gain access to the right side, which it then could ascend from level to level. The top four divided levels contained plastic rectangular platforms (30 × 12 × 1 cm) each with a recessed food well (2 cm in depth), over which stimulus objects could be placed. One platform was placed on each level on the left side of the apparatus, whereas on the right side of the apparatus, two platforms were placed on each level with the food wells 9 cm apart. All platforms were positioned near the middle barrier wall, in line with the passageway that provided access to the level. The room contained dim lights (20 lx) and three video cameras were used to record test sessions –one was positioned in front of the apparatus and two on the test side.
Diagram of the apparatus used for mDNMS testing depicting a session on (a–c) Pre-training stage 1, 2, and 3, respectively, and (d) mDNMS task acquisition, training at progressively longer delays, and pseudo-mixed delay testing. A loading cage provided access to the apparatus, and passageways on both sides of the apparatus allowed rats to access the different levels. The top four levels contained plastic platforms with recessed food wells in which objects could be placed over. During pre-training, the rat descended the left side of the apparatus encountering either (a) four copies of one sample object on stage 1, (b) two copies of two distinct sample objects on stage 2, or (c) two copies of one sample object and two distinct sample objects on stage 3. During (d) mDNMS task acquisition and subsequent testing, the rat encountered four unique sample objects as it descended the left side. For all stages (a–d) once the rat reached the bottom level, it traversed to the right side where it ascended each level encountering four different tests. On each test a copy of the sample object was paired with a unique novel object. During training at progressively longer delays and pseudo-mixed delay testing, a delay was imposed by temporarily blocking access to the test by inserting a removable barrier into the passageway
Stimulus objects
A total of 285 different objects were used as stimuli for the mDNMS task. Objects were made of plastic, metal, glass, or glazed ceramic, and ranged in size from 4 to 18 cm in height, and from 4 to 13 cm in width. Each object was large enough to cover the food well but light enough to be easily displaced by a rat. There were two copies of each object – one for the learning phase and one for the test. The objects were cleaned after every trial on which they were used, by wiping with a damp paper towel. At the end of each day the objects were cleaned using a diluted bleach solution (1:20 concentration ratio).
Behavioral procedures
The new paradigm involves a series of training stages, each of which the rat had to reach a specific criterion before moving onto the next stage. There were three stages: (1) habituation, (2) pre-training, and (3) mDNMS task acquisition.
Habituation
The rats were handled for ~10 min daily for 1 week before they were habituated to the apparatus. The goal of habituation was to have rats complete an entire circuit of the apparatus (start on the top left level and finish on the top right level), with relatively little hesitation. Rats received two habituation sessions per day. On the first 20 sessions, all ten rats were placed in the apparatus for 30 min with no stimulus objects present, and ~20 Cheerios (1.8 g, General Mills) were placed on each level near and inside the food wells. On the final two habituation sessions, the rats were placed in the apparatus in pairs for 5 min, and only five Cheerios were placed inside each food well. By this point, each rat was consistently eating Cheerios from each food well and reliably completing the entire circuit. During this stage and subsequent stages, the experimenter left the room after placing the rats in the apparatus and watched the session on a TV monitor in an adjacent room.
Pre-training
Pre-training consisted of three stages; stages 1, 2, and 3 (Fig. 1a-c). The rats were now tested individually and they were introduced to stimulus objects. The purpose of the pre-training stages was to gradually familiarize the rats to the procedural aspects of the task (e.g., learn to displace objects from over food wells and to dig for a buried Cheerio placed in food wells) and to teach them that the visual/tactile object features were key to predicting food location. Pre-training stages 1, 2, and 3 differed in the number of distinct sample objects that were presented to the rat: one, two or three, respectively. The rat had to reach a performance criterion at each stage before advancing to the next stage.
On each stage, a rat received one session per day, which consisted of two phases: a sample phase and a test phase. On the sample phase, the rat descended the left side of the apparatus and encountered either four copies of one sample object (stage 1, see Fig. 1a), two copies of two different sample objects (stage 2, see Fig. 1b), or three different sample objects –two copies of one object on the top two levels, and two distinct sample objects on the bottom two levels (stage 3, see Fig. 1c). One Cheerio was placed in each food well to encourage the rat to approach and investigate the sample objects. On the test phase, the rat ascended each level on the right side of the apparatus encountering a different novel object paired with a copy of the sample object and one Cheerio was placed in the food well under each novel object. Thus, the test phase consisted of four separate “trials,” one for each level. On stages 2 and 3 the sample objects on the test phase were presented in the same order that the rat had encountered them on the sample phase (i.e., the first sample object appeared on the first test level). The sample and test phase were separated by a short retention interval in which the rat spent traversing the bottom level of the apparatus. On the first few stage 1 sessions, the objects only partially covered the food well to encourage timid rats to displace objects. As sessions continued, the objects were gradually positioned to cover the entire food well. During stage 2, the Cheerios on the sample and test phase were gradually buried beneath woodchip until the food well was entirely filled to the top (2 cm deep) and rats were consistently digging for the Cheerio. Burying the Cheerio was done in an attempt to reduce the likelihood that a rat would rely on olfactory cues to locate the reward. Moreover, by making the rat dig for the Cheerio we increased both the delay and amount of effort necessary to retrieve the Cheerio from beneath the novel object.
A correct choice on a test trial was defined as the rat either displacing the novel object before displacing the sample object, or only displacing the novel object. An incorrect choice was defined as the rat only displacing the sample object, or displacing the sample object before the novel object. If a rat did not displace either object on a particular test, it was considered a non-trial. For a particular rat, we began recording its accuracy on the test phase once all objects fully covered the food well. Different sample and novel objects were used on each session. On stage 1 a total of 15 different object sets were used, each containing eight copies of one sample object and four unique novel objects. After 15 sessions, rats re-encountered the objects again in the same sequence, starting with the first object set. On stages 2 and 3 four new object sets were introduced – each containing four copies of two distinct sample objects and four unique novel objects. These objects sets were used in combination with the stage 1 sets. The location of the novel object on the test phase was counterbalanced in a pseudorandom order.
After the rat completed the final test, the experimenter entered the room and removed the rat from the top right side of the apparatus. Between each rat, the woodchip on every level was redistributed to spread any potential odor cues left by a previous rat and each object platform was cleaned using a 70% ethanol solution. A rat advanced to the next pre-training stage once it reached a performance criterion of at least 80% of trials correct on five consecutive sessions (i.e., at least 16 correct trials out of 20 trials). A rat was given a maximum of 50 sessions at each stage to reach the performance criterion.
mDNMS task acquisition
During the final training stage, rats encountered four distinct sample objects, one on each of the divided levels of the sample phase (see Fig. 1d). Thus, this stage was similar to conventional DNMS tasks in that each sample object was encountered only once during the sample phase and was subsequently paired with a unique novel object for the test phase. Similar to pre-training, a session consisted of a sample and test phase. On the sample phase, a rat descended the apparatus to familiarize itself with four distinct sample objects, encountering a different one on each level. One Cheerio was buried in the food well under each sample object. During the test phase, a copy of each sample object was presented next to a novel object. A Cheerio was buried under the novel object on each test level. Each session consisted of four trials (as there were four distinct sample objects in the apparatus).
