Abstract
Variation in reward responsiveness has been linked to psychopathology. Reward responsiveness is a complex phenomenon that encompasses different temporal dimensions (i.e., reward anticipation or consumption) that can be measured using multiple appetitive stimuli. Furthermore, distinct measures, such as neural and self-report measures, reflect related but distinct aspects of reward responsiveness. To understand reward responsiveness more comprehensively and better identify deficits in reward responsiveness implicated in psychopathology, we examined ways multiple measures of reward responsiveness jointly contribute to distinct psychological problems by using latent profile analysis. Specifically, we identified three profiles of reward responsiveness among 139 female participants based on their neural responses to money, food, social acceptance, and erotic images and self-reported responsiveness to reward anticipation and consumption. Profile 1 (n = 30) exhibited blunted neural responses to social rewards and erotic images, low self-reported reward responsiveness, but average neural responses to monetary and food rewards. Profile 2 (n = 71) showed elevated neural response to monetary rewards, average neural responses to other stimuli, and average self-reported reward responsiveness. Profile 3 (n = 38) showed more variable neural responses to reward (e.g., hypersensitivity to erotic images, hyposensitivity to monetary rewards), and high self-reported reward responsiveness. These profiles were differentially associated with variables generally linked to aberrations in reward responsiveness. For example, Profile 1 was most strongly associated with anhedonic depression and social dysfunction, whereas Profile 3 was associated with risk-taking behaviors. These preliminary findings may help to elucidate ways different measures of reward responsiveness manifest within and across individuals and identify specific vulnerabilities for distinct psychological problems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aberrations in the ability to respond to reward (i.e., reward responsiveness) have received substantial attention due to their link to outcomes, such as depression (Burkhouse et al., 2017; Sandre et al., 2019; Sherdell et al., 2012; Weinberg et al., 2016), risky behaviors (Bart et al., 2021; Freeman et al., 2020; Huggins et al., 2019; Joyner et al., 2019), and disrupted social functioning (Banica et al., 2022; Weinberg, Ethridge, et al., 2021b). Reward responsiveness is a complex phenomenon that has distinct temporal dimensions (e.g., anticipation or consumption: Berridge & Robinson, 2003; Treadway & Zald, 2011), which can be measured by using multiple appetitive stimuli (e.g., money, food, social acceptance, erotic images). Furthermore, different types of measurement, such as neural and self-report measures of reward responsiveness, reflect related but distinct aspects (e.g., neural vs. phenomenological) of this multifaceted construct (Banica et al., 2022; Bress & Hajcak, 2013). To understand the role of reward responsiveness more comprehensively and to better identify deficits in reward responsiveness implicated in psychopathology, it will be crucial to account for this complexity. However, previous studies have rarely addressed the complex nature of reward responsiveness, because they have primarily examined individual measures of reward responsiveness in isolation (Bart et al., 2021; Burkhouse et al., 2017; Klawohn, Burani, et al., 2021b; Sandre et al., 2019; Weinberg et al., 2016) and in response to one type of reward (typically monetary; Bart et al., 2021; Burkhouse et al., 2017; Freeman et al., 2020; Huggins et al., 2019; Joyner et al., 2019). Thus, our understanding of the associations between reward responsiveness and psychological outcomes is incomplete, because it is not clear how multiple measures of reward responsiveness manifest within and across individuals and jointly contribute to clinical outcomes.
However, there is reason to believe that an ensemble of measures of reward responsiveness function together within individuals (Baskin-Sommers & Foti, 2015; Nusslock & Alloy, 2017; Shankman et al., 2014). For example, individuals may show distinct patterns of associations among multiple temporal dimensions of reward responsiveness that confer risk for different outcomes. That is, whereas individuals with blunted responses to anticipated reward but intact responses to reward receipt may be prone to depression, individuals with intact responses to anticipated reward but excessive responses to reward receipt may be susceptible to substance abuse (Baskin-Sommers & Foti, 2015). Alternatively, some may show blunted responses to all types of reward, whereas others only show reduced responses to one type of reward (Shankman et al., 2014). These conceptualizations are partially supported by the sizable independence among different reward responsiveness measures (e.g., modest correlations among self-reported and/or neural responses to different incentives including money, food, and social acceptance: Banica et al., 2022; Ethridge et al., 2017; Khazanov et al., 2020) and a previous finding that depressive symptoms are differentially associated with motivation to approach reward across different types of incentives (i.e., decreased motivation to approach monetary and social reward but increased motivation to approach food reward: Fussner et al., 2018). In other words, accumulating evidence suggests that there may be unique patterns of associations among multiple measures of reward responsiveness and these patterns can characterize different subgroups of individuals who are at risk for distinct psychological problems.
Few studies have directly examined this important possibility, in part due to the reliance on variable-centered approaches in previous studies (Banica et al., 2022; Bart et al., 2021; Burkhouse et al., 2017; Ethridge et al., 2017; Freeman et al., 2020; Fussner et al., 2018; Huggins et al., 2019; Joyner et al., 2019; Klawohn, Burani, et al., 2021b; Nelson & Jarcho, 2021; Sandre et al., 2019). Variable-centered approaches assume that the population is homogeneous regarding the associations among variables of interest (Bergman & Magnusson, 1997). This assumption precludes the possibility of identifying heterogeneous patterns of associations among multiple measures of reward responsiveness. Person-centered approaches are free from such assumptions and can address heterogeneity in the population by identifying homogeneous subgroups that have distinct patterns of associations among variables of interest (i.e., profiles: Masyn, 2013). Initial attempts at person-centered approaches to examine reward responsiveness have suggested the presence of distinct profiles of reward responsiveness or unique patterns of associations among different reward responsiveness measures. However, these previous studies have included relatively few measures of reward responsiveness (Da Silva et al., 2018), capturing only a part of the complex phenomenon, or have included measures that were not directly related to reward responsiveness (Bounoua et al., 2021), prohibiting inferences specific to reward processing.
To that end, the objective of the current study was to better delineate the multifaceted phenomenon of reward responsiveness and identify specific deficits in reward responsiveness that are linked to distinct psychosocial problems using a person-centered approach. Specifically, a primary aim of the current study was to examine whether there are distinct subgroups of individuals that show unique patterns of associations among multiple measures of reward responsiveness using latent profile analysis, a model-based person-centered approach. To include a wider coverage of measures of reward responsiveness, the current study measured reward responsiveness multimodally by using both self-report and neural measures that involved multiple appetitive stimuli (i.e., money, food, social acceptance, erotic images). Here, we used self-report measures to assess two temporal dimensions of reward responsiveness (i.e., reward anticipation and consumption). In addition, we used two reliable neural indicators of reward responsiveness, the reward positivity (RewP) and the late positive potential (LPP), to assess neural responses to reward receipt across four different types of appetitive stimuli (money, food, social acceptance, and erotic images). The RewP and the LPP are two event-related potentials (ERPs) that are direct measures of brain function related to reward processing and motivated attention, respectively (Hajcak et al., 2009; Klawohn, Brush, & Hajcak, 2021a; Klawohn, Burani, et al., 2021b; Proudfit, 2015; Weinberg, 2022; Weinberg et al., 2022; Weinberg, Correa, et al., 2021a).
