Abstract
Nonreinforced exposure to a cue tends to attenuate subsequent conditioning with that cue—an effect referred to as latent inhibition (LI). In the two experiments reported here, we examined LI effects in the context of conditioned taste aversion by examining both the amount of consumption and the microstructure of the consummatory behavior (in terms of the mean size of lick clusters). The latter measure can be taken to reflect affective responses to, or the palatability of, the solution being consumed. In both experiments, exposure to a to-be-conditioned flavor prior to pairing the flavor with nausea produced by lithium chloride attenuated both the reduction in consumption and the reduction in lick cluster sizes typically produced by taste aversion learning. In addition, we observed a tendency (especially in the lick cluster measure) for nonreinforced exposure to reduce neophobic responses to the test flavors. Taken together, these results reinforce the suggestion from previous experiments using taste reactivity methods that LI attenuates the effects of taste aversion on both consumption and cue palatability. The present results also support the suggestion that the failure in previous studies to see concurrent LI effects on consumption and palatability was due to a context specificity produced by the oral taste infusion methods required for taste reactivity analyses. Finally, the fact that the pattern of extinction of conditioned changes in consumption and in lick cluster sizes was not affected by preexposure to the cue flavors suggests that LI influenced the quantity but not the quality of conditioned taste aversion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established in rats that pairing a novel taste with illness induced by the injection of an emetic drug (e.g., lithium chloride, LiCl) results in decreased consumption of the taste when it is subsequent contacted, a learning paradigm that is termed conditioned taste aversion (see Reilly & Schachtman, 2009, for a review of this phenomenon). Although taste aversions produced by different methods are often considered together, it has been argued by Parker and colleagues (see Parker, 2003; Parker, Limebeer, & Rana, 2009) that a reduction in the consumption of a taste previously paired with aversive consequences may be motivated by two different processes: the association of the taste with nausea, or its association with a potential danger (e.g., that produced by a novel change in a rat’s physiological state). This distinction is largely based on the presence or absence of aversive (rejection) reactions in the taste reactivity test introduced by Grill and Norgren (1978). In this test, rats are infused with a flavored solution via a cannula implanted in their oral cavity, and the orofacial reactions elicited by the flavor are recorded. Rats usually display rejection reactions, such as gaping, chin rubbing, and paw treading, when infused with unpalatable solutions such as bitter-tasting quinine. Critically, rats also display the same rejection reactions to otherwise palatable tastes (such as sweet sucrose) that have been previously paired with nausea produced by LiCl administration, reflecting a shift in the hedonic value, or palatability, of the taste (e.g., Parker, 1982; Pelchat, Grill, Rozin, & Jacobs, 1983). In contrast, when sucrose is paired with peripheral pain (electric shock), the consumption of that solution is reduced to a degree comparable to that induced by pairing the solution with LiCl, but it does not produce a change in the palatability of the taste stimulus as measured by the taste reactivity test (e.g., Pelchat et al., 1983). This was interpreted in terms of the solution becoming a danger signal without a change in its affective properties. Further evidence that taste aversion learning is mediated both by internal nausea linked to disgust reactions and by other mechanisms includes the fact that rats can suppress the intake of flavors paired with rewarding drugs, such as cocaine or amphetamine, that do not result in the production of rejection reactions to the conditioned stimulus flavors (e.g., Parker, 1982, 1995), and that antiemetic drugs that can interfere with the establishment of disgust reactions to an LiCl-paired flavor without affecting the amount consumed of the flavored solution (e.g., Limebeer & Parker, 2000).
It is also well established that exposure to a stimulus prior to its being paired with some reinforcing event will attenuate (or even prevent) learning about the cue–event relationship. This phenomenon is referred to as latent inhibition (LI) and has been demonstrated with a wide variety of preparations, including when the cue stimulus is a flavor and the subsequent event is the administration of LiCl (for reviews, see Lubow, 1989, 2009). But whereas it has long been known that flavor preexposure reduces conditioned taste aversion as measured by voluntary fluid ingestion in simple consumption tests, its effects on taste palatability are not well understood. In particular, the possibility that LI might affect the quality of learning produced by taste aversion learning (i.e., whether or not taste aversion learning produces changes in the palatability of the cue flavor) has yet to be conclusively addressed. In a recent study conducted to evaluate whether flavor preexposure concurrently attenuates the effects of taste aversion on both fluid consumption and conditioned disgust reactions as an index of palatability, we found that preconditioning flavor exposure not only disrupts suppressed consumption, but also attenuates the establishment of conditioned disgust reactions to a flavor paired with LiCl (López et al., 2010). However, the effects of preconditioning exposure to saccharin on acquired consumption and disgust reactions differed as a function of how the saccharin exposure was performed. That is, when rats were given intraoral infusions of saccharin prior to conditioning with LiCl, saccharin preexposure resulted in attenuated conditioned disgust reactions in the taste reactivity test, but did not attenuate the reduction in flavor ingestion during a voluntary consumption test; in contrast, when preexposed to the solution by bottle, the taste-aversion-induced reduction in the consumption of saccharin was attenuated, but there was no effect of exposure on the acquisition of conditioned disgust reactions to saccharin. In short, LI effects on either consumption or disgust reactions required a common method of fluid delivery during preexposure and testing.
This apparent dissociation in LI effects on consumption and taste reactivity measures might relate to the context specificity of LI, whereby a change of context between exposure and test will attenuate or abolish the LI effect (e.g., Boakes, Westbrook, Elliot, & Swinbourne, 1997; Hall & Channell, 1986; Lovibond, Preston, & Mackintosh, 1984). In the experiments of López et al. (2010), taste reactivity analyses were performed during intraoral fluid delivery, whereas consumption was assessed by giving free access to the test solution in a bottle. On the grounds that the method by which fluid access was given would presumably be highly salient to the rats, López et al. suggested that it would act as a contextual cue, and so exposure to the flavor before training should only influence conditioned taste aversion when the exposure and test methods of fluid delivery matched (which was exactly the pattern of results that was observed). However, whereas context-based LI effects certainly offer an account of the apparent dissociation in the taste reactivity and consumption measures, this account cannot be tested by traditional taste reactivity methods because the reliance on intraoral infusion means that the fluid delivery context will be perfectly correlated with the type of response being assessed.Footnote 1 Moreover, it is at least possible that consumption and taste reactivity reflect two different aspects of the conditioned response, and that flavor exposure might influence them independently. In terms of Konorski’s (1967) distinction between preparatory and consummatory conditioning, bottle-based consumption tests afford preparatory responses (e.g., approach or withdrawal from the bottle), whereas intraoral infusion does not, but intraoral infusion does afford consummatory responses (including hedonic reactions). This division between consummatory and preparatory responses has previously been considered in light of the fact that the hedonic effects of conditioned taste aversion appear to extinguish faster than the effects on consumption (Cantora, López, Aguado, Rana, & Parker, 2006; Dwyer, 2009).