From this point forward a new collection of object sets was used. The objects changed on each session, however, the same objects served as the sample objects and novel objects for all rats. Once a particular object was used on a session, it was not used again until all of the objects in each set were used. This resulted in a particular object re-occurring approximately every 20 sessions. Moreover, an object that served as a sample the first time a rat encountered it, served as a novel the next time it was encountered (and vice versa). The sample and novel object on each trial were paired based on similarities in size, weight, and material. The location of the novel object on each test was counterbalanced in a pseudorandom order. A rat was required to reach a performance criterion of at least 80% of trials correct on five consecutive sessions (16 trials correct out of 20). The average delay between the sample and test phase was 30 s (s = 22.47). A rat was given a maximum of 50 sessions at this stage to reach the performance criterion. Rats received one session per day and were tested no fewer than 5 days per week. The dependent measures were mean percent correct choices and mean number of sessions required to reach the performance criterion.
Training at progressively longer delays
Once a rat met the performance criterion at the 30-s delay, the delay between the sample and test phase was increased to 70 then 90, 220, 330, 440, and then 630 s.Footnote 1 To impose a longer retention delay, the passageway leading to the first test was blocked with a removable barrier. The passageway leading to the last sample object was also blocked to prevent a rat from going back to the sample phase once it reached the bottom level. At the end of a particular delay the experimenter entered the room and unblocked the passageway leading to the first test to allow the rat to start the test phase. For each delay, the rat was required to reach the same performance criterion as before (16 trials correct out of 20 trials), or it received a maximum of 30 sessions.
Pseudo mixed-delay testing
The final stage of DNMS testing consisted of presenting sessions with different retention delays (100, 220, 330, and 630 s) in a mixed fashion. The goal was to compare performance across the range of retention delays used during the preceding stage of training, without the confounding effects of practice (Mumby, 2005). The shortest retention interval that could be achieved at this stage was 100 s, in contrast to the 30-s delay during acquisition. It appeared that after the rats received training at longer delays, they became accustomed to waiting in the delay area, and no longer quickly traversed to the test phase. All rats received ten sessions at each delay, administered in blocks of ten such that each rat received ten consecutive sessions with one delay before moving onto a different delay. The type of delay administered first and the sequence of the delays were randomized for each rat. Once a rat completed the pseudo mixed-delay tests, it received ten sessions using a 4-h delay. Thus, all 4-h delay tests were conducted last.
Probe tests
Following testing, probe tests were administered to confirm the rats were not relying on olfactory cues to correctly locate the food reward buried under the novel object on the test phase. Two types of probe tests were conducted: (1) the food reward was omitted on the test (No Reward) and (2) the sample object was baited on the test (Sample Baited). Two sessions (eight trials) of each type of probe test were performed and compared to two normal test sessions conducted contemporaneously.
Results
One rat failed to reach the performance criterion within the allotted 50 sessions during mDNMS task acquisition. Thus, the results for this rat were excluded from all analyses.
Pre-training
On stage 1, rats reached an accuracy of 92.02% following an average of 6.67 sessions (s = 5). On stage 2, rats reached an accuracy of 83.34% following an average of 6.11 sessions (s = 1.36). Lastly, on stage 3, rats reached an accuracy of 85% following an average of 5.89 sessions (s = 1.83).
mDNMS task acquisition
Figure 2a depicts DNMS scores on the first and last five sessions at each delay during acquisition. Performance during the first training session at the 30-s delay was significantly above chance (M = 67.44%, s = 32.4%). Rats reached a mean accuracy of 84.48% following an average 6.11 sessions (s = 7.24). A dependent-samples t-test revealed a statistically significant improvement in scores from the first to the last five sessions of acquisition (t(8) = -2.82, p = .01, Hedge’s g = -1.26). The highest level of accuracy for the rat that failed to reach the performance criterion was 75% by Session 16.
Mean scores (± SEM) on the (a) first and last five sessions of the DNMS acquisition phase and (b) mean number of sessions required to reach the performance criterion at each of the seven delays in Experiment 1. The white numerical values on the bars represent the number of rats that attained the performance criterion during that delay
Each time the delay was increased, performance initially declined and then improved with more testing at the new delay. Figure 2b depicts the mean number of sessions rats required to reach the performance criterion at increasing delays. Not all rats reached the performance criterion at increasing delays. One rat failed to reach the criterion at the 70-s and 220-s delay, four rats failed to reach the criterion at the 90-s and 330-s delay, and three rats failed to reach the criterion at the 440-s and 630-s delay.
Pseudo mixed-delay testing
Figure 3 depicts the mean retention curves. The length of the retention intervals increased from 100 to 630 s during this stage of testing, and then to 4 h in a separate block of trials. The results of a repeated measures analysis of variance (ANOVA) indicated that performance declined significantly with increases in the retention delay (F(4,32) = 3.41, p = .02, η2 = 0.43). Follow-up t-tests (Bonferroni corrected) revealed a significant difference between the 100-s and 4-h delay (p = .03). Performance at the 4-h delay was not significantly above chance (t(8) = 1.83, p = .05, Hedge’s g = .86). Considering the novelty of the 4-h delay procedure may have contributed to the near chance score at the 4-h delay, a separate repeated measures analysis of variance (ANOVA) was conducted on scores ranging from the 100-s to the 630-s delay. This revealed no significant difference in scores (F(3,24) = 1.76, p = .18, η2 = 0.22), suggesting no decline in accuracy with increasing delays.
Mean scores (± SEM) on the pseudo mixed-delay sessions in Experiment 1. Dashed line represents chance performance
Probe tests
One rat died prior to probe testing, thus the results reported are only for eight rats (Fig. 4). We compared rats’ scores on the probe tests to scores on the normal tests that were administered contemporaneously. Rats’ scores were not significantly different from chance on the “No Reward” probe (t(7) = .00, p > .05, Hedge’s g = .00) and the “Sample Baited” probe (t(7) = .26, p > .05, Hedge’s g = .13). The scores on the normal tests were also not significantly above chance (t(7) = 1.41, p > .05, Hedge’s g = .71). In addition, a one-way ANOVA revealed no significant difference between scores on the probe and normal tests (F(2, 21) = .91, p > .05, η2 = .08).