A second aim of the current study was to examine associations between our observed profiles and variables that have been theoretically and empirically associated with individual differences in reward processing (i.e., validation variables) to shed light on ways multiple measures of reward responsiveness may confer risk for psychopathology in tandem. These variables included symptoms of depression (Burkhouse et al., 2017; Weinberg et al., 2016), risk-taking behaviors (Bart et al., 2021; Baskin-Sommers & Foti, 2015; Freeman et al., 2020; Huggins et al., 2019), and disrupted social functioning (Banica et al., 2022; Morgan et al., 2015; Weinberg, Ethridge, et al., 2021b). Due to the novel nature of the current study, we only hypothesized that individuals would be grouped into several interpretable profiles that would show differential associations with the validation variables in theory-consistent ways (e.g., profiles characterized by greater reward responsiveness across different measures would be associated with reduced anhedonic depression) without specific predictions about the characteristics of the profiles the LPA would identify.
Method
Participants
This study involved secondary data analysis. Portions of data from the current sample were reported elsewhere (Banica et al., 2023; Banica et al., 2022; Schell et al., 2021). Specifically, Banica et al. (2022) used a variable-centered approach to investigate bivariate associations between self-reported reward responsiveness and the RewP elicited following monetary, food, and social feedback. In another study, Banica et al. (2023) reported on associations between neural response to food reward and factors that may increase its salience, such as hunger. Finally, Schell et al. (2021) described relationships between behavioral responses to positive and negative social feedback and eating disorder symptoms. A list of all measures and tasks used in the current study is publicly available at https://osf.io/gvhnp/.
One hundred forty-one female participants who were aged 18 years or older were recruited by using online advertisements, McGill University’s online pool for psychology research participants, and flyers posted in the Montreal area. All participants were able to read and write in English. Of the initial sample, two participants were excluded due to their previous knowledge of the lab tasks that involved deception, leaving a final sample of 139 participants (mean age = 20.66, SD = 2.20, range = 18–29). In terms of race/ethnicity, 40.3% identified as white, 38.2% as Asian, 5.0% as Middle Eastern, 2.9% as Latin American, 0.7% as black, 3.6% as other, and 9.3% did not provide this information. Most of the participants were college students (95%), 3.6% were not students, and 1.4% did not disclose this information. The sample’s median yearly household income was less than $10,000 CAD, and the median parental guardians’ combined yearly income was $76,000-$100,000 (ranging from “unemployed or disabled” to “more than $200,000”), although 35.0-36.0% of the participants did not provide this information.
Procedure
Participants who signed up for the study reviewed the study protocol and provided informed consent online. They completed self-report questionnaires on an online Qualtrics survey (details provided below) before completing four electroencephalogram (EEG) tasks. During the lab visit, participants were first given a brief overview of the lab session and provided informed consent for the lab portion of the study. EEG sensors were then attached, and detailed instructions for the EEG tasks were provided. For the EEG tasks, participants first completed three tasks in which they won monetary or high-calorie food rewards or got positive social feedback, with the order of the tasks counterbalanced across participants. Following these three tasks, participants completed a task in which they were asked to passively view erotic or neutral images. Participants received either course credit or $30 CAD for their participation. The McGill University Research Ethics Board approved all study procedures.
Measures
Self-report questionnaires for reward responsiveness
The participants completed the following questionnaires that assess reward responsiveness, which served as indicators in LPA.
Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006)
The TEPS is an 18-item questionnaire assessing experiences of anticipatory pleasure and consummatory pleasure (i.e., two temporal dimensions of reward responsiveness) on a 6-point scale (1 = very false for me to 6 = very true for me). The TEPS provides summed subscale scores for levels of both anticipatory and consummatory pleasure, with higher scores indicating greater pleasure experience. The Anticipatory Pleasure and Consummatory Pleasure subscales showed acceptable internal consistency and good test-retest reliability (Gard et al., 2006). Cronbach’s alphas for the subscales in the current sample were 0.74 and 0.67, respectively.
Reward Responsiveness Scale (RR scale; Van den Berg et al., 2010)
The RR scale is an 8-item questionnaire measuring tendency to engage in (recurrent) reward-related behavior upon anticipation of reward or the receipt of reward on a 4-point scale (1 = strong disagreement to 4 = strong agreement). All items are summed together into one total reward responsiveness score; higher scores indicate greater reward responsiveness. The RR scale showed acceptable to good internal consistency and good test-retest reliability (Van den Berg et al., 2010). Cronbach’s alpha for this scale in the current sample was 0.75.
Self-report questionnaires for validation variables
Mood and Anxiety Symptoms Questionnaire (MASQ-D30; Wardenaar et al., 2010)
The MASQ-D30 is a 30-item questionnaire assessing the shared and distinct symptoms of depression and anxiety during the past 2 weeks on a 5-point scale (1 = not at all to 5 = extremely). The MASQ-D30 provides summed subscale scores of general distress (i.e., shared symptoms of depression and anxiety), anhedonic depression (i.e., distinct symptoms of depression), and anxious arousal (i.e., distinct symptoms of anxiety); higher scores indicate greater symptomology. The three subscales showed good to excellent internal consistency (Wardenaar et al., 2010). Cronbach’s alphas for the subscales ranged from 0.79 to 0.92 in the current sample.
Revised Social Anhedonia Scale – Short Form (RSAS-SF; Winterstein et al., 2011)
The RSAS-SF is a 15-item subscale of the Wisconsin Schizotypy Scales (Winterstein et al., 2011) that assesses degree of a lack of pleasure from social experiences on a binary scale (i.e., “true” or “false”). All items are summed to compute a total score; higher scores indicate greater social anhedonia. This scale showed acceptable internal consistency (Winterstein et al., 2011). Cronbach’s alpha for the scale was 0.72 in the current sample.
Ostracism Experience Scale for Adolescents (OES-A; Gilman et al., 2013)
The OES-A is an 11-item scale that assesses experiences with being excluded and ignored by others on a 5-point scale (1 = never to 5 = always). We modified the instructions to ask participants to answer the items regarding their entire lifetime, rather than the past year. The Excluded and Ignored subscales were used to assess the extent to which participants were socially excluded and ignored, respectively. Higher scores indicate greater experiences of being excluded and ignored. The subscales showed excellent internal consistency (Gilman et al., 2013). Cronbach’s alphas for the subscales were 0.89 and 0.91 in the current sample.