With these issues in mind, the goal of the present experiments was to determine whether LI in taste aversion has concurrent effects on consumption of, and hedonic reactions to, the target taste when the possibility of context effects produced by the fluid delivery methods is removed. This was achieved by the microstructural analysis of licking behavior during voluntary consumption (for reviews of this methodology, see Davis, 1973, 1989; Dwyer, 2012). The ingestive behavior of rats consuming fluids consists of sustained runs of rapidly occurring rhythmic licks (referred to here as clusters) separated by pauses of varying lengths. It has consistently been observed that palatable sugar solutions increase the quantity of fluid consumed, the number of licks, and the number of licks per cluster (e.g., Davis & Perez, 1993; Davis & Smith, 1992); in contrast, the aversive taste of quinine reduces the rate of licking and the size of licking clusters (e.g., Spector & St. John, 1998). Also, pairing an otherwise palatable taste with LiCl results in a reduction of the lick cluster size similar to that produced by quinine (Baird, John, & Nguyen, 2005; Dwyer, 2009). Moreover, amphetamine-based aversions do not produce the same degree of change in lick cluster size as does the aversion produced by LiCl (Dwyer, Boakes, & Hayward, 2008; but see also Arthurs, Lin, Amodeo, & Reilly, 2012; Lin, Arthurs, Amodeo, & Reilly, 2012). In addition, it has been demonstrated that the administration of benzodiazepine drugs, which modulate ingestion responses in the taste reactivity test and enhance hedonic reactions to food in humans, enhance lick cluster size (e.g., Cooper, 2005; Higgs & Cooper, 1998). All of these findings indicate that the analysis of the microstructure of licking behavior can be taken as an effective indicator of rodents’ hedonic reactions. Moreover, the measurement of licking behavior does not affect the means of fluid delivery (it relies on simple electrical means to record the time of each lick to a freely available bottle), and so does not produce a context change similar to that created by the use of intraoral fluid delivery. Thus, in the present experiments, using the LI paradigm, we examined both the amount of consumption and the microstructure of licking behavior in order make an unambiguous assessment of concurrent changes in consumption and taste palatability following flavor preexposure in taste aversion learning.
Experiment 1
The design of Experiment 1 is shown in Table 1. Half of the animals (Group LI) were exposed to 0.1% (w/w) saccharin without any experimentally defined consequences across four drinking sessions, whereas the remainder (Group Control) received water. Following this exposure phase, all of the animals received two sessions in which saccharin was paired with intraperitoneal injections of LiCl. The responses to saccharin were then examined across ten drinking sessions in extinction. Throughout the initial exposure, conditioning, and test phases, the timing of all licks was recorded to allow for the analysis of lick cluster sizes. On the basis of previous analyses of LI in conditioned taste aversion, the rats in Group LI should consume more saccharin than do those in Group Control during the test phase (i.e., after saccharin was paired with LiCl). Furthermore, to the extent that LI also attenuates the degree to which conditioned taste aversion influences hedonic reactions, the lick cluster sizes elicited by saccharin should be larger in Group LI than in Group Control.
Method
Subjects
A group of 24 male Lister hooded rats (Rattus norvegicus) were obtained from Harlan, Bicester, UK, for the purposes of the study. Their weights before the beginning of the experiment ranged from 289 to 361 g, with a mean weight of 333 g. The rats were housed in pairs in a room illuminated between the hours of 0800 and 2000, during which time they had ad-lib access to food and received 60 min of access to water per day, approximately 1 h after the experimental sessions.
Apparatus and stimuli
The rats were trained and tested in 12 custom-made drinking chambers (Med Associated Inc., St Albans, USA). These chambers measured 32 × 15 × 12 cm (L × W × H), with steel mesh flooring and white acrylic walls. The drinking chambers were located in a room separate from that containing the home cages. Fluids were made accessible through drinking spouts made of stainless steel, attached to 50-ml cylinders. These could be inserted on the left- or right-hand side of the lid (made of wire mesh). The distance between the holes for the bottles was 8 cm. Only the left-hand side was used for the present experiments. A contact-sensitive lickometer registered the time of each lick to the nearest 0.01 s. This was recorded by a computer using MED-PC software (Med Associates, Inc., St. Albans, VT). The amount of fluid consumed by each rat was measured by weighing the drinking bottle before and after each session. The stimuli were tap water or solutions of 0.1% (w/w) saccharin.
Procedure
All of the experimental drinking sessions were 15 min in duration, with one session being held on each day. To acclimatize the rats to the experimental apparatus, they were given two 15-min sessions with access to water. The following four sessions comprised the exposure phase: The rats in Group LI received saccharin in each session, whereas those in Group Control received water (see Table 1). Following the exposure phase, all of the rats received a 2-day conditioning phase in which exposure to saccharin was followed by an intraperitoneal injection of LiCl (0.15 M at 5-ml/kg body weight) on both days. The test phase consisted of ten drinking sessions in which saccharin was presented without any experimental consequences.
Data analysis
In addition to the consumption data, the mean cluster size for each rat was extracted from the record of licks for analysis. A cluster was defined as a set of licks, each separated by an interlick interval of no more than 0.5 s. This criterion was used by Davis and his coworkers (e.g., Davis & Perez, 1993; Davis & Smith, 1992) and in the majority of our previous studies using lick analysis techniques (for a review, see Dwyer, 2012). Although other criteria have been used (e.g., Dwyer, Pincham, Thein, & Harris, 2009; Spector, Klumpp, & Kaplan, 1998), parametric analyses suggested that there is little practical difference between them, as most pauses greater than 0.5 s are also greater than 1 s (e.g., Davis & Smith, 1992; Spector et al., 1998). Mixed analyses of variance (ANOVAs) were used to analyze the test data with the factors Exposure Condition (LI vs. control) and Session (Conditioning 1 and 2, Tests 1–10). All tests reported here used a criterion for significance of p = .05.