Mean scores (± SEM) on the probe and normal test sessions in Experiment 1. Dashed line represents chance performance
Discussion
One goal of the present experiment was to develop a DNMS procedure that rats could learn more quickly than conventional DNMS tasks, while still performing accurately with retention intervals lasting several minutes. Rats required an average of 24 trials to reach the performance criterion of 84% correct choices on five consecutive sessions (criterion trials not included). By comparison, rats trained using the DNMS procedure described by Mumby and colleagues required on average 174–420 trials to reach a criterion of at least 85% of trials correct on two consecutive sessions (see Supplemental Table 1 for relevant comparisons between the data reported here and in several previous DNMS studies) (Clark, West, Zola, & Squire, 2001; Duva et al., 1997; Kesner, Bolland, & Dakis, 1993; Kornecook, Kippin, & Pinel, 1999; Mumby et al., 1996; Mumby, Pinel, & Dastur, 1993; Mumby et al., 1990; Mumby, Wood, & Pinel, 1992; Mumby, Mana, Pinel, David, and Banks, 1995; Mumby & Pinel, 1994; Mumby, Pinel, Kornecook, Shen, and Redila, 1995; Wiig & Bilkey, 1995; Wood, Mumby, Pinel, & Phillips, 1993). Rats trained with the Y-maze DNMS task described by Aggleton required on average 130–190 trials to reach a criterion of at least 80% correct trials across five consecutive sessions (Aggleton, Hunt, & Rawlins, 1986; Aggleton, 1985). Moreover, mDNMS task acquisition rate was faster compared to previous studies despite: (1) a longer retention delay (30 s compared to 0 s in Aggleton (1985) and 4 s in Mumby et al. (1990)) and (2) the presentation of four distinct sample objects compared to one sample object. Thus, rats retained more item information over a longer delay compared to rats in previous studies and were capable of reaching comparable choice accuracy levels in significantly fewer trials. It would appear that rats’ performance on the mDNMS task is more robust compared to conventional DNMS tasks. In line with our objectives, these findings confirm that the new mDNMS task can be mastered much quicker than conventional DNMS tasks, while requiring little human intervention when administering trials.
During the final stage of retention testing, rats scored 67%, 59%, 66%, and 63% at delays of 100, 220, 330, and 630 s, respectively (Fig. 3). These levels of asymptotic performance compare favorably with the asymptotic levels observed at similar retention delays on conventional DNMS tasks (see Supplemental Table 1). Thus, compared to conventional DNMS tasks, the modified DNMS task was easier for rats to learn, and they maintained a good level of performance at delays lasting up to 630 s. Accuracy following the 4-h delay, however, was not significantly above chance levels. These results could be due to potential disruptive effects of the procedure used for the 4-h delay. Unlike the procedure used for the other delays, during the 4-h delay the rats were handled, returned to their cage, and transported back to the colony room. This manipulation may have acted as a distraction – a factor known to disrupt performance on DNMS tasks (Hurst & West, 2010; Zola-Morgan & Squire, 1985; Zola-Morgan, Squire, & Amaral, 1989). Another explanation for the observed impairment is that the rats simply forgot the sample objects over the retention interval. This is plausible considering they only briefly encountered each sample object (e.g., between 4 and 10 s, 90% of trials). Perhaps this amount of exposure to four distinct objects was not long enough for object information to be held over several hours. Indeed, providing rats with more time to investigate stimulus objects on the sample phase does increase accuracy on DNMS tests (Beck & Kalynchuk, 1992). Regardless of the reason, this finding indicated that we had to modify the procedure to assess object-recognition following delays lasting more than several minutes (the aim of Experiment 2).
During DNMS acquisition training with progressively longer delays, whenever the delay was increased, there was a transient disruption of choice accuracy followed by a significant recovery (Fig. 2a). This suggests that rats either gradually learned to avoid distraction for increasing periods of time, or they became more efficient at encoding the attributes of the sample objects as training progressed. In any case, this pattern shows the importance of controlling for the extent of prior training when comparing performance across different retention delays. Providing extensive training at different delays can help rats master other skills that are required for good performance at longer delays that may otherwise mask normal object-recognition abilities (Mumby, 2001).
Unlike the conventional DNMS tasks on which the reward is delivered only after the correct choice has been made, in the present study, the reward was placed under the novel object prior to the choice phase. In order to rule out the possibility that rats were locating the food reward by detecting its odor, we administered probe tests. Rats’ performance on both types of probe tests and the normal tests was not significantly different from chance. The low scores on the normal tests make it difficult to interpret the probe test results. If rats were relying on olfactory cues, however, one would predict the accuracy of selecting an object would correspond to the session type (i.e., a bias for selecting the novel on normal tests and the sample on the “Sample Baited” probe tests), but this was not the case. Additionally, relying on olfactory cues would not be expected to produce delay-dependent changes in performance, as we observed on the pseudo mixed-delay tests between the 100-s and 4-h delay. Animals can anticipate features of a trial (e.g., the quantity and probability of a reward) and studies have shown that modifying these characteristics on the task can disrupt performance on the test despite intact recognition abilities (Honig & Dodd, 1986). We suspect that introducing these changes to the reward contingency during probe testing disrupted rats’ performance on both the normal tests and probe tests, resulting in a decline in accuracy scores. Consequently, we decided to modify the probe test procedure for Experiment 2 in an attempt to reduce the disruptive effects of the probe tests.
Experiment 2
The goal of Experiment 2 was to determine if the mDNMS task could be used to successfully measure rats’ memory for objects following longer retention intervals: 72 h and 3 and ~45 weeks. To accomplish this, we used a procedure that we previously found promotes long-lasting memories for sample objects on the NOP test. For example, by modifying the familiarization phase on the NOP test such that rats receive repeated exposures to the sample object over several consecutive days, rats can display novelty preferences after delays lasting as long as 5 weeks (Gaskin et al., 2003; Mumby et al., 2002, 2005, 2007).Footnote 2 Accordingly, for Experiment 2 we modified the procedure to incorporate this method of object familiarization. To accomplish this, two changes were made: (1) the sample phase was conducted in a different apparatus to provide longer, distributed sample object exposures, and (2) the food reward was no longer provided on the sample phase as a means to prevent rats from forming a preference for the sample object on the test phase following repeated pairings with food. The test phase was still administered in the mDNMS task apparatus.
For the ~45-week delay, rats were familiarized to sample objects before they were trained on the mDNMS task. Starting in peri-adolescence (7 weeks old) the rats encountered different sample objects for several hours per day over several weeks while placed together in an apparatus that was similar to the mDNMS apparatus. Once the rats were ~50 weeks old, after they received mDNMS training, their memory for those sample objects was tested.
Rats were tested on the 72-h and 3-week delay shortly after the test phase for the ~45-week delay. To familiarize rats to objects for the 72-h and 3-week delay, we exposed them individually to several sample objects over four consecutive days in a circular-track apparatus that we previously used in a modified-NOP test (Piterkin, Cole, Cossette, Gaskin, & Mumby, 2008). Using a different apparatus for the sample phase allowed us to present multiple objects to a rat concurrently for extended periods of time. This would not have been feasible in the mDNMS task apparatus following training as it would have disrupted the rats’ prior shaping – a facet that needed to remain intact for the test phase (i.e., move with little hesitation from one level to the next in one direction only). While collecting data for each long delay, we also administered tests using a short delay to confirm rats accurately discriminated between objects following a less taxing retention interval.
Materials and method
Subjects
The subjects were 11 male Long-Evans rats (Charles River, Kingston, ON, Canada), 450–550 g at the start of mDNMS training (~22 weeks old). The housing and feeding conditions were the same as Experiment 1. Prior to the start of mDNMS task training, rats received 14 weeks of environmental enrichment starting on post-natal day 28. Environmental enrichment entailed placing all 11 rats in a large apparatus, similar to the one used on the mDNMS task, for 5 h/day, 5 days/week. During environmental enrichment, the rats were familiarized to sample objects for extended periods of time for the ~45-week delay and were exposed to different events as part of an unrelated experiment. Specifically, rats had the opportunity to socialize, forage for novel foods, and on occasion encounter aversive stimuli (e.g., a lithium chloride injection following the ingestion of a novel food and a collar infused with cat odor). Following enrichment, the rats were used in a series of brief unrelated experiments involving exposure to aversive stimuli (e.g., receiving a foot-shock in a conditioning chamber or being placed in a water maze).