Domain-Specific Risk-Taking (Adult) Scale (DOSPERT; Blais & Weber, 2006)
The DOSPERT is a 30-item questionnaire measuring the likelihood that individuals would engage in risky behaviors in several life domains on a 7-point scale (1 = extremely unlikely to 7 = extremely likely). The DOSPERT has five subscales, each of which indicates risk-taking tendency in five life domains: social, recreational, financial, health/safety, and ethical risks. Higher scores indicate a greater tendency to engage in risk-taking behavior in the corresponding life domains. We excluded six items that might not be applicable for our sample (e.g., “Starting a new career in your mid-thirties”) and added one item tapping an ethical risk from the original, 40-item DOSPERT (i.e., “Cheating on an exam”; Weber et al., 2002) to make the questionnaire more suitable for our sample. The DOSPERT subscales showed acceptable to good internal consistency (Blais & Weber, 2006). In the current sample, Cronbach’s alphas for the subscales ranged from 0.48 to 0.80, with particularly low internal consistency for the Health/Safety and Ethical Risk-Taking subscales (0.48 for both subscales). Given such low internal consistency, we excluded these two subscales from the analyses, which resulted in the inclusion of three remaining subscales in the analyses (i.e., social, recreational, and financial risk-taking tendency). Cronbach’s alphas for the social, recreational, and financial risk-taking subscales were 0.55, 0.80, and 0.76 respectively.
Task and materials
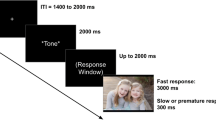
The current study included four different tasks to elicit neural responses to multiple appetitive stimuli. Specifically, we used the RewP elicited following monetary rewards, food rewards, and positive social feedback in three tasks (Doors, Plates, and Island Getaway Tasks). The RewP is a positive deflection in the ERP waveform typically observed 250 to 350 ms following positive feedback (e.g., monetary gains). The RewP has shown convergent validity by its correspondence with other measures of reward responsiveness as well as other aspects of reward processing (see Proudfit, 2015 for a review). In line with the aims of our study, the RewP can be assessed using different appetitive stimuli (Banica et al., 2022; Ethridge et al., 2017; Nelson & Jarcho, 2021; Pegg et al., 2021), and RewPs to different appetitive stimuli appear to reflect distinct but related aspects of reward responsiveness (Banica et al., 2022; Freeman et al., 2022, 2023; Huggins et al., 2019; Sandre et al., 2019; Weinberg, Ethridge, et al., 2021b; Weinberg, Liu, et al., 2015a). We also used the LPP elicited by erotic images (compared to neutral images) in the Images Task. The LPP is a sustained, positive-going slow wave in the ERP waveform that is sensitive to motivationally or affectively salient stimuli (Cuthbert et al., 2000; Weinberg & Hajcak, 2010; Wilson & MacNamara, 2021). Variation in the LPP to appetitive/rewarding pictures, such as erotic images, has particularly been associated with outcomes, such as depression (Klawohn, Burani, et al., 2021b; Sandre et al., 2019; Weinberg et al., 2016) and risky behaviors (Franken et al., 2003; van de Laar et al., 2004; Prause et al., 2014). More details for each task are presented below. Tasks were presented to participants on an Intel Core i7 computer. We used Presentation software (Neurobehavioral Systems, Inc.; Albany, CA) to control the presentation and timing of task stimuli, which were displayed on a 19-inch (48.3 cm) computer monitor. Please see Fig. 1 for a graphical depiction of all tasks.
Doors (a), Plates (b), Island Getaway (c), and Images (d) tasks. Each panel shows one trial of each task. Figure created with Bio.Render.com
Doors task (Proudfit, 2015)
We used the Doors task to elicit neural responses to monetary rewards (i.e., the “monetary RewP”). Participants were asked to view images of two doors on the screen in a total of 40 rounds and indicate which door they thought was hiding a prize by using the right or left mouse buttons. Research assistants (RAs) told participants that there would be a monetary reward for every correct guess (i.e., winning $0.50 for choosing a door hiding a prize behind it), and they would lose $0.25 for each incorrect guess. Following door selection, a fixation cross was displayed for 1,000 ms, and then either a green arrow pointing up or a red arrow pointing down appeared on the screen for 2,000 ms to inform participants of whether they won or lost money in the corresponding round (i.e., a green arrow indicating winning and a red arrow indicating losing). Another fixation cross was then displayed for 1,500 ms, after which an instruction “Click for next round” appeared on the screen. Note that green arrows and red arrows appeared on 50% of the trials, respectively, but in a random order. Participants were given a break after the 20th trial, during which they were given $1.50 and learned that this amount was their task earnings thus far. At the end of the task, all participants received an additional $1.50 for their earnings in the second half of the task.
Plates task (Banica et al., 2022)
To elicit neural responses to food rewards (i.e., the “food RewP”), the Plates task was designed after the Doors task (Banica et al., 2022). Thus, the structure and timing of the task was identical to that of the Doors task described above, but participants saw an image of two covered chef’s plates instead of doors and later received food rewards instead of monetary rewards. Participants were instructed to play a game during which they could win or lose snack foods of their choice and were shown bowls containing one portion of M&Ms (3), Skittles (3), Pringles (1 chip), or Doritos (1 chip). Participants chose one of these snacks as their food reward during the task and were told that they would obtain one portion of food for every plate they chose correctly (i.e., choosing a plate hiding a prize behind it) and lose half a portion of food every time they chose a plate incorrectly. As with the Doors task, participants were given a break following the 20th trial and were provided with half of their anticipated food winnings (e.g., 3 Pringles chips out of 6) and were told that these were their snack earnings so far. Participants could choose to consume their snack during the break or save it for later. At the end of the task, participants received the rest of their total anticipated food earnings. All participants were given a total of 15 M&Ms, 15 Skittles, 6 Pringles chips, or 6 Doritos chips, depending on the food choice they made earlier.
Island Getaway Task (IG: Kujawa et al., 2014; Ethridge et al., 2017)
We used the IG task to elicit neural responses to social rewards (i.e., the “social RewP”). The RewP elicited by social acceptance during the IG task is temporally and morphologically similar to the RewP elicited by both monetary and food rewards (Banica et al., 2022; Freeman et al., 2023; Pegg et al., 2019, 2021), suggesting the task is effectively capturing social-reward-related processes. The original task code can be found at https://arfer.net/projects/survivor. The task used in the present study was modified from previous versions. Our code can be found at https://osf.io/gvhnp/.
Participants were initially told that they would play a “Survivor”-style game against 11 other research participants in labs across North America, with a goal to be one of the last six players to make it to the “big island.” In each of the six rounds of the game, participants were asked to vote to indicate whether they would like each co-player to remain in the game or be removed from the game. After each vote, participants also received feedback about whether the co-player they just voted for or against decided to keep them or send them home. Unbeknownst to participants, all other co-players were computer-generated, and the task was programmed such that all participants would make it to the final destination (i.e., big island) regardless of their responses. More detailed descriptions of the task are provided below and elsewhere (Banica et al., 2022).