On several occasions, no licks were recorded for individual rats (Test Session 1 [three rats from Group Control], Test Session 2 [two rats from Group Control], and Test Sessions 6, 8, and 9 [one rat from Group Control]). Consumption was correspondingly very low at these times, suggesting that these were genuine absences of licking, rather than a failure of the recording equipment. Because lick cluster size measures are undefined in the absence of any recorded licks, these empty cells were replaced with the relevant group means for that session in the analyses reported below. A preliminary analysis using only the animals for which data were available for every test session revealed the same general pattern of effects, suggesting that this treatment of the data did not generate spurious effects.
Results
Table 2 shows the data averaged across the exposure phase. The consumption of saccharin in Group LI was higher than the consumption of water in Group Control, t(22) = 5.63, SED = 0.55, p < .001, but the mean lick cluster sizes did not differ between groups, t < 1.
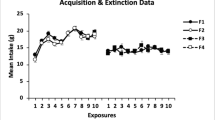
Figure 1 shows the data from the conditioning and tests sessions (consumption in panel A and lick cluster size in panel B). Inspection of panel A suggests that the consumption of saccharin was generally lower for the control than for the LI group, and that consumption in both groups dropped from the level seen in Conditioning Session 1, before partially recovering across testing in extinction. ANOVAs conducted on the amount consumed revealed significant effects of exposure condition (LI vs. control), F(1, 22) = 9.90, MSE = 3.67, p = .005, and session, F(11, 242) = 73.43, MSE = 2.93, p < .001, but no interaction between these two factors, F(11, 242) = 1.41, MSE = 2.93, p = .167. Simple effects analyses revealed that the difference between Groups LI and Control was significant in every session [lowest F(1, 22) = 4.35, MSE = 8.79, p = .049, for Test Session 10] except Test Session 1 [F(1, 22) = 2.08, MSE = 3.31, p = .164]. In addition, consumption was significantly lower than in Conditioning Session 1 in all subsequent sessions for both Group LI [lowest F(1, 22) = 24.85, MSE = 0.82, p < .001, for the comparison to Test Session 10] and Group Control [lowest F(1, 22) = 31.08, MSE = 0.82, p < .001, for the comparison to Test Session 10].
Experiment 1: Mean consumption (A) and lick cluster size (B) per session for both the LI and control groups (error bars indicate SEMs). C1 and C2 on the x-axes refer to Conditioning Sessions 1 and 2, whereas T1–T10 refer to Extinction Test Sessions 1–10
Inspection of panel B suggests similar results for the analysis of mean lick cluster sizes. These were generally lower for the control than for the LI group, and the mean lick cluster sizes in both groups dropped from the level seen on Conditioning Session 1. Unlike with consumption, this recovery did approach initial levels of lick cluster size by the end of extinction testing. ANOVAs conducted on the lick cluster size data once again revealed significant effects of exposure condition (LI vs. control), F(1, 22) = 13.58, MSE = 62.13, p = .001, and session, F(11, 242) = 20.76, MSE = 96.27, p < .001, but no interaction between these two factors, F < 1. Simple effects analyses revealed that the difference between Groups LI and Control was significant in Conditioning Session 2 and Test Sessions 3–9 [lowest F(1, 22) = 5.33, MSE = 161.04, p = .031, for Test Session 5], but not in Conditioning Session 1 and Test Sessions 1, 2, and 10 [highest F(1, 22) = 3.94, MSE = 90.55, p = .060, for Conditioning Session 1]. In addition, in Group LI the lick cluster size was significantly lower than in Conditioning Session 1 in Conditioning Session 2 and Test Sessions 1–5 and 7 [lowest F(1, 22) = 5.29, MSE = 26.80, p = .031, for the comparison to Conditioning Session 2], but it was not significantly different from the initial level in Test Sessions 6 or 8–10 [highest F(1, 22) = 3.44, MSE = 32.9, p = .077, for the comparison to Test Session 6]. In Group Control, the lick cluster size was significantly lower than in Conditioning Session 1 in Conditioning Session 2 and Test Sessions 1–9 [lowest F(1, 22) = 5.33, MSE = 17.46, p = .031, for the comparison to Test Session 9], but it was not significantly different from the initial level in Test Session 10 (F < 1).
In summary, exposure to saccharin prior to taste aversion conditioning with LiCl resulted in both higher levels of consumption and higher lick cluster sizes than were found in a nonexposed control. This is consistent with exposure producing an LI effect that was apparent in both consumption and lick microstructure measures. In addition, the effects of the taste aversion treatment on consumption were more resistant to extinction treatment than were the effects of taste aversion on lick cluster size in both the LI and control conditions. It should also be noted that the effects produced by stimulus preexposure were not affected by the stage of conditioning or by extinction test. The fact that the difference between the LI and control groups remained consistent across sessions suggests that exposure may have attenuated a neophobic reaction to the saccharin solution.Footnote 2 In this light, it is interesting that the studies of neophobia reduction that have been based on taste reactivity (Neath, Limebeer, Reilly, & Parker, 2010) and lick microstructure (Lin, Amodeo, Arthurs, & Reilly, 2012) methods have produced inconsistent effects. The evidence of an attenuation of neophobia raises the question of whether preconditioning exposure to the flavor stimulus affected learning of the CS–US relationship or simply changed the preconditioning baseline against which learning took place. Before considering the theoretical implications of the results of Experiment 1 in more detail, we sought to replicate and extend them to a within-subjects design.
Experiment 2
In Experiment 1, the fact that exposure influenced consumption (and, to an extent, lick cluster size) at the start of the conditioning phase meant that it was hard to completely disentangle any effects of neophobia attenuation from LI more generally. The design of Experiment 2 is shown in Table 1. Half of the animals (Group LI) were exposed to two separate CS flavors (NaCl and maltodextrin, although both were presented without consequences in the exposure phase, they were later counterbalanced between the CS + and CS–), whereas the remainder (Group Control) received water. Following this exposure phase, all of the animals received a 2-day conditioning phase in which the CS + flavor was paired with the IP injection of LiCl on one day, and the CS– flavor was paired with the IP injection of NaCl on the other. The responses to the CS + and CS– were then examined across 16 drinking sessions in extinction (these alternated between the CS + and CS–). As in Experiment 1, both consumption and mean lick cluster size measures were taken throughout. Using a within-subjects manipulation of the taste aversion manipulation means that we were able to compare both conditioned and unconditioned differences in the responses to the flavored solutions. The comparison between the LI and control conditions of the responses averaged across the CS + and CS– during the conditioning phase allowed for an assessment of unconditioned differences in the responses to the stimuli. More importantly, the CS + and CS– would be equally familiar in the LI group and equally unfamiliar in the control group. Therefore, if CS exposure merely affects the preconditioning baseline, no difference should arise in the sizes of the differences between the CS + and CS– across exposure conditions. However, if preexposure to the CSs affects learning, then the size of the CS + −versus-CS– difference should be lower in the LI than in the control condition.