Apparatuses
Enrichment apparatus
The apparatus dimensions were the same as the mDNMS task apparatus in Experiment 1. The design was similar such that rats entered the environment via a loading cage placed on top of the apparatus, and there were five levels, the top four of which had divider walls. The floor substrate varied across levels and consisted of wood chips, sand, or wood pellets.
mDNMS task
The same mDNMS apparatus that was used in Experiment 1 was used in Experiment 2, but it was placed in a different test room with different lighting (40 lx).
Circular-track apparatus
Figure 5 illustrates the apparatus used for the sample phase on the 72-h and 3-week delay. The floor of the track was 30 cm wide, and formed a circle with an outside diameter of 270 cm. The floor of the track was covered with wood chips. The inside and outside walls of the track extended from the floor to a height of 40 cm, and both walls had a slight concave curvature. Modular divider-walls separated the track into eight equally-sized compartments. One compartment was used as a “start location” where the rat was placed at the beginning of each trial, and the remaining compartments were either used to present a single object (locations depicted in Fig. 5) or empty. A rat could circulate the track in either direction via small doors located at the base of the divider walls. The testing room contained dim lights (30 lx) and a video camera was positioned above the apparatus to record the sessions for later analysis.
Circular-track apparatus depicting a sample phase trial for the 72-h and 3-week delay in Experiment 2. The apparatus was divided into eight equally sized compartments with one start location compartment and seven object compartments, four of which were used to present objects, and three remained empty. A rat could circulate the track in either direction via passageways located at the base of each divider wall
Stimulus objects
A total of 384 different objects were used and the object material and sizes were the same as Experiment 1, as was the cleaning procedure. The sample objects used for the sample phase for the 72-h, 3- and ~45-week delay had a small container (2.5 cm high) that was attached to the bottom of the object with epoxy. The objects were then fixed in place by screwing the containers into inverted lids that were attached to a ceramic tile (10 × 10 cm), which was then buried under 2.5 cm of wood chips to stabilize it.
Behavioral procedures
Figure 6 depicts a timeline for testing.
Timeline depicting the sequence and average duration of each phase of the procedure for Experiment 2. Gray bars represent the phase length. Shaded portion of the enrichment phase bar depicts the sample phase for the ~45-week delay. Diagonal line portion of the mixed-delay testing phase bar depicts the test phase period for the ~45-week delay. The sample and test phase for the 72-h and 3-week delay were conducted immediately after the ~45-week delay tests over the course of an 8-week period
Sample phase for the ~45-week delay
During the last 12 weeks of environmental enrichment, rats received the sample phase for the ~45-week delay by exposing them to a total of 48 objects in the enriched environment. To ensure rats spent an approximately equal time investigating each object, the objects were staggered such that only four objects were placed in the environment at a time. Over the course of 10 days, rats were exposed to a total of eight different objects (two sets of four objects on alternating days). On Days 1, 3, 5, 7, and 9 rats encountered Objects 1–4 and on Days 2, 4, 6, 8, and 10 rats encountered Objects 5–8. On each day, rats were exposed to the set of objects for 5 h. This procedure was repeated five times throughout the 12-week period, using different object sets each time. The objects were fastened to the floor on the left side of the apparatus, one on each of the top four levels placed in the same spot where sample objects appear on the mDNMS task sample phase. The level in which an object appeared was varied each day, such that an object appeared at least once on each level. Twenty of these objects later served as the sample objects on the test phase for the ~45-week delay, whereas the remaining 28 sample objects were not used. The same objects served as the sample for all rats.
mDNMS task acquisition
The habituation, pre-training, and mDNMS task acquisition were the same as in Experiment 1, with the exception that rats received one 30-min habituation session per day, rather than two, and rats were tested a minimum of four days per week. Once a rat reached the performance criterion of at least 80% of trials correct on five consecutive sessions, it received probe tests.
Probe tests
Following mDNMS task acquisition we confirmed that the rats were not relying on olfactory cues to correctly locate the food reward buried underneath the novel object on the test phase. The same two types of probe tests were administered (“No Reward” and “Sample Baited”), except now each type of probe test was administered within normal test sessions, rather than simultaneously in one session, in an attempt to reduce the disruptive effects of probe tests. All rats received ten trials on each probe test, and the tests occurred on consecutive sessions.
Mixed-delay testing
Test phase for the ~45-week delay
Rats received 20 trials on the ~45-week delay, which occurred immediately after the probe tests. The ~45-week delay tests were administered in the mDNMS apparatus and were conducted concurrently with short delay trials, such that on each session, a rat received two tests using a ~45-week delay and two tests using a short delay. To administer the short delay trials, the apparatus was setup in the typical manner but with only two sample objects on the left side of the apparatus – each randomly placed on one level – and two tests on the test side. The two remaining test levels were setup with objects for the ~45-week delay tests (Supplemental Fig. 1). The short retention interval was on average 80 s (s = 58.11). The test location was counterbalanced across sessions, such that a test for each delay occurred equally often on each of the four test levels. The location of the novel object was counterbalanced across trials such that it occurred equally often on the left and right side. The object sets used for the short delays were presented in a similar fashion as in Experiment 1, such that once a particular object was used on a session, it was not used again until all of the objects in each set were used. Moreover, an object that served as a sample the first time a rat encountered it, served as a novel the next time it was encountered (and vice versa). Conversely, none of the novel objects used for the ~45-week delay tests had been previously encountered by the rats, and thus were trial-unique.
Sample phase for the 72-h and 3-week delays
The sample phase for the 72-h and 3-week delay was administered concurrently in the circular-track apparatus. Over four consecutive days, rats were familiarized to eight distinct sample objects. On Days 1 and 3 rats encountered Objects 1–4 and on Days 2 and 4 they encountered Objects 5–8. On each day, a rat received three distributed 10-min trials, each separated by 1 h, and the objects changed location in a clockwise fashion across trials. Of the eight objects, half were used for the 72-h delay and the remaining half for the 3-week delay. This procedure was repeated four times, using different objects each time. Thus, rats encountered a total of 40 objects (20 objects for each delay). The objects were pseudo-counterbalanced between rats, such that the sample objects for approximately half of the rats were used as the novel objects for the remaining rats. All of the sample and novel objects used for the 72-h and 3-week delay were trial-unique, except for some sample objects that had been encountered one time as a novel object on the ~45-week delay tests. After placing the rat in the apparatus, the experimenter left the room and watched the session on a TV monitor in an adjacent room.
The rats were considered to be investigating an object if their head was 4 cm away from the object and oriented towards the object, or away from the object at no more than a 45° angle. A rat standing on its hind legs and touching the object with at least one forepaw was also considered to be investigating. Chewing, climbing, or sitting on top of an object was not considered investigation.
Test phase for the 72-h and 3-week delays
Rats received 20 trials on both the 72-h and 3-week delay in a mixed fashion. Similar to the ~45-week delay tests, the tests were administered two at a time concurrently with short-delay trials, such that on the test phase there were two tests set up for either a 72-h or 3-week delay and two for the 80-s delay (Supplemental Fig. 1).