Participants were provided with each co-player’s profile (in a random order) whenever they decided whether they would like to “keep” or “kick out” the corresponding co-player. A screen asking, “Should we keep [coplayer name] or kick [him/her] out?” was displayed with each profile, as were buttons saying, “Kick Out” and “Keep.” Following the decision (via a mouse click), a fixation cross was displayed for 1,000 ms, and the message “Waiting for [coplayer name] to vote…” was then shown if the participant voted faster than a simulated voting time to increase task authenticity (Ethridge et al., 2017; Ethridge & Weinberg, 2018; Kujawa et al., 2014, 2017). Subsequently, either acceptance feedback (i.e., a green thumbs up) or rejection feedback (i.e., a red thumbs down) from the “coplayer” was displayed to the participant for 2,000 ms. Participants were then asked to use visual analogue scales to indicate how much they liked, and how much they thought other coplayers would like, the coplayer they voted on a scale from 1 (not at all) to 9 (extremely). The scales were displayed until the participant made an answer, and a blank screen was then presented for 1,500 ms. At the end of each round, participants were shown the name and photo of the coplayer who they were told received the most votes to leave the game and was kicked out. The next voting round then started. There was a total of 51 feedback trials in which participants received, on average, 26.84 “keep” votes and 24.16 “kick out” votes. At the end of the task, RAs asked participants to rate how much they believed that they were playing against real peers on a scale from 1 (not at all) to 5 (completely). The average and median of task believability ratings were 2.95 (SD = 1.30) and 3, respectively, in the current sample.
Images task
To elicit neural responses to erotic images, participants completed the Images task, a passive viewing paradigm with pictures from the International Affective Picture System (IAPS; Lang et al., 1997). Participants were instructed to simply view the images, which were presented one at a time in three blocks—erotic, aversive, and neutral—randomly counterbalanced between participants. Participants first completed a practice block where they were shown three erotic, three neutral, and three aversive images. The actual task consisted of 20 erotic images of heterosexual couples, 20 neutral pictures (i.e., objects), and 20 aversive pictures (i.e., images depicting bodily harm or threat of harm). Before each block, a message was displayed for 4,000 ms indicating whether the upcoming pictures would be neutral, erotic, or aversive. All images were displayed for 1,500 ms, followed by an intertrial interval ranging between 2,000 to 2,500 ms consisting of a white fixation cross in the middle of a black screen. Between blocks, a message saying, “Break. Press the space bar when you are ready to continue.” was displayed until the participant advanced the task. According to IAPS normative ratings that used a 9-point scale (1 = low pleasure, 9 = high pleasure), the aversive images used were less pleasant (mean valence = 1.78) than the neutral images (mean valence = 5.05), and the neutral images were less pleasant than the erotic images (mean valence = 6.67).
Electroencephalogram Recording and Data Processing
We recorded continuous EEG with a 32-electrode cap based on the standard 10/20 layout using a BrainVision actiCHamp system (Brain Products, Munich, Germany) with a ground electrode at site Fpz. We recorded data at a sampling rate of 1,000 Hz. Offline analyses were done with BrainVision Analyzer software (Brain Products, Munich, Germany). Unsegmented data were band-pass filtered with low and high cutoffs of 0.01 and 30 Hz, respectively, with a Butterworth zero phase filter with a 24 db/octave roll-off.
For each trial in the Doors, Plates, and IG tasks, the EEG was segmented into 2,500-ms windows starting 1,000 ms before feedback onset and continuing for 1,500 ms post-feedback. For each trial in the Images task, the EEG was segmented into 3,000-ms windows starting 1,000 ms before image presentation and continuing for 2,000 ms after image presentation. In each of the four tasks, data were then referenced to the average of the left and right mastoids (TP9 and TP10, respectively). Ocular and eye-blink corrections were done using the FT9 site for HEO and the FP1 site for VEO, and a modification of the Miller et al. (1988) formula.
We conducted artifact rejection with a semiautomatic procedure where individual channels were removed from a trial if they had a voltage difference of more than 175 μV within 400-ms intervals, a voltage difference of less than 0.50 μV within 100-ms intervals, and a voltage step of more than 50.0 μV between sample points. Visual inspection was then conducted to detect and remove remaining artifacts. Data from the Images task were next re-segmented into 1,200 ms windows starting 200 ms before image presentation and ending 1,000 ms after image presentation. Gain and loss trials (from Doors and Plates), acceptance and rejection trials (from IG), and erotic, aversive, and neutral image trials (from Images) were then averaged separately. The mean voltage in the time window from −200 ms to 0 ms pre-feedback/image served as a baseline and was subtracted from each data point.
In the Doors and Plates tasks, we quantified the RewP as the average activity from 250 to 350 ms following gain feedback at electrode site Cz. This decision was made based on previous research (Ethridge & Weinberg, 2018; Proudfit, 2015; Weinberg et al., 2014) as well as visual inspection of the grand averaged waveforms. Guided by time windows identified in previous research (Babinski et al., 2019; Ethridge et al., 2017; Kujawa et al., 2017; Rappaport et al., 2019) and previous work that scored the social RewP using principal components analysis (PCA; Ethridge et al., 2017; Kujawa et al., 2017; Weinberg, Ethridge, et al., 2021b), we quantified the IG task RewP as the average activity from 275 to 375 ms following acceptance feedback at site Cz. Finally, based on previous work (Baskin-Sommers et al., 2013; Hajcak et al., 2007; Liu et al., 2012; Sandre et al., 2018; Sandre et al., 2019) and visual inspection of the grand averaged waveforms, we quantified the LPP as the average neural activity from 400 to 1,000 ms at electrode site Pz.