Method
Subjects, apparatus, and stimuli
A group of 24 male Lister hooded rats, obtained from the same source and maintained in the same fashion as in Experiment 1, were used. These animals had previously been used in an unrelated flavor preference experiment, in which they were exposed to fructose and Kool Aid flavors (Kraft Foods USA, Rye Brook, NY) in different experimental chambers than were used here. Their weights before the beginning of the study ranged from 354 to 441 g, with a mean weight of 400 g. The drinking chambers used were the same as those described for Experiment 1. To ensure that the rats’ previous experience with sweet tastes did not interfere with the present study, the stimuli were tap water or solutions of 1% (w/w) NaCl or 4% (w/w) maltodextrin (C*Dry MD 01904; Cerestar-UK, Manchester, UK).
Procedure
All of the experimental drinking sessions were 15 min in duration, and one session was presented each day. To acclimatize the rats to the experimental apparatus, they were given one 15-min session with access to water. The following eight sessions comprised the exposure phase: The rats in Group LI received alternating sessions with NaCl and maltodextrin, whereas those in Group Control received water (see Table 1). Following the exposure phase, all of the animals received a 2-day conditioning phase. On the first conditioning day, all rats received NaCl in the drinking session: For half of the rats in both Groups LI and Control, this was followed by an intraperitoneal injection of LiCl (0.15 M at 5 ml/kg body weight); the remainder of the rats received an intraperitoneal injection of NaCl (0.9% at 5 ml/kg body weight). On the second conditioning day, all rats received maltodextrin in the drinking session: The rats that had received LiCl on the first conditioning session now received an injection of NaCl, whereas the remainder received an injection of LiCl. Thus, for both the LI and control groups, the CS + and CS– were counterbalanced between NaCl and maltodextrin. A single pairing of the CS + with LiCl was used because Experiment 1 had indicated that one pairing was sufficient to produce changes in consumption and lick cluster size in this general protocol. The test phase consisted of 16 drinking sessions alternating between the CS + and the CS–.
Data analysis
The data were prepared for analysis in the same general manner as in Experiment 1. In addition, as will be seen below, large unconditioned differences in the lick cluster sizes were elicited by NaCl and maltodextrin during the exposure phase (these continued into the conditioning and test phases). Thus, the factor Solution Counterbalance (CS + = NaCl vs. CS + = maltodextrin) was added to the analysis of the consumption and lick cluster size data from the conditioning and test phases.
Results
Table 2 shows the data averaged across the exposure phase. Taking the LI group first, whereas consumption of the solutions to become the CS + and the CS– was equivalent, we observed a tendency for the consumption of maltodextrin to be lower than that of NaCl. These trends were stronger in the lick cluster size data. An ANOVA was performed on the consumption data from the LI group, with the factors of whether or not that solution was to be paired with LiCl (CS + vs. CS–) and the nature of the solution (NaCl vs. maltodextrin). This revealed no main effect of CS, F < 1, a main effect of solution type that approached standard levels of significance, F(1, 10) = 3.66, MSE = 2.32, p = .085, and no interaction between these factors, F < 1. A similar analysis of the lick cluster size data revealed no main effect of CS, F < 1, a significant effect of solution type, F(1, 10) = 24.88, MSE = 49.77, p = .001, and no interaction between these factors, F < 1. In addition, consumption of the flavored solutions as a whole in Group LI was higher than the consumption of water in Group Control, t(22) = 6.90, SED = 0.61, p < .001, but the mean lick cluster sizes did not differ between the groups, t(22) = 1.64, SED = 2.99, p = .115.
Figure 2 shows the data from the conditioning and test sessions (consumption in panel A and lick cluster size in panel B). Inspection of panel A suggests that, in both the LI and control groups, consumption of the CS + dropped following the conditioning session before recovering across extinction testing—with the initial reduction being smaller in the LI than in the control group. That is, preexposure to the CS + and CS– attenuated, but did not prevent, the formation of a conditioned taste aversion. The consumption data were subjected to a mixed ANOVA with the within-subjects factors CS (CS + vs. CS–) and test session, plus the between-subjects factors exposure group (LI vs. control) and stimulus assignment (CS + = NaCl vs. CS + = maltodextrin). The most theoretically relevant results from the analysis were as follows: We found a main effect of CS, F(1, 20) = 93.14, MSE = 25.60, p < .001, a session × CS interaction, F(8, 160) = 34.18, MSE = 4.25, p < .001, and an exposure × CS interaction, F(1, 20) = 5.77, MSE = 25.60, p = .026. Respectively, these confirmed that pairing the CS + with LiCl produced an aversion, that this aversion reduced over extinction testing, and that the size of the conditioned difference between the CS + and CS– was attenuated by exposure to the CS solutions. The remainder of the full four-way ANOVA was as follows: A main effect of test session emerged, F(8, 160) = 42.39, MSE = 4.53, p < .001, as well as interactions between session and exposure condition, F(8, 160) = 2.77, MSE = 4.53, p = .007, and session and stimulus assignment, F(8, 160) = 2.39, MSE = 4.53, p = .019. We also observed an interaction between CS and stimulus assignment, F(1, 20) = 21.75, MSE = 25.60, p < .001, such that the CS + −versus-CS– difference was attenuated, but still significant, when NaCl was the CS + solution (means not shown). No interaction was apparent between CS, exposure condition, and stimulus assignment, F(1, 20) = 1.63, MSE = 25.60, p = .217, indicating that the theoretically important exposure × CS interaction was not influenced by stimulus assignment. Finally, we found no significant interactions between session, CS, and exposure condition, F(8, 160) = 1.83, MSE = 4.25, p = .076; between session, CS, and stimulus assignment, F(8, 160) = 1.71, MSE = 4.25, p = .099; nor between session, CS, exposure condition, and stimulus assignment, F < 1.