Results
mDNMS task
During pre-training stage 1, three rats had positional biases that could not be removed (i.e., the rat consistently displaced objects according to their location on the test (right or left side), and not whether it was novel or familiar). Additionally, one rat failed to reach the performance criterion within the allotted 50 sessions during mDNMS task acquisition. Thus, the results for these four rats were excluded from all analyses. Furthermore, due to human error, four trials for one rat and two trials for another rat were excluded from the 72-h delay analyses, and one trial for two rats was excluded from the 3-week delay analyses.
Pre-training
On stages 1, 2, and 3 rats reached an accuracy of 80.26%, 80.83%, and 80%, respectively, following an average of 15.43 (s = 10.29) sessions on stage 1, 15.71 sessions (s = 13.96) on stage 2, and eight sessions (s = 5.20) on stage 3.
mDNMS task acquisition
Performance during the first training session was significantly above chance (M = 71.43%, s = 26.73%). Rats reached a mean accuracy of 81.43% following an average of 19.86 sessions (s = 14.92).
Probe tests
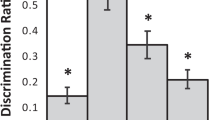
We compared rats’ scores on the probe tests to scores obtained on the normal tests that were administered concurrently (Fig. 7). Rats’ scores were significantly above chance on the “No Reward” probe (t(6) = 2.76, p = .02, Hedge’s g = 1.47), the “Sample Baited” probe (t(6) = 1.99, p = .04, Hedge’s g = 1.06), and the normal tests administered at the same time as the probe tests (t(6) = 2.25, p = .03, Hedge’s g = 1.20). A one-way ANOVA revealed no significant difference between scores on the probe and normal tests (F(2, 18) = .89, p > .05, η2 = .09).
Mean scores (± SEM) on the probe and normal test trials in Experiment 2. Dashed line represents chance performance
Mixed-delay testing
Figure 8 depicts the average time rats spent investigating objects during the sample phase for each delay. On average, rats spent 7.17 s (s = 1.82), 90.61 s (s = 16.51), and 99.89 s (s = 28.59) investigating objects during the 80-s, 72-h, and 3-week delay, respectively.
Mean (± SEM) time spent investigating objects during the sample phase for the 80-s, 72-h, and 3-week delay in Experiment 2
Figure 9 depicts rats’ performance on the mDNMS task at each delay. Accuracy scores were significantly above chance at the 80-s delay (t(6) = 4.64, p = .004, Hedge’s g = 2.47), the 72-h delay (t(6) = 5.41, p = .002, Hedge’s g = 2.90), the 3-week delay (t(6) = 12.88, p < .001, Hedge’s g = 6.99), and the ~45-week delay (t(6) = 6.22, p = .001, Hedge’s g = 3.33).
Mean scores (± SEM) during mixed-delay testing across the four delays in Experiment 2. Dashed line represents chance performance
General discussion
The goals of the present experiments were to develop an object-recognition task that rats could master more quickly than conventional DNMS tasks and could be used to assess long-term object-recognition memory in rats. Experiment 1 revealed that rats required an average of 24 trials to reach the performance criterion of 84% correct choices on five consecutive sessions (criterion trials not included). This is a significant improvement compared to conventional DNMS tasks. Although the rats in Experiment 2 required more sessions to reach criterion compared to those in Experiment 1, they nevertheless required significantly fewer trials compared to previous studies. Including the pre-training sessions in the calculation for the average number of trials needed to master the task, it was revealed that rats required on average 99 and 236 trials for Experiments 1 and 2, respectively. This is still significantly fewer trials compared to the average number of pre-training and training trials required on conventional DNMS tasks (~400 trials on average; see Supplemental Table 1). The results from Experiment 1 demonstrated that rats maintained a good level of performance at delays lasting up to 630 s, and Experiment 2 revealed that modifying the sample phase procedure led to accuracy scores significantly above chance following a 72-h, 3-week, and ~45-week delay. Thus, unlike conventional DNMS tasks, the mDNMS task requires significantly fewer trials to train rats on it and can be used to assess both short- and long-term object-recognition memory.
The results from Experiment 2 are, to our knowledge, the first successful attempt to employ a reinforcement paradigm to assess object-recognition memory in rats following very long retention intervals. To date, there have been few studies examining rats’ long-term recognition abilities and this is in part due to a lack of appropriate tests available. As previously stated, modifying the familiarization phase of the NOP test can promote long-lasting memories of the sample object, lasting up to several weeks (Gaskin et al., 2003; Mumby et al., 2002, 2005, 2007). This is important because in order to study the effects of experimental manipulations on long-term object recognition memory, control animals must show intact recognition abilities at delays lasting at least a few days. This modified familiarization method has been used in a few experiments to examine the effects of lesions made to different medial temporal lobe (MTL) structures on rats’ memory for objects encountered before the damage. Those studies reported that rats with either hippocampal (HPC) or perirhinal cortex (PRh) lesions failed to exhibit significant novel object preferences on tests following learning-surgery intervals ranging between 24 h and 5 weeks, whereas control rats still exhibited novelty preferences (Broadbent, Gaskin, Squire, & Clark, 2010; Gaskin et al., 2003; Mumby et al., 2002). From these experiments, it is clear that the control rats recognized the sample objects for long periods as indicated by their novel-object-preference on the test. It is difficult, however, to determine why the treatment rats failed to show a novelty preference on the test. When a rat fails to show a novel-object-preference following some form of treatment, a common interpretation is that the sample object has been forgotten. However, an equally plausible interpretation is that the treatment has simply altered or suppressed a rat’s natural exploratory bias for the novel object. The number of potential reasons for why a rat does not display a novelty preference on the test is complicated by the fact that the NOP test does not require the rat to make an explicit choice response based on memory. Thus, NOP tests, and incidental learning tests alike, do not provide a straightforward interpretation of behavior in regards to the status of object-recognition abilities. Because there is no evidence to indicate that the magnitude of the novel-object-preference reflects the strength in memory for the sample object, object investigation scores can only be analyzed as binary data (yes/no investigation bias). This is problematic in determining the strength of a rat’s recognition abilities and, in cases of null preferences, whether the manipulation affected object-recognition abilities or the natural exploratory bias for novel objects. Conversely, administering many trials that each consist of an independent test of object-recognition memory while requiring rats to make an instrumental response based on memory and providing a reward for accurate choices, provides a less ambiguous interpretation of the status of object-recognition memory.
The fact that rats showed intact memory for objects encountered up to 45 weeks earlier is intriguing because it indicates that this task can be used to study the effects of experimental manipulations on the consolidation of long-term object memory. Damage made to the MTL can cause temporally-graded retrograde amnesia whereby memories formed long before the damage are intact, whereas more recently formed memories are disrupted. This task can prove useful for examining questions regarding memory for objects learned at various time points over the course of 1 year. This is something that has not been feasible with existent object-recognition tests. Overall, the results from Experiment 2 indicate that the mDNMS task is a promising tool to assess the effects of experimental manipulations on long-term object-recognition memory in rats.