Unstandardized residual RewP scores (RewPresid) were calculated separately for neural activity elicited during the Doors, Plates, and IG tasks (Ethridge & Weinberg, 2018; Pegg et al., 2019; Rappaport et al., 2019). Specifically, participants’ ERPs to reward loss or social rejection were modeled as the predictor and ERPs to reward gain or social acceptance as the dependent variable in a regression analysis. We then saved the unstandardized residual scores from each regression analysis and used these values as the monetary RewPresid, the food RewPresid, or the social RewPresid. The residual RewP values adjust for neural activity associated with reward loss or social rejection, thus isolating activity specific to reward gain or social acceptance. We also calculated a residual LPP erotic score to adjust for neural activity associated with the processing of neutral images and isolate activity associated with the processing of erotic images (Levinson et al., 2018; Speed et al., 2015; Weinberg, Venables, et al., 2015b). To do this, we modeled participants’ ERPs to neutral images as the predictor and ERPs to erotic images as the dependent variable, saved the unstandardized residual scores, and used them as LPP residual values (i.e., LPPresid). We calculated split-half reliability for the ERP residual scores between even and odd trials to estimate internal consistency, which are displayed in Table 1. Overall, ERP responses to favorable and not-favorable feedback in Doors, Plates, and IG Tasks, as well as ERP responses to erotic images in Images Task showed high internal consistency (Spearman-Brown coefficients = 0.78-0.92). Residual RewP and LPP scores showed more variable and lower internal consistency, ranged from poor to acceptable (Spearman-Brown coefficients = 0.41-0.70), with particularly low internal consistency for the residual RewP scores obtained from Doors and IG Tasks. These results are consistent with prior findings that residual-based measures show worse internal consistency than ERP responses to each type of feedback or stimuli (e.g., gain/loss and acceptance/rejection: Ethridge & Weinberg, 2018; Luking et al., 2017). However, these residualized scores typically display better psychometric properties than subtraction-based difference scores, which have been used to investigate neural responses to gain in contrast to another condition (Ethridge & Weinberg, 2018; Meyer et al., 2017; Weinberg, Liu, et al., 2015a). Moreover, subtraction-based difference scores can be difficult to interpret as the difference score remains correlated with both conditions, making it challenging to determine which condition is driving associations with individual differences. For these reasons, the current study used the residualized scores.
Data analysis
Power and sample size estimations
Power analysis and estimation of required sample size in LPA are complex tasks and still developing areas (Ferguson et al., 2020; Spurk et al., 2020). For example, statistical power for identifying the correct number of latent profiles using LPA depends on multiple factors, including sample size, number of indicators in LPA, and distance between latent profiles (Tein et al., 2013). Indeed, simulation studies typically suggest a minimum sample size of 250 to 500, but they also indicate that sample size has limited impact on power, as statistical power for a given sample size depends on other data characteristics (e.g., number of indicators, model fit indices used, inter-profile distance; Nylund et al., 2007; Tein et al., 2013). Here, inter-profile distance is deemed to be more important for determining power than other factors, but inter-profile distance often is unknown before analysis, and no previous information about it may be available for novel studies like the current study (Spurk et al., 2020; Tein et al., 2013). Given the novelty of the current study and difficulties collecting EEG data from a sample as large as what is typically recommended, therefore, our sample size was based on the availability of existing data, with the intent to inform future research with our preliminary findings.
Profile identification and associated analyses
To identify profiles of reward responsiveness, we conducted LPAs with robust maximum likelihood estimators (MLR) that account for missing data without biasing parameter estimates and model non-normal data appropriately. Specifically, we first compared competing models in terms of model fit, conceptual interpretability of the extracted profiles, and the size of the emerged profiles to identify the optimal model. When comparing models, we evaluated multiple fit indices, including Bayes information criterion (BIC), sample-size adjusted BIC (SABIC), Lo-Mendell-Rubin adjusted likelihood ratio test (LMR), bootstrap likelihood-ratio test (BLRT), and entropy. Here, lower values of BIC and SABIC as well as significant LMR and BLRT (i.e., p < .05) indicate a better fit, and an entropy value of 0.80 or greater suggests that individuals are classified into profiles with minimum error (Ferguson et al., 2020; Tein et al., 2013). In addition, we estimated the mean probabilities of profile membership in each candidate model to ensure that the optimal solution has profiles that are separate from one another (Ferguson et al., 2020; Masyn, 2013). We also evaluated sizes of the extracted profiles because profiles that are too small (e.g., <5% of the sample size) are unlikely to be reliably estimated or replicated (Ferguson et al., 2020; Nylund-Gibson & Choi, 2018). Regarding the interpretability of the extracted profiles, we computed the model-estimated profile-specific mean scores of the indicator variables both in raw scores and z-scores to make it easier to compare means across indicators with different scales. To describe the characteristics of the profiles and label them objectively and appropriately, we used the guidelines Park and Naragon-Gainey (revise and resubmit) suggested based on the interpretation of Cohen’s d: e.g., |z| < 0.2 = average; 0.2 < |z| < 0.5 = above/below average; 0.5 < |z| < 0.8 = moderately high/low; 0.8 < |z| = high/low; 1.2 < |z| = very high/low; 2.0 < |z| = extremely high/low. To explore whether the identified profiles were mainly due to self-report measures of reward responsiveness, we also re-ran the LPA using only self-reported measures of reward responsiveness post-hoc and reported the results in the Online Resource 1 (Table B; Figure A).
Once we determined the optimal number of latent profiles, we tested whether the profiles indeed differed from one another with regard to the validation variables theoretically or empirically related to reward processing, using the manual BCH approach (Asparouhov & Muthén, 2021). Specifically, we tested whether the profiles are differentially associated with symptoms reflective of depression, anxiety, and their co-occurrence (i.e., anhedonic depression, anxious arousal, general distress), social functioning (e.g., social anhedonia, experiences of ostracism), and risk-taking tendency. We opted for the manual BCH method over other methods used in the literature (e.g., pseudo-class draws, the 3-step approach), because, unlike other methods, the BCH method yields unbiased parameter estimates, avoids any shifts in latent profiles in the analysis, and performs well when variances of these validation variables are allowed to vary across profiles (Asparouhov & Muthén, 2021). All analyses were conducted by using Mplus 8.4 (Muthén & Muthén, 1998-2017). Note that, in Mplus, the effects of latent profiles on these variables are estimated as mean scores of the variables that vary across profiles (i.e., mean differences), instead of directly regressing these variables on the latent profile variable. Because examining the associations of the profiles with these variables involved multiple testing, we used the Benjamini-Hochberg (B-H) procedure to reduce the false-positive rate (Benjamini & Hochberg, 1995). To enhance interpretability of the mean differences and estimate their effect sizes, we also calculated Cohen’s d scores for each pair of comparison.
Results
Table 2 shows the overall sample means, standard deviations, range, and zero-order correlations of the seven indicators of reward responsiveness. Zero-order correlations among all the study variables are provided in Online Resource 1 (Table A). The correlations among the indicators were generally not significant (|r|s = .03-.17, ps > .05), although some were significantly and modestly to moderately correlated (|r|s = .18-.50, ps < .05) with greater positive correlations among self-reported indicators. Additionally, the neural measures were not significantly correlated with the self-report measures, except that neural responses to erotic images were positively and significantly associated with self-reported anticipatory pleasure (r = .18, p < .05). ERP waveforms and topographic maps for the neural indicators of reward responsiveness are presented in Fig. 2.