Experiment 2: Mean consumption (A) and lick cluster size (B) per session for both the CS + and CS– flavors for the LI and control groups (error bars indicate SEMs). C on the x-axes refers to the conditioning session, and T1–T8 refer to Extinction Test Sessions 1–8
In order to further explore the effects of exposure on conditioning, simple effect tests were performed in order to compare the LI and control groups for consumption of both the CS + and CS–. These revealed that consumption of the CS + was greater in the LI than in the control group on Test Sessions 1–4 [lowest F(1, 20) = 4.94, MSE = 9.63, p = .038, for Test Session 2], but consumption of the CS + did not differ between groups at any other time [highest F(1, 20) = 1.45, MSE = 16.17, p = .242, for Test Session 5]. Consumption of the CS– did not differ between Groups LI and Control on any session [highest F(1, 20) = 2.24, MSE = 6.20, p = .150, for Test Session 6]. In addition, in Group LI the consumption of the CS + was significantly reduced relative to the conditioning session baseline on Test Sessions 1–4 [lowest F(1, 20) = 5.22, MSE = 1.01, p = .033, for the comparison to Test Session 4], but it was not significantly different from the baseline on Test Sessions 5–8 [highest F(1, 20) = 2.45, MSE = 1.22, p = .133, for the comparison to Test Session 4]. In Group LI, the consumption of the CS– did not differ from the conditioning session baseline during any subsequent test [highest F(1, 20) = 2.94, MSE = 0.80, p = .102, for the comparison to test session 5]. In Group Control, consumption of the CS + was significantly reduced relative to the conditioning session baseline on Test Sessions 1–5 [lowest F(1, 20) = 5.33, MSE = 1.35, p = .032, for the comparison to Test Session 5] but was not significantly different from the baseline on Test Sessions 6–8 [highest F(1, 20) = 2.19, MSE = 1.22, p = .155, for the comparison to Test Session 6]. In contrast to Group LI, in Group Control the consumption of the CS– did exceed that in the conditioning session baseline on Test Sessions 2–8 [lowest F(1, 20) = 8.40, MSE = 0.80, p = .009, for the comparison to Test Session 5] but was equivalent to baseline consumption on Test Session 1 (F < 1). Finally, with respect to the possibility of neophobia reduction, an analysis of consumption during the conditioning phase averaged across the CS + and CS– conditions revealed no difference between the LI and control groups, F(1, 20) = 1.56, MSE = 5.91, p = .222.
Turning to the lick cluster size data in panel B of Fig. 2, for both the LI and control groups, the mean lick cluster size elicited by the CS + reduced following the conditioning session, before recovering across extinction testing—with the initial reduction being smaller in the LI than in the control group. Thus, preexposure to the CS + and CS– attenuated, but did not prevent, taste-aversion-produced changes in affective responses to the flavored solutions. In addition, the mean lick cluster sizes (across both the CS + and CS–) appeared somewhat lower for the control group than for the LI group during the conditioning session—an effect consistent with a neophobic response. The lick cluster data were subjected to the same mixed ANOVA as the consumption data, with the within-subjects factors CS (CS + vs. CS–) and test session, plus the between-subjects factors exposure group (LI vs. control) and stimulus assignment (CS + = NaCl vs. CS + = maltodextrin). The most theoretically relevant results from the analysis were as follows: We observed a main effect of CS, F(1, 20) = 2625, MSE = 395.80, p < .001, a session × CS interaction, F(8, 160) = 8.36, MSE = 140.69, p < .001, and an exposure × CS interaction, F(1, 20) = 16.59, MSE = 395.80, p = .001. Respectively, these confirmed that pairing the CS + with LiCl reduced the lick cluster size for the CS+, that this reduction decreased over extinction testing, and that the size of the CS + −versus-CS– difference was attenuated by exposure to the CS solutions. In addition to these theoretically critical effects, a main effect of test session also emerged, F(8, 160) = 5.11, MSE = 212.07, p < .001, but no interaction between session and exposure condition, F(8, 160) = 1.16, MSE = 212.07, p = .325, or between session and stimulus assignment, F(8, 160) = 1.15, MSE = 212.07, p = .333. We also found an interaction between CS and stimulus assignment, F(1, 20) = 43.71, MSE = 395.80, p < .001, such that the CS + −versus-CS– difference was only present when maltodextrin was the CS + solution (means not shown). An interaction between CS, exposure condition, and stimulus assignment was also apparent, F(1, 20) = 6.74, MSE = 395.80, p = .017, reflecting the fact that the exposure condition × CS interaction was carried by the conditions in which maltodextrin was the CS+. Finally, we observed no significant interaction between session, CS, and exposure condition, F < 1; a significant interaction between session, CS, and stimulus assignment, F(8, 160) = 4.59, MSE = 140.69, p < .001; but no four-way interaction between session, CS, exposure condition, and stimulus assignment, F < 1.
In order to further explore the effects of exposure on conditioning, simple effect tests were performed to compare the LI and control groups for the mean lick cluster sizes elicited by the CS + and CS–. These revealed that the lick cluster size for the CS + was greater in the LI than in the control group during the conditioning session, as well as during Test Sessions 1–5 [lowest F(1, 20) = 4.56, MSE = 301.40, p = .045, for Test Session 4], but that the lick cluster sizes for the CS + did not differ between groups at any other time [highest F(1, 20) = 4.08, MSE = 549.27, p = .057, for Test Session 7]. Lick cluster sizes for the CS– did not differ between the LI and control groups on any session [highest F(1, 20) = 2.98, MSE = 274.77, p = .099, for the conditioning session]. In addition, in Group LI the lick cluster sizes for the CS + were significantly reduced relative to the conditioning session baseline on Test Sessions 1–3 [lowest F(1, 20) = 7.75, MSE = 20.58, p = .011, for the comparison to Test Session 3], but were not significantly different to the baseline on Test Sessions 4–8 [highest F(1, 20) = 2.98, MSE = 23.75, p = .100, for the comparison to Test Session 4]. In Group LI, the lick cluster sizes for the CS– did not differ from the conditioning session baseline during any subsequent test [highest F(1, 20) = 3.06, MSE = 32.94, p = .096, for the comparison to Test Session 8]. In Group Control, the lick cluster size for the CS + was significantly reduced relative to the conditioning session baseline on Test Sessions 1–3 [lowest F(1, 20) = 5.03, MSE = 20.58, p = .036, for the comparison to Test Session 3] but was not significantly lower than the baseline on Test Sessions 4–8 [highest F(1, 20) = 1.62, MSE = 23.75, p = .218, for the comparison to Test Session 4]. In Group Control, the lick cluster sizes for the CS– did not differ from the conditioning session baseline on any test session [highest F(1, 20) = 1.61, MSE = 33.51, p = .219, for the comparison to Test Session 2]. Finally, with respect to the possibility of neophobia reduction, an analysis of the lick cluster sizes during the conditioning phase, averaged across the CS + and CS–, revealed that these were lower in Group Control and in Group LI, F(1, 20) = 5.35, MSE = 240.03, p = .031.