Rats performed significantly above chance on both the probe tests and normal tests administered in Experiment 2, suggesting they were not relying on olfactory cues to successfully locate the food reward on the test. The scores on the normal tests and probes, however, declined relative to scores obtained on the criterion sessions during task acquisition. This disruption in performance may reflect the disruptive effects of probe tests. Although an attempt was made to reduce the disruptive effects of the probe tests, administering ten consecutive probe test sessions in combination with regular tests likely led rats to learn that the novel object was no longer consistently rewarded, leading to a decline in performance. Previous delayed matching-to-sample studies suggest that animals can anticipate trial features such as the quantity and probability of a reinforcer, especially when trial features remain constant over many trials (Honig & Dodd, 1986). Consequently, changes made to features of a trial can affect an animal’s performance, such that task accuracy may decline despite intact recognition abilities. Thus, the decline in task accuracy during probe testing may have reflected the rat’s incentive to respond accurately, and not necessarily memory abilities or the ability to detect the odor of the reward. In the future, a better design may include baiting both the sample and novel objects with a reward on the test but only making the reward underneath the novel object accessible, thus eliminating the need to conduct probe tests.
Several factors are likely to have contributed to making the new mDNMS task relatively easy for rats to learn and perform, and for experimenters to administer in a consistent manner. On conventional DNMS procedures developed in the 1980s, the experimenter plays an interactive role in administering individual trials. Rats probably perceive humans as large, noisy, smelly potential predators, and a rat that perceives the experimenter as the most interesting thing in the room will pay more attention to the experimenter than to the task at hand. This is often the most difficult and frustrating aspect of DNMS testing for someone inexperienced with the behavior of laboratory rats and how they react to movements and sounds. The mDNMS task apparatus has the advantage that the objects can be set up before each trial, and after the rat is placed in the loading cage, the experimenter can quietly leave the room, allowing the rat to “self-administer” trials. Eliminating the presence of the experimenter lessens the potential for distraction.
The pre-training stage also likely contributed to the above-chance level of initial performance. On conventional DNMS tasks, pre-training typically consists of administering object-discrimination problems, which entail repeatedly presenting the same two distinct objects to the rat where selection of one of the objects is rewarded and selection of the other is not (Kesner et al., 1993; Mumby et al., 1990). This teaches the rat both the instrumental-response requirements of the task (i.e., displace objects for food) and that the visual/tactile object features are key to predicting food location. The pre-training procedure in the present study incorporated these task characteristics in addition to teaching the rat that displacing the sample object on the test phase would not provide a reward. Presenting multiple copies of the same sample object within sessions increased the opportunity of the rat to learn this feature, which may have further facilitated task acquisition.
In Experiment 1, scores on pseudo mixed-delay sessions were lower than on the earlier blocked-trial sessions, which used the same delays. We suspect this discrepancy is more likely to reflect an effect of the different testing procedures on performance than on memory. During the blocked-trial sessions with progressively longer delays, there was a transient disruption of choice accuracy during the initial sessions with a new retention delay, followed by a significant recovery to asymptotic levels with continued training at the same delay. This pattern suggests that over several successive trials with a specific retention delay, certain aspects of performance become habitual, and the slight change in procedure that occurs when the delay is lengthened is enough to transiently disrupt them. On the pseudo mixed-delay sessions, the delay changes considerably and on fewer successive sessions, so the disruptive effects are magnified, resulting in lower overall scores during the latter stage of testing. A similar explanation has been previously offered to explain why pigeon’s performance on free-operant delayed matching-to-sample is more accurate on trials with long delays than on trials with an unexpected short delay, if they originally learned the task and received baseline trials with long rather than short delays (Honig & Dodd, 1986; Honig & Wasserman, 1981). This is contrary to what would be expected when measuring working memory, as one would presume accuracy should increase as the working memory demands decrease. These findings suggest that a prospective process (using past experiences to anticipate future responses), and not just memory for trial-specific information may be reflected in task performance (Zentall, 2010). Although the exact mechanisms of this process remain unclear, the findings in the present experiment provoke similar questions about measuring working memory in nonhuman animals; namely, whether a decline in task accuracy as a function of increasing delays truly reflects a loss in working memory capacity (Zentall, 1997).
Although the scores on the pseudo mixed-delay tests in Experiment 1 and the 80-s delay in Experiment 2 were comparable to those on conventional rodent DNMS tasks, there may yet be an opportunity to refine the procedure. Perhaps accuracy scores would be even higher using trial-unique stimuli, rather than stimuli that reoccur every ~20 sessions. This would eliminate the potential for objects becoming increasingly familiar over time, thus making it easier to discriminate between familiar and unfamiliar objects on the test. The extent to which a rat would have difficulty applying the nonmatching rule on the test for a sample object it encountered minutes earlier and a “novel” object it encountered 20 sessions (~1 month) earlier is unknown. Considering rats can recognize objects they encountered ~45 weeks earlier, it does not however, make it implausible that prior brief exposures to an object can cause it to become so familiar that a rat has difficulty distinguishing between it and an object it encountered moments earlier. Moreover, we cannot rule out the possibility that prior experience with aversive stimuli somehow influenced performance on the mDNMS task in Experiment 2.
A related task called the “missing-object recognition task” developed by Cohen and colleagues is also relatively easy for rats to acquire and maintain above-chance object-recognition accuracy over long delays (e.g., 30 min), and generates some theoretically interesting data about rats’ visuo-spatial working memory (Arain & Cohen, 2013; Arain, Parameswaran, & Cohen, 2012; Cohen et al., 2010; Keshen & Cohen, 2016). For the sake of space we will only describe one possible condition used on this task. On this task, a rat encounters a cluster of three distinct or identical objects in a large square open field arena, and receives a seed for displacing each object. Following a delay, the rat receives a test whereby each object is replaced with a copy and a new “jackpot” object is randomly positioned within the array with six seeds underneath it. A correct choice on this task is selecting the “jackpot” object first. The task can yield insights into which cues (non-spatial or spatial) rats rely on to find the previously missing object as a function of the relative position of the object within both the array and open field when it remains either the same or changes between the learning and test phase. A feature of this task that differs from the mDNMS task is that the same objects are presented across trials, thus, the task does not use trial-unique stimuli. While the missing-object recognition task may consist of a more ecologically valid foraging design by presenting rats with the same few objects (proximal landmark resource sites), it might not prevent build-up of long-term proactive interference (PI) as would tasks that present different objects over trials (Jitsumori, Wright, & Shyan, 1989; Wright, 2006). Any task designed to examine declines in working or reference memory must control for such long-term build-up of PI. Accordingly, the mDNMS task with pseudo trial-unique stimuli eliminates some of the possible pitfalls inherent in the missing-object paradigm and as such, may provide a better control for long-term build-up of PI when used to examine age-related neuro-degeneration (i.e. rodent models of Alzheimer’s disease).
Although the rats in the present experiments acquired the mDNMS task in significantly fewer trials compared to conventional DNMS tasks, the mDNMS task still required a considerable number of weeks to collect data. Compared to using the NOP test, the mDNMS task is less practical in that it will require more time to conduct an experiment. However, given the concerns about internal validity when the NOP test is used to make inferences about object-recognition abilities, the choice of which task to use in a memory experiment would appear to be a choice between getting dubious data quickly versus taking a bit more time in order to get high-quality data.