ERP waveforms and topographic maps. Stimulus-locked ERP grand-averaged waveforms for gain and loss trials (or acceptance and rejection trials), as well as the gain minus loss (or acceptance minus rejection) difference waves, at electrode Cz for monetary (a), food (b), and social reward (c). Topographic maps depict the average difference (μV) between gain and loss responses from 250 ms to 350 ms post-stimulus onset for monetary (a) and food reward (b) and from 275 ms to 375 ms post-stimulus onset for social reward (c). Stimulus-locked ERP grand-averaged waveforms for neural responses to erotic and neutral images, as well as the erotic minus neutral difference waves, at electrode Pz are presented in (d), along with the topographic map depicting the average difference (μV) between erotic and neutral images from 400 ms to 1,000 ms post-stimulus onset. Figure created with Bio.Render.com
Table 3 shows model fit information and profile proportions of each candidate model tested. We first evaluated LPA solutions with one to three profiles to determine the optimal model. Note that models with four or more profiles were not well-identified and thus excluded from consideration. Among the three model solutions, BIC, LMR, and BLRT favored the 2-profile model, as this model had the lowest value of BIC and statistically significant LMR and BLRT values. On the other hand, SABIC and Entropy suggested that the 3-profile model fit better than other solutions as the 3-profile model had the lowest SABIC value and classified individuals into the profiles with minimum uncertainty (i.e., error). Mean probabilities of profile membership in the 2-profile model and the 3-profile model showed that both models have adequate separation between its extracted profiles: 93.1% for Profile 1 (n = 78) and 90.6% for Profile 2 (n = 61) in the 2-profile model; 91.8% for Profile 1 (n = 30), 89.4% for Profile 2 (n = 71), and 94.9% for Profile 3 (n = 38) in the 3-profile model. These values indicate, for example, that individuals who had most likely profile membership in Profile 1 of the 3-profile model had an average 91.8% chance of being classified in Profile 1. Furthermore, all the profiles in the two candidate models were of adequate size.
In addition, we visually inspected the profiles in the 2- and 3-profile models and concluded that the 3-profile model would provide more meaningful theoretical implications (e.g., possible interactions between indicators of reward responsiveness across neural and self-report measures). For example, the extracted profiles of the 2-profile model differed from one another mostly in terms of the self-reported reward responsiveness, suggesting that the profiles may not reflect meaningful patterns of associations among all indicators of reward responsiveness across measures of different modality. In contrast, the profiles of the 3-profile model provided clearer patterns of associations among the indicators as the profiles differed in terms of both self-reported measures of reward responsiveness and neural measures of reward responsiveness in a meaningful way. Based on all these considerations, we retained the 3-profile model. More detailed interpretation of these profiles is presented below.
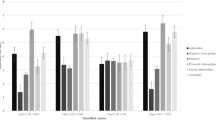
Fig. 3 shows the profile-specific means of the indicators in Z-scores. Profile 1 (n = 30, 21.6%) was characterized by below average neural responses to social rewards and erotic images (zs = −0.25), very low self-reported anticipatory pleasure (z = −1.41), as well as moderately low self-reported consummatory pleasure and reward-related behavior (zs = −0.71, −0.68, respectively). However, Profile 1 still showed average neural responses to monetary rewards and food rewards (zs = −0.003, −0.18, respectively). Profile 2 (n = 71, 51.1%) was characterized by average neural responses to food rewards, social rewards, and erotic images (|z|s = 0.04-0.14), average levels of self-reported anticipatory pleasure, consummatory pleasure, and reward-related behavior (|z|s = 0.04-0.15), but elevated neural responses to monetary rewards (z = 0.24). Profile 3 (n = 38, 27.3%) was characterized by blunted neural responses to monetary rewards (z = −0.45), average neural responses to food and social rewards (zs = 0.07, 0.03, respectively), elevated neural responses to erotic images (z = 0.47), and elevated levels of self-reported anticipatory pleasure, consummatory pleasure, and reward-related behavior (zs = 0.75-1.19).
Table 4 shows the associations of the profiles with variables related to emotional and social functioning. Here, we focused on the results that were significant after adjusting for multiple comparisons. Uncorrected results are provided in Online Resource 1 for descriptive purposes. First, Profile 1 was most strongly associated with anhedonic depression as it showed greater anhedonic depression than Profile 3 (|d| = 1.02, p < .0001). Profile 1 also was most closely associated with social dysfunction, in that showed greater social anhedonia (|d| = 0.77, p = .008) than Profile 2 and more experiences of being socially excluded than both Profile 2 (|d| = 0.94, p = .001) and Profile 3 (|d| = 0.73, p = .005). Interestingly, Profile 3 reported similar levels of experiences of being ignored as Profile 1 (|d| = 0.06, p > .05). Regarding risk-taking tendencies, Profile 3 showed greater recreational risk-taking (|d| = 0.68, p = .009) than Profile 1. Profiles 2 and 3 did not significantly differ in terms of these risk-taking tendencies (|d|s = 0.14–0.18, ps > .05). Finally, none of the three profiles differed significantly from one another in general distress, anxious arousal, or financial risk-taking tendency, although the effect sizes varied (|d|s = 0.09-0.40, ps > .05).
Discussion
The current study identified subgroups of individuals with distinct patterns of associations among neural and self-reported measures of reward responsiveness (i.e., profiles of reward responsiveness), using a person-centered approach in a sample of young female adults. As hypothesized, participants were grouped into interpretable profiles that were differentially associated with anhedonic depression, social anhedonia/social stress, and risk-taking behaviors. Specifically, the profiles differed from one another in relatively straightforward ways in terms of self-reported measures of reward responsiveness (e.g., high, average, and low levels of anticipatory and consummatory pleasure), but each was characterized by unique patterns of associations among neural measures of reward responsiveness. For example, participants in Profile 1, who showed the lowest self-reported anticipatory and consummatory reward responsiveness, exhibited blunted neural responses to stimuli of interpersonal nature (i.e., social rewards and erotic images), but their neural responses to monetary rewards and food rewards were intact (i.e., average). This is consistent with evidence that reward responsiveness differs depending on incentive types (Ethridge et al., 2017; Khazanov et al., 2020; Nelson & Jarcho, 2021; Pegg et al., 2021; Shankman et al., 2014) and further suggests that individuals with subjectively reduced reward responsiveness may still show normative responses to the receipt of some types of reward.
Moreover, participants in Profile 3, who subjectively reported elevated anticipatory and consummatory reward responsiveness, exhibited both hypersensitivity to erotic images and hyposensitivity to monetary rewards at the neural level. Previous discussions of reward responsiveness have often considered the role of excessive reward responsiveness and blunted reward responsiveness separately (Joyner et al., 2019; Nusslock & Alloy, 2017), positing that these two opposite levels of reward responsiveness would characterize different groups of individuals. However, the characteristics of Profile 3 suggest that an individual can exhibit both enhanced and blunted responses to rewards, depending on appetitive stimuli. Moreover, unlike those in Profiles 1 and 3, participants in Profile 2 (the largest subgroup) showed average levels of reward responsiveness across most of the measures included in the analysis, suggesting that some individuals also may have little variability in their responses to reward across different measures of reward responsiveness. Still, it should be noted that Profile 2 showed slightly elevated neural response to monetary rewards. This may indicate that monetary rewards were somewhat more salient among other types of rewards to this subgroup of individuals with overall average levels of reward responsiveness. Taken together, the current findings suggest that the unique combination of variations in these measures of reward responsiveness should be considered to understand the phenomenon of reward responsiveness more deeply.