In summary, exposure to the cue flavors prior to taste aversion conditioning with LiCl resulted in a reduction in the subsequent differences between the CS + and CS– flavors for both the consumption and lick cluster size measures relative to nonexposed controls. Although we also found some evidence for exposure reducing neophobic responses (especially in terms of the differences between Groups LI and Control for the lick cluster measure during the conditioning phase), the use of a within-subjects manipulation of aversion conditioning meant that any neophobia reduction could be parceled out of the exposure effect on learning itself. In addition, the effects of taste aversion persisted for longer on consumption than they did on lick cluster size, but in neither case did the effects of extinction interact with the effects of exposure condition.
General discussion
The main purpose of these experiments was to provide a demonstration of the attenuating effects of flavor preexposure (i.e., LI) on taste aversion learning as assessed by a microstructural analysis of licking behavior, as a means to ascertain whether LI has concurrent effects on consumption and hedonic responses. Although LI effects in taste aversion have been examined extensively using consumption tests (i.e., prior exposure attenuates subsequent suppressed consumption of an illness-paired flavor), the effect of flavor preexposure on taste palatability is not well known. In Experiment 1, nonreinforced exposure to saccharin prior to aversive conditioning with LiCl resulted in attenuated conditioned taste aversion, as assessed by the amount consumed from a bottle containing the solution (i.e., the typical LI effect in taste aversion learning). More interestingly, the preexposure treatment also reduced the effects of taste aversion on the sizes of licking clusters, as compared to a nonexposed control, indicating that the effects of taste aversion on hedonic reactions had also been attenuated. That is, LI attenuates the effects of taste aversion on both consumption and taste palatability. In addition, we found in this experiment that conditioned changes in taste palatability extinguished more rapidly than did those in consumption. Experiment 2 used a within-subjects design to preclude any interpretation of the above-described pattern of results in terms of attenuating neophobia to the cue flavor. As in Experiment 1, flavor preexposure attenuated the formation of a conditioned taste aversion, as measured by consumption and lick cluster size. More specifically, the exposure to the cue flavors (CS + and CS–) prior to aversive conditioning with LiCl resulted in a reduction in the subsequent differences between the CS + and CS– flavors for both the consumption and lick cluster size measures. Again, conditioned changes extinguished more rapidly in taste palatability than in consumption. Therefore, the concurrent effects of LI on lick cluster size and consumption indicated that preconditioning exposure to the CS flavors attenuated the changes in both consumption and taste palatability produced by conditioned taste aversion in a way that was independent of exposure effects on neophobia.
The present results are largely consistent with those of previous experiments (López et al., 2010) that used the taste reactivity methodology to examine changes in cue palatability following flavor preexposure in the taste aversion learning paradigm. López et al. demonstrated for the first time that flavor preexposure not only disrupts suppressed consumption, but also attenuates the establishment of conditioned disgust reactions to an LiCl-paired taste. However, the attenuating effects of flavor preexposure on both consumption and taste reactivity appeared to depend on a common method of fluid delivery during preexposure and testing. As we noted in the introduction, the methods of flavor presentation differentially affected the consumption of the flavor and the display of disgust reactions. When the rats were intraorally infused with the flavor during preexposure, they did not display rejection reactions, but showed a reduction in flavor consumption; in contrast, when the solution was provided by bottle during the preexposure phase, the rats displayed disgust reactions, but they drank the solution in the consumption test. López et al. interpreted this pattern of results as being consistent with the idea that the contextual cues provided by the fluid delivery method (especially the intraoral infusion) can modulate the expression of LI in taste aversion learning. Some evidence has already emerged that changing the fluid delivery method between preexposure and conditioning attenuates the LI effect on consumption measures in taste aversion learning (e.g., Fouquet, Oberling, & Sandner, 2001; Yamamoto, Fresquet, & Sandner, 2002), as the strength of the taste aversion is weakened by changing the method of fluid exposure between conditioning and testing (e.g., Limebeer & Parker, 2006). The present experiments, which have demonstrated concurrent effects of LI on consumption and palatability without the contextual confound of different fluid delivery methods, are thus consistent with the suggestion that the absence of concurrent LI effects on consumption and palatability observed in the previous study by López et al. was due to a context effect produced by the oral taste infusion method required for taste reactivity analyses.
Considered in this way, LI appears to produce the same general pattern of effects on lick cluster and taste reactivity measures in the context of conditioned taste aversion. Thus, LI joins a number of other manipulations that have parallel effects on these two measures (for a review, see Dwyer, 2012). Such results suggest that microstructural analysis of lick patterns and taste reactivity may be complementary measures that both assess taste palatability or hedonic responses. However, it should be noted that in at least some places taste reactivity and lick microstructure measures diverge. This is apparent in the present context when the effects of flavor exposure on neophobia are considered. As was previously noted, a study by Neath et al. (2010) using the taste reactivity method found that repeated intraoral exposure to saccharin caused an increase in consumption in an intake test, but not an increase in hedonic reactions to the fluid in the taste reactivity test. In contrast, a recent study by Lin, Amodeo, et al. (2012) found that repeated exposure to saccharin results in an attenuation of the neophobic response to this solution, as revealed by an increase in consumption and, importantly, an increase in the size of lick clusters. Although they were not designed as an explicit test of the effects of flavor exposure on neophobia, our own experiments reflect this pattern of results: Both Experiments 1 and 2 here provided at least some suggestion that lick cluster sizes were indeed larger following flavor exposure, whereas our previous study of LI in taste aversion (López et al., 2010) did not see any evidence of an influence of flavor novelty on unconditioned taste reactivity responses. Taken at face value, these results appear to represent a dissociation between taste reactivity and lick microstructure measures, with the former suggesting that the reduction in neophobia with exposure does not affect the palatability of a taste, whereas the latter suggests that it does. Although it is premature to offer a definitive interpretation here, it is worth noting that (broadly speaking) taste reactivity analyses are aimed at making a qualitative distinction as to whether a pattern of facial responses are appetitive or aversive, whereas lick microstructure analyses provide a more quantitative measure. It is thus possible that release from neophobia might not change a taste from being aversive to being appetitive (hence, the lack of a taste reactivity change), but merely change the degree to which it is appetitive (or aversive).