In summary, the findings from the present experiments demonstrate some of the advantages of using an alternative approach to the conventional DNMS tasks and NOP test to assess rats’ object-recognition memory abilities. The mDNMS task was easier for rats to learn compared to conventional DNMS tasks, and unlike conventional DNMS tasks, it can be used to assess object-recognition memory following retention intervals lasting up to one year. An advantage of a paradigm that requires a rat to make an explicit choice response based on memory is that it provides a less ambiguous interpretation of the status of object-recognition memory. Additionally, implementing a DNMS task to assess memory in rats is advantageous, not only because of the limitations inherent with the NOP test and concerns regarding its internal validity, but because it can help bridge the findings in nonhuman primates and humans, which rely almost exclusively on DNMS paradigms to study object-recognition memory. The goal of the present article is not to persuade other experimenters to adopt and use the specific protocol presented here, but rather to see the advantages in using this general approach to test object-recognition in rats. Specifically, an approach that requires rats to make an explicit choice response based on memory, and one that rewards accurate responses on the test while reducing the presence of the experimenter in administering individual trials.
Notes
In actuality the 220, 330, and 630-s delays were ~10 s shorter on average, but to avoid confusion when comparing scores obtained during training to those during pseudo mixed-delay testing, we made the delay values the same. The increase in delays presumably occurred because the rats became accustomed to waiting for prolonged periods in the delay area by the end of the 630-s delay training.
It is important to note that although increasing the amount of sample object exposure does increase the likelihood of observing a novelty preference, it fails to do so in a linear fashion, such that it does not predict the degree of novelty preference.
References
Aggleton, J. P. (1985). One-trial object recognition by rats. The Quarterly Journal of Experimental Psychology, 37, 279–294. https://doi.org/10.1080/14640748508401171
Aggleton, J. P., & Brown, M. W. (1999). Episodic memory, amnesia, and the hippocampal – anterior thalamic axis. The Behavioral and Brain Sciences, 22, 425–444. https://doi.org/10.1017/S0140525X99002034
Aggleton, J. P., Hunt, P. R., & Rawlins, J. N. P. (1986). The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research, 19, 133–146. https://doi.org/10.1016/0166-4328(86)90011-2
Arain, M., & Cohen, J. (2013). Hierarchical use of cues in the missing object recognition task by rats (Rattus norvegicus). Behavioural Processes, 97, 41–52. https://doi.org/10.1016/j.beproc.2013.04.007
Arain, M., Parameswaran, V., & Cohen, J. (2012). Changing within-trial array location and target object position enhances rats’ (Rattus norvegicus) missing object recognition accuracy. Animal Cognition, 15(5), 771–782. https://doi.org/10.1007/s10071-012-0501-2
Beck, C. H. M., & Kalynchuk, L. E. (1992). Analysis of the ongoing behavior of rats in non-matching-to-sample: Improved acquisition and performance is related to facilitation of investigation. Behavioural Brain Research, 48, 171–176. https://doi.org/10.1016/S0166-4328(05)80154-8
Berlyne, D. E. (1950). Novelty and curiosity as determinants of exploratory behaviour. British Journal of Psychology, 41, 68–80. https://doi.org/10.1111/j.2044-8295.1950.tb00262.x
Besheer, J., & Bevins, R. A. (2000). The role of environmental familiarization in novel-object preference. Behavioural Processes, 50, 19–29. https://doi.org/10.1016/S0376-6357(00)00090-5
Broadbent, N. J., Gaskin, S., Squire, L. R., & Clark, R. E. (2010). Object recognition memory and the rodent hippocampus. Learning & Memory, 17, 5–11. https://doi.org/10.1101/lm.1650110
Brown, M. W., Warburton, E. C., & Aggleton, J. P. (2010). Recognition memory: Material, processes, and substrates. Hippocampus, 1244, 1228–1244. https://doi.org/10.1002/hipo.20858
Clark, R. E., West, A. N., Zola, S. M., & Squire, L. R. (2001). Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus, 186, 176–186. https://doi.org/10.1002/hipo.1035
Cohen, J., Han, X., Matei, A., Parameswaran, V., Zuniga, R., & Hlynka, M. (2010). Rats’ visual-spatial working memory: New object choice accuracy as a function of number of objects in the study array. Learning and Motivation, 41(2), 125–140. https://doi.org/10.1016/j.lmot.2010.01.003
Duva, C. A., Floresco, S. B., Wunderlich, G. R., Lao, T. L., Pinel, J. P. J., & Phillips, A. G. (1997). Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behavioral Neuroscience, 111, 1184–1196. https://doi.org/10.1037/0735-7044.111.6.1184
Ennaceur, A., & Delacour, J. (1988). A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research, 31, 47–59. https://doi.org/10.1016/0166-4328(88)90157-X
Gaskin, S., Tardif, M., Cole, E., Piterkin, P., Kayello, L., & Mumby, D. G. (2010). Object familiarization and novel-object preference in rats. Behavioural Processes, 83, 61–71. https://doi.org/10.1016/j.beproc.2009.10.003
Gaskin, S., Tremblay, A., & Mumby, D. G. (2003). Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus, 13, 962–969. https://doi.org/10.1002/hipo.10154
Gervais, N. J., Brake, W. G., & Mumby, D. G. (2013). Systemic and intra-rhinal-cortical 17- β estradiol administration modulate object-recognition memory in ovariectomized female rats. Hormones and Behavior, 64, 642–652. https://doi.org/10.1016/j.yhbeh.2013.08.010
Gervais, N. J., Hamel, L. M., Brake, W. G., & Mumby, D. G. (2016). Intra-perirhinal cortex administration of estradiol, but not an ER b agonist, modulates object-recognition memory in ovariectomized rats. Neurobiology of Learning and Memory, 133, 89–99. https://doi.org/10.1016/j.nlm.2016.06.012
Herremans, A., Hijzen, T., & Slangen, J. (1995). The object delayed non-matching to sample task in rats does not depend on working memory. NeuroReport, 6, 1963–1965. https://doi.org/10.1097/00001756-199510010-00003
Honig, W. K., & Dodd, P. W. D. (1986). Anticipation and intention in working memory. In D. F. Kendrick, M. E. Rilling, & M. R. Denny (Eds.), Theories of Animal Memory (pp. 77–207). Hillsdale, New Jersey: LEA.