The profiles also generally showed theory-consistent associations with the validation variables, which supports the validity of the profiles. For example, the profiles were differentially associated with anhedonic depression, but not with anxiety or general distress, consistent with previous findings (Burkhouse et al., 2017; Weinberg et al., 2016). Also, individuals in Profile 1, who showed blunted neural responses to interpersonal rewards as well as blunted self-reported reward responsiveness, reported the highest levels of social anhedonia and social stress, which is in line with previous research showing the link between blunted reward responsiveness (especially in response to social rewards) and disrupted social functioning (Banica et al., 2022; Weinberg, Ethridge, et al., 2021b). However, the current study also showed that individuals with both hyposensitivity and hypersensitivity to rewards at the neural level in addition to high self-reported reward responsiveness (i.e., Profile 3) may be prone to social maladjustment and risk-taking behaviors. These findings suggest that the link between reward responsiveness and some psychological problems may be rather complex. On the one hand, this is consistent with work showing that blunted reward responsiveness (assessed with the RewP in the Doors task) was associated with both risk-prone and risk-averse behaviors (Huggins et al., 2019). Similarly, other researchers have suggested that both hypersensitivity and hyposensitivity to rewards might be positively linked to risk-taking behaviors (e.g., substance use: Nusslock & Alloy, 2017). On the other hand, the current finding that the same individuals could exhibit both hyposensitivity and hypersensitivity depending on the appetitive stimuli indicates a need to further clarify the function of this unexpected combination of hypo- and hypersensitivity to reward within individuals. More work will be necessary to elucidate the nature and the underlying mechanisms of the associations between multiple measures of reward responsiveness and variables, including social functioning and risk-taking behaviors.
It is noteworthy that the profiles did not show strong clustering of indicators by method type (i.e., self-report vs. neural measures), which is not surprising given the generally modest or nonsignificant correlations among the indicators within the same method type. The largest correlation among the indicators was still moderate (r = .50, p < .001), suggesting that indicators reflect related, but distinct, aspects of reward responsiveness. However, it should also be noted that the identified profiles were most strongly differentiated by self-reported indicators of reward responsiveness. When we re-ran the LPA using only self-reported measures of reward responsiveness post-hoc, it suggested a 3-profile solution to be the best-fitting model. This self-report-based, 3-profile model yielded profiles that had similar characteristics (i.e., high, average, and low levels of self-reported reward responsiveness) compared with the 3-profile model identified based on both neural and self-reported indicators of reward responsiveness. The consistency of the results across solutions with and without neural measures of reward responsiveness raises the possibility that neural measures might make limited contributions to the classification of participants. However, even if the three profiles emerged primarily from different patterns of associations among the self-reported measures of reward responsiveness, the inclusion of neural measures helps to further clarify the characteristics of individuals in the profiles, by providing descriptive information about how individuals in each profile responded to the receipt of different types of appetitive stimuli at the neural level. For example, one might assume that individuals in the profile characterized by low self-reported reward responsiveness (Profile 1) also would show blunted neural responses to rewards. However, the current findings revealed that these individuals could show intact responses to some types of rewards at the neural level. Furthermore, the additional information neural measures provide can help to clarify the association between the profiles and the validation variables. For example, Profile 1 was most strongly associated with variables reflective of social dysfunction (e.g., social anhedonia, experiences of ostracism) among the three profiles. This is consistent with the finding that individuals in this profile showed blunted neural responses to interpersonal stimuli (social acceptance feedback, erotic images). Considering that the self-report measures included in the current study do not specifically evaluate responsiveness to social rewards, the inclusion of neural measures using different appetitive stimuli can offer insight into domain-specific deficits in reward responsiveness that characterize each profile.
Despite the preliminary nature of the work, the present findings also could inform clinical assessments of reward responsiveness and help individualize interventions targeting deficits in reward responsiveness. Indeed, researchers have introduced treatment approaches specifically designed to increase reward responsiveness and/or pleasure by encouraging individuals to identify, visualize, and engage in pleasurable activities (Barnes-Horowitz et al., 2023; Craske et al., 2019; Taylor et al., 2017). Our findings suggest that it may be helpful to evaluate distinct profiles of reward responsiveness at the beginning of therapy and use the information to individualize these interventions accordingly. For example, if a patient seeking treatment for depression shows a profile consistent with the Profile 1 identified in the current study, it might be more effective to focus on helping the patient to identify and engage in pleasurable social activities rather than activities involving financial gains or food gains. Furthermore, given the association between Profile 1 and variables reflective of social dysfunction, targeted treatments might consider identifying and addressing barriers that prevent successful social interactions (e.g., lack of social skills).
Limitations of the study suggest future directions for research. First, our sample was relatively small and homogeneous and only included female participants, most of whom were from an elite college campus. The small sample size might have prevented us from identifying additional profiles that exist in the population, while the sample’s homogeneity and idiosyncratic features may limit generalizability of the profiles and their associations with validation variables. In particular, it should be noted that students at elite universities may exhibit unique reward function such that excessive reward responsiveness and high striving may be associated with psychopathology. For example, the current finding that individuals with enhanced reward responsiveness across multiple measures (i.e., Profile 3) may be prone to social maladjustment and risk-taking behaviors may be reflective of the idiosyncratic features of the current sample. Thus, the current findings need to be interpreted in light of the unique cultural context of the sample. Furthermore, the particularly low proportion of participants from racial and ethnic minority groups, including black participants, also limits our ability to generalize our findings to the general population. In addition, two of the identified profiles were fairly small (e.g., n = 30 and n = 38), which might present further challenges to generalizability. Finally, although the nonsignificant associations between the profiles and general distress and anxious arousal are consistent with previous findings that showed specific associations between reward responsiveness and anhedonic depression, the use of a nonclinical sample might have limited power to detect weaker associations between the profiles and these variables. It will be crucial for future studies to recruit larger samples that are more representative of the general population and clinical population.
Additionally, the majority of the participants in the existing data set the current study utilized were young adults. Thus, it will be crucial to examine whether the profiles identified in the current study and their associations with depression, social anhedonia, ostracism, and risk-taking behaviors are replicable in other age groups. Regarding potential effects of age on reward responsiveness, researchers have shown age-related changes in neural responses to reward from childhood to late adolescence (Feldmann et al., 2021; Gibb et al., 2022), and a previous study using a sample of a wide range of ages (18-60 years: Bounoua et al., 2021) found that older adults show a different profile of reward and emotional functioning from younger adults. This suggests that age may play a role in the way multiple measures of reward responsiveness manifest within and across individuals. To examine the role of age, researchers may consider comparing profiles of reward responsiveness across different age groups as well as examining how reward responsiveness assessed with multiple measures emerges and changes across development using longitudinal data (e.g., longitudinal latent profile analysis).