Finally, the results of the present experiments may also provide some information about the hedonic processes underlying extinction of conditioned taste aversions. Previous studies examining the microstructure of licking during extinction of a taste aversion have shown that the reduction in lick cluster size associated with a learned change in palatability extinguishes more quickly than does the avoidance of the flavor previously paired with the lithium (Dwyer, 2009). That is, the suppressed consumption appears to be more resistant to extinction than are learned changes in taste palatability, as indicated by the lick cluster size. Similarly, taste reactivity experiments have shown that a conditioned palatability shift precedes extinction of suppressed consumption (Cantora et al., 2006). The pattern of results obtained in the present study is consistent with these results: In Experiment 1, consumption was significantly lower in the last extinction trial than in the first conditioning session for both Groups LI and Control, but lick cluster size did return to baseline levels for both groups; in Experiment 2 the differences in consumption between the CS + and CS– reduced more slowly than did the differences in lick cluster size (for both Groups LI and Control). We (Cantora et al., 2006; Dwyer, 2009) have previously suggested that the difference in extinction rates for hedonic and consumption measures might result from preparatory responses associated with approaching the drinking bottle being more resistant to extinction than are the consummatory responses (including hedonic ones) directed to the taste itself (e.g., Konorski, 1967; Wagner & Brandon, 1989). The present data are entirely consistent with this general idea, and the fact that prior exposure to the conditioned flavors has little or no effect on the relative speed of extinction suggests that there is little reason to think that LI differentially influences preparatory and consummatory responses in taste aversion. That is, LI appears to have affected the amount of learning about the CS–US relationship in conditioned taste aversion without affecting the nature of what was learned.
To summarize, we found that LI attenuates the effects of taste aversion on both consumption and taste palatability, as assayed by the size of licking clusters. That is, nonreinforced exposure to a flavor to be associated with illness resulted in faster recovery of the size of licking clusters and consumption after taste aversion treatment. The fact that the lick cluster and consumption changes were seen concurrently, and that exposure did not materially affect the relative speeds of extinction in consumption and lick cluster measures, suggests that LI influences taste aversion through a single mechanism, rather than having separate effects on preparatory and consummatory processes; in short, LI appears to have had quantitative but not qualitative effects on conditioned taste aversion. That said, differences do remain between studies that have used taste reactivity and lick microstructure methods. Although some of these differences might well be attributable to context effects based on fluid delivery methods, further studies will be needed to determine conclusively how the type of measure (e.g., amount consumed, lick microstructure, and taste reactivity) are related to the processes involved in taste aversion learning.
Notes
Although taste reactivity analyses can be performed under free-consumption conditions, this produces confounds based on the amount of consummatory contact with the cue flavor: If the solution is aversive, then rats do not consume enough to reliably elicit aversive behaviors, and if the solution is not aversive, then appetitive responses can be swamped by actual consumption. Although the connection between the fluid delivery context and the type of response being assessed in theory could be broken, this would come at the cost of reducing the sensitivity of the taste reactivity assessment.
In the present case, the difference in lick cluster sizes between the exposed and nonexposed groups during the initial conditioning session (i.e., when the control group first had access to saccharin) did not reach standard levels of statistical significance (albeit that at p = .06, it would have been significant on a one-tailed test).
References
Arthurs, J., Lin, J.-Y., Amodeo, L. R., & Reilly, S. (2012). Reduced palatability in drug-induced taste aversion: II. Aversive and rewarding unconditioned stimuli. Behavioral Neuroscience, 126, 433–444. doi:10.1037/a0027676
Baird, J. P., John, S. J. S., & Nguyen, E. A. N. (2005). Temporal and qualitative dynamics of conditioned taste aversion processing: Combined generalization testing and licking microstructure analysis. Behavioral Neuroscience, 119, 983–1003. doi:10.1037/0735-7044.119.4.983
Boakes, R. A., Westbrook, R. F., Elliott, M., & Swinbourne, A. L. (1997). Context dependency of conditioned aversions to water and sweet tastes. Journal of Experimental Psychology: Animal Behavior Processes, 23, 56–67. doi:10.1037/0097-7403.23.1.56
Cantora, R., López, M., Aguado, L., Rana, S., & Parker, L. A. (2006). Extinction of a saccharin–lithium association: Assessment by consumption and taste reactivity. Learning & Behavior, 34, 37–43. doi:10.3758/BF03192869
Cooper, S. J. (2005). Palatability-dependent appetite and benzodiazepines: new directions from the pharmacology of GABAA receptor subtypes. Appetite, 44, 133–150. doi:10.1016/j.appet.2005.01.003
Davis, J. D. (1973). The effectiveness of some sugars in stimulating licking behavior in the rats. Physiology & Behavior, 11, 39–45. doi:10.1016/0031-9384(73)90120-0
Davis, J. D. (1989). The microstructure of ingestive behavior. Annals of the New York Academy of Sciences, 575, 39–45. doi:10.1016/0031-9384(73)90120-0
Davis, J. D., & Perez, M. C. (1993). The acquired control of ingestive behavior in the rat by flavor-associated postingestional stimulation. Physiology & Behavior, 54, 1221–1226. doi:10.1016/0031-9384(93)90352-G
Davis, J. D., & Smith, G. P. (1992). Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience, 106, 217–228. doi:10.1037/0735-7044.106.1.217
Dwyer, D. M. (2009). Microstructural analysis of ingestive behaviour reveals no contribution of palatability to the incomplete extinction of a conditioned taste aversion. Quarterly Journal of Experimental Psychology, 62, 9–17. doi:10.1080/17470210802215152
Dwyer, D. M. (2012). Licking and liking: The assessment of hedonic responses in rodents. Quarterly Journal of Experimental Psychology, 65, 371–394. doi:10.1080/17470218.2011.652969
Dwyer, D. M., Boakes, R. A., & Hayward, A. J. (2008). Reduced palatability in lithium- and activity-based, but not in amphetamine-based, taste aversion learning. Behavioral Neuroscience, 122, 1051–1060.