Honig, W. K., & Wasserman, E. A. (1981). Performance of pigeons on delayed simple and conditional discriminations under equivalent training procedures. Learning and Motivation, 12(2), 149–170. https://doi.org/10.1016/0023-9690(81)90016-3
Hurst, J. L., & West, R. S. (2010). Taming anxiety in laboratory mice. Nature Methods, 7. https://doi.org/10.1038/NMETH.1500
Jitsumori, M., Wright, A. A., & Shyan, M. R. (1989). Buildup and release from proactive interference in a rhesus monkey. Journal of Experimental Psychology. Animal Behavior Processes, 15(4), 329–337. https://doi.org/10.1037/0097-7403.15.4.329
Keshen, C., & Cohen, J. (2016). Rats (Rattus norvegicus) flexibly retrieve objects’ non-spatial and spatial information from their visuospatial working memory: Effects of integrated and separate processing of these features in a missing-object recognition task. Animal Cognition, 19(1), 91–107. https://doi.org/10.1007/s10071-015-0915-8
Kesner, R. P., Bolland, B. L., & Dakis, M. (1993). Memory for spatial locations, motor responses, and objects: Triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Experimental Brain Research, 93, 462–470. https://doi.org/10.1007/BF00229361
Kornecook, T. J., Kippin, T. E., & Pinel, J. P. J. (1999). Basal forebrain damage and object-recognition in rats. Behavioural Brain Research, 98, 67–76. https://doi.org/10.1016/S0166-4328(98)00053-9
Mumby, D. G. (1995). Assessing working memory for objects in rats: No one said it was easy. NeuroReport, 6, 1960–1962. https://doi.org/10.1097/00001756-199510010-00002
Mumby, D. G. (2001). Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research, 127, 159–181. https://doi.org/10.1016/S0166-4328(01)00367-9
Mumby, D. G. (2005). Object Recognition. In I. Q. Whishaw & B. Kolb (Eds.), Behaviour of the Laboratory Rat: A Handbook with Tests (pp. 383–391). New York, NY: Oxford University Press.
Mumby, D. G., Glenn, M. J., Nesbitt, C., & Kyriazis, D. A. (2002). Dissociation in retrograde memory for object discriminations and object recognition in rats with perirhinal cortex damage. Behavioural Brain Research, 132, 215–226. https://doi.org/10.1016/S0166-4328(01)00444-2
Mumby, D. G., Kornecook, T. J., Wood, E. R., & Pinel, J. P. (1995). The role of experimenter-odor cues in the performance of object-memory tasks by rats. Animal Learning & Behavior, 23, 447–453. https://doi.org/10.3758/BF03198944
Mumby, D. G., Mana, M. J., Pinel, J. P. J., David, E., & Banks, K. (1995). Pyrithiamine-induced thiamine deficiency impairs object recognition in rats. Behavioral Neuroscience, 109, 1209–1214. https://doi.org/10.1037/0735-7044.109.6.1209
Mumby, D. G., Pinel, J. P., & Wood, E. R. (1990). Nonrecurring-items delayed nonmatching-to-sample in rats: A new paradigm for testing nonspatial working memory. Psychobiology, 18, 321–326. https://doi.org/10.3758/BF03327250
Mumby, D. G., & Pinel, J. P. J. (1994). Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience, 108, 11–18. https://doi.org/10.1037/0735-7044.108.1.11
Mumby, D. G., Pinel, J. P. J., & Dastur, F. N. (1993). Mediodorsal thalamic lesions and object recognition in rats. Psychobiology, 21, 27–36. https://doi.org/10.3758/BF03327123
Mumby, D. G., Pinel, J. P. J., Kornecook, T. J., Shen, M. J., & Redila, V. A. (1995). Memory deficits following lesions of hippocampus or amygdala in rat: Assessment by an object-memory test battery. Psychobiology, 23, 26–36. https://doi.org/10.3758/BF03327055
Mumby, D. G., Piterkin, P., Lecluse, V., & Lehmann, H. (2007). Perirhinal cortex damage and anterograde object-recognition in rats after long retention intervals. Behavioural Brain Research, 185, 82–87. https://doi.org/10.1016/j.bbr.2007.07.026
Mumby, D. G., Tremblay, A., Lecluse, V., & Lehmann, H. (2005). Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus, 15, 1050–1056. https://doi.org/10.1002/hipo.20122
Mumby, D. G., Wood, E. R., Duva, C. A., Kornecook, T. J., Pinel, J. P. J., & Phillips, A. G. (1996). Ischemia-induced object-recognition deficits in rats are attenuated by hippocampal ablation before or soon after ischemia. Behavioral Neuroscience, 110, 266–281. https://doi.org/10.1037/0735-7044.110.2.266
Mumby, D. G., Wood, E. R., & Pinel, J. P. J. (1992). Object-recognition memory is only mildly impaired in rats with lesions of the hippocampus and amygdala. Psychobiology, 20, 18–27. https://doi.org/10.3758/BF03327156
Piterkin, P., Cole, E., Cossette, M., Gaskin, S., & Mumby, D. G. (2008). A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learning & Memory, 15, 785–791. https://doi.org/10.1101/lm.1035508
Rothblat, L. A., & Hayes, L. L. (1987). Short-term object recognition memory in the rat: Nonmatching with trial-unique junk stimuli. Behavioral Neuroscience, 101, 587–590. https://doi.org/10.1037/0735-7044.101.4.587
Warburton, E. C., & Brown, M. W. (2015). Neural circuitry for rat recognition memory. Behavioural Brain Research, 285, 131–139. https://doi.org/10.1016/j.bbr.2014.09.050
Wiig, K. A., & Bilkey, D. K. (1995). Lesions of rat perirhinal cortex exacerbate the memory deficit observed following damage to the fimbria-fornix. Behavioral Neuroscience, 109, 620–630. https://doi.org/10.1037/0735-7044.109.4.620
Winters, B. D., Saksida, L. M., & Bussey, T. J. (2008). Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and Biobehavioral Reviews, 32, 1055–1070. https://doi.org/10.1016/j.neubiorev.2008.04.004
Wood, E. R., Mumby, D. G., Pinel, J. P. J., & Phillips, A. G. (1993). Impaired object recognition memory in rats following ischemia-induced damage to the hippocampus. Behavioral Neuroscience, 107, 51–62. https://doi.org/10.1037/0735-7044.107.1.51
Wright, A. A. (2006). Memory processing. In E. A. Wasserman & T. R. Zentall (Eds.), Comparative cognition: Experimental explorations of animal intelligence (pp. 164–185). New York: Oxford University Press.
Zentall, T. R. (1997). Animal memory: The role of “Instructions”. Learning and Motivation, 28, 280–308. https://doi.org/10.1006/lmot.1996.0968
Zentall, T. R. (2010). Coding of stimuli by animals: Retrospection, prospection, episodic memory and future planning. Learning and Motivation, 41, 225–240. https://doi.org/10.1016/j.lmot.2010.08.001
Zola-Morgan, S., & Squire, L. R. (1985). Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behavioral Neuroscience, 99, 22–34. https://doi.org/10.1037/0735-7044.99.1.22
Zola-Morgan, S., Squire, L. R., & Amaral, D. G. (1989). Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. The Journal of Neuroscience, 9, 1922–1936. Retrieved from http://www.jneurosci.org/content/9/6/1922.short
Acknowledgements
The researchers thank Devon Merza, Lianne Trigiani, Joelle Ziadé, Jonathan Cuthbert, and Francis Carter for their assistance in the behavioral data collection. We are grateful to Aileen Murray and the Animal Care Facility staff for their care of the rats. This work was supported by the National Science and Engineering Research Council of Canada Discovery Grants to DGM (156937-212); a group grant from the Fonds de recherche Québec—santé to the Center for Studies in Behavioral Neurobiology, and an NSERC scholarship to EC.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 364 kb)
Rights and permissions
About this article
Cite this article
Cole, E., Simundic, A., Mossa, F.P. et al. Assessing object-recognition memory in rats: Pitfalls of the existent tasks and the advantages of a new test. Learn Behav 47, 141–155 (2019). https://doi.org/10.3758/s13420-018-0347-9
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-018-0347-9