The present study aimed to improve coverage of the construct of reward responsiveness by using both neural and self-report measures. Future studies should expand upon this by incorporating additional measures of reward responsiveness. For instance, our ERP tasks did not capture anticipatory processes. Thus, it would be advisable to measure neural responses to both reward anticipation and reward receipt, incorporating tasks that could assess both temporal dimensions of reward responsiveness (e.g., incentive delay tasks; Ait Oumeziane et al., 2019) and other ERPs, such as cue-P300, stimulus-preceding negativity (SPN), and feedback-P300. Although the current study included a relatively larger set of rewarding stimuli, it will also be important to use a wider range of rewarding stimuli including stimuli that are personally rewarding to each participant to increase ecological validity of the measures (e.g., pictures of loved ones or dream vacation destinations). Finally, the current study focused on neural and self-report measures, but future work may benefit from the inclusion of measures of other modalities (e.g., behavioral measures) that reflect additional aspects of reward responsiveness (e.g., reward learning). Incorporating additional measures will also help increase the statistical power to detect the correct number of profiles, because the number of indicators is an important factor contributing to the statistical power in LPAs and the number of indicators used in the current study is relatively small (Tein et al., 2013).
In addition, the current study focused on a few validation variables that have shown significant associations with reward responsiveness in the literature, to identify specific deficits in reward responsiveness that may be associated with distinct psychological problems. However, it should be noted that we could not examine other more relevant variables that were not available in the existing data set that we utilized in this secondary data analysis (e.g., social functioning, social anxiety and other internalizing symptoms, stress/early life experiences, substance use). Thus, future studies should carefully select a range of variables to better delineate deficits in reward responsiveness that may confer risk for psychological problems, including more direct measures of social functioning. Finally, all study variables were assessed cross-sectionally. Thus, we cannot infer causality regarding associations between profiles and the variables used to validate the profiles (e.g., anhedonic depression, social anhedonia, ostracism, risk-taking behaviors). In other words, it is not clear whether variability in the variables used to validate the profiles contributes to the formation of the profiles, is affected by of the profiles, or both. It will therefore be crucial for future research to clarify the directionality of the association between the profiles and these validation variables using longitudinal designs.
Conclusions
The current study identified unique profiles of reward responsiveness that provided insight into how different measures of reward responsiveness may manifest within and across individuals. These profiles particularly highlighted independence among neural measures of reward responsiveness. We also found that the profiles were associated with anhedonic depression, social dysfunction, and risk-taking behaviors in ways consistent with theory and previous empirical findings. Still, the directionality and the underlying mechanisms of those associations need to be further examined, particularly to answer how the combination of hypersensitivity and hyposensitivity to reward within individuals contributes to mental health issues, such as risk-taking behaviors. Future work should also explore the extent to which neural measures can make important contributions to the profiles. Again, it should be noted that the profiles identified in the current study are most likely to reflect characteristics specific to the current sample (e.g., elite university students, female), which limits our ability to generalize the current findings. Future work will be needed to replicate these distinctive profiles of reward responsiveness and elucidate how these profiles confer risk for different mental health outcomes by using more representative samples and incorporating other neural, behavioral, and self-reported indicators of reward responsiveness. This line of research should advance our understanding of reward responsiveness and better inform psychological interventions that target aberrations in reward processing.
Data availability
The dataset generated and/or analyzed during the current study is available on the Open Science Framework at https://osf.io/gvhnp/.
References
Ait Oumeziane, B., Jones, O., & Foti, D. (2019). Neural sensitivity to social and monetary reward in depression: Clarifying general and domain-specific deficits. Frontiers in Behavioral Neuroscience, 13, 199. https://doi.org/10.3389/fnbeh.2019.00199
Asparouhov, T. & Muthén, B. (2021). Auxiliary variables in mixture modeling: Using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes: No. 21. May 14, 2014. Revised February 4, 2021.
Babinski, D. E., Kujawa, A., Kessel, E. M., Arfer, K. B., & Klein, D. N. (2019). Sensitivity to peer feedback in young adolescents with symptoms of ADHD: Examination of neurophysiological and self-report measures. Journal of Abnormal Child Psychology, 47, 605–617. https://doi.org/10.1007/s10802-018-0470-2
Banica, I., Schell, S. E., Racine, S. E., & Weinberg, A. (2022). Associations between different facets of anhedonia and neural response to monetary, social, and food reward in emerging adults. Biological Psychology, 172, 108363. https://doi.org/10.1016/j.biopsycho.2022.108363
Banica, I., Allison, G., Racine, S., Foti, D., & Weinberg, A. (2023). All the Pringle ladies: Neural and behavioural responses to high-calorie food rewards in young adult women. Psychophysiology, 60(3), e14188. https://doi.org/10.1111/psyp.14188.
Barnes-Horowitz, N. M., Echiverri-Cohen, A., Ruiz, J., Zbozinek, T. D., Kim, R., Treanor, M., Rosenfield, D., Ritz, T., Meuret, A. M., & Craske, M. G. (2023). Exploratory study of threat sensitivity as a moderator of positive affect treatment and negative affect treatment for depression and anxiety. Journal of Experimental Psychopathology, 14(1), 1–14. https://doi.org/10.1177/20438087231161188
Bart, C. P., Nusslock, R., Ng, T. H., Titone, M. K., Carroll, A. L., Damme, K. S. F., Young, C. B., Armstrong, C. C., Chein, J., & Alloy, L. B. (2021). Decreased reward-related brain function prospectively predicts increased substance use. Journal of Abnormal Psychology, 130(8), 886–898. https://doi.org/10.1037/abn0000711
Baskin-Sommers, A. R., Curtin, J. J., & Newman, J. P. (2013). Emotion-modulated startle in psychopathy: Clarifying familiar effects. Journal of Abnormal Psychology, 122(2), 458–468. https://doi.org/10.1037/a0030958
Baskin-Sommers, A. R., & Foti, D. (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2), 227–239. https://doi.org/10.1016/j.ijpsycho.2015.01.011
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Bergman, L. R., & Magnusson, D. (1997). A person-oriented approach in research on developmental psychopathology. Development and Psychopathology, 9(2), 291–319. https://doi.org/10.1017/S095457949700206X
Berridge, K. C., & Robinson, T. E. (2003). Parsing reward. Trends in Neurosciences, 26(9), 507–513.
Blais, A.-R., & Weber, E. U. (2006). A Domain-Specific Risk-Taking (DOSPERT) scale for adult populations. Judgment and Decision Making, 1(1), 33–47.
Bounoua, N., Church, L., & Sadeh, N. (2021). Alterations in