Dwyer, D. M., Pincham, H. L., Thein, T., & Harris, J. A. (2009). A learned flavor preference persists despite the extinction of conditioned hedonic reactions to the cue flavors. Learning & Behavior, 37, 305–310. doi:10.3758/LB.37.4.305
Fouquet, N., Oberling, P., & Sandner, G. (2001). Differential effect of free intake versus oral perfusion of sucrose in conditioned taste aversion in rats. Physiology & Behavior, 74, 465–474. doi:10.1016/S0031-9384(01)00585-6
Grill, H. C., & Norgren, R. (1978). The taste reactivity test. I: Mimetic responses to gustatory stimuli in neurobiologically normal rats. Brain Research, 143, 263–279. doi:10.1016/0006-8993(78)90568-1
Hall, G., & Channell, S. (1986). Context specificity of latent inhibition in taste aversion learning. Quarterly Journal of Experimental Psychology, 38B, 121–139. doi:10.1080/14640748608402224
Higgs, S., & Cooper, S. J. (1998). Effects of benzodiazepine receptor ligands on the ingestion of sucrose, intralipid and maltodextrin: An investigation using a microstructural analysis of licking behavior in a brief contact test. Behavioral Neuroscience, 112, 447–457. doi:10.1037/0735-7044.112.2.447
Konorski, J. (1967). Integrative activity of the brain. Chicago, IL: University of Chicago Press.
Limebeer, C. L., & Parker, L. A. (2000). The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. Journal of Experimental Psychology: Animal Behavior Processes, 26, 371–384. doi:10.1037/0097-7403.26.4.371
Limebeer, C. L., & Parker, L. A. (2006). Effect of conditioning method and testing method on strength of lithium-induced taste aversion learning. Behavioral Neuroscience, 120, 963–969. doi:10.1037/0735-7044.120.4.963
Lin, J.-Y., Amodeo, L. R., Arthurs, J., & Reilly, S. (2012). Taste neophobia and palatability: The pleasure of drinking. Physiology & Behavior, 106, 515–519. doi:10.1016/j.physbeh.2012.03.029
Lin, J.-Y., Arthurs, J., Amodeo, L. R., & Reilly, S. (2012). Reduced palatability in drug-induced taste aversion: I. Variations in the initial value of the conditioned stimulus. Behavioral Neuroscience, 126, 423–432. doi:10.1037/a0027674
López, M., Gasalla, P., Vega, M., Limebeer, C. L., Rock, E. M., Tuerke, K. J., Bedard, H., & Parker, L. A. (2010). Latent inhibition of conditioned disgust reactions in rats. Learning & Behavior, 38, 177–186. doi:10.3758/LB.38.2.177
Lovibond, P. F., Preston, G. C., & Mackintosh, N. J. (1984). Context specificity of conditioning, extinction, and latent inhibition. Journal of Experimental Psychology: Animal Behavior Processes, 10, 360–375. doi:10.1037/0097-7403.10.3.360
Lubow, R. E. (1989). Latent inhibition and conditioned attention theory. New York, NY: Cambridge University Press.
Lubow, R. E. (2009). Conditioned taste aversion and latent inhibition: A review. In S. Reilly & T. R. Schatchman (Eds.), Conditioned taste aversion. Behavioral and neural processes (pp. 37–57). New York, NY: Oxford University Press.
Neath, K. N., Limebeer, C. L., Reilly, S., & Parker, L. A. (2010). Increased liking for a solution is not necessary for the attenuation of neophobia in rats. Behavioral Neuroscience, 124, 398–404. doi:10.1037/a0019505
Parker, L. A. (1982). Non-consummatory and consummatory behavioral CRs elicited by lithium-paired and amphetamine-paired flavors. Learning and Motivation, 13, 281–303.
Parker, L. A. (1995). Rewarding drugs produce taste avoidance, but not taste aversion. Neuroscience and Biobehavioral Reviews, 19, 143–151. doi:10.1016/0149-7634(94)00028-Y
Parker, L. A. (2003). Taste avoidance and taste aversion: Evidence for two different processes. Learning & Behavior, 31, 165–172. doi:10.3758/BF03195979
Parker, L. A., Limebeer, C. L., & Rana, S. A. (2009). Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In S. Reilly & T. R. Schachtman (Eds.), Conditioned taste aversion. Behavioral and neural processes (pp. 92–113). New York, NY: Oxford University Press.
Pelchat, M. L., Grill, H. J., Rozin, P., & Jacobs, J. (1983). Quality of acquired responses to tastes by Rattus norvegicus depends on type of associated discomfort. Journal of Comparative Psychology, 97, 140–153. doi:10.1037/0735-7036.97.2.140
Reilly, S., & Schachtman, T. R. (2009). Conditioned taste aversion: Behavioral and neural processes. New York, NY: Oxford University Press.
Spector, A. C., Klumpp, P. A., & Kaplan, J. M. (1998). Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience, 112, 678–694. doi:10.1037/0735-7044.112.3.678
Spector, A. C., & St. John, J. (1998). Role of taste in the microstructure of quinine ingestion by rats. American Journal of Physiology, 274, 1687–1703.
Wagner, A. R., & Brandon, S. E. (1989). Evolution of a structured connectionist model of Pavlovian conditioning (AESOP). In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory (pp. 149–189). Hillsdale, NJ: Erlbaum.
Yamamoto, J., Fresquet, N., & Sandner, G. (2002). Conditioned taste aversion using four different means to deliver sucrose to rats. Physiology & Behavior, 75, 387–396. doi:10.1016/S0031-9384(01)00671-0
Author note
This research was partly supported by grants from the Ministry of Science and Innovation of Spain to M.L. (Grant No. MICINN-09-08074) and to P.G. (Grant No. FICYT-BP10-016). The authors express their thanks to Rebecca Wright for her support in conducting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dwyer, D.M., Gasalla, P. & López, M. Nonreinforced flavor exposure attenuates the effects of conditioned taste aversion on both flavor consumption and cue palatability. Learn Behav 41, 390–401 (2013). https://doi.org/10.3758/s13420-013-0114-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-013-0114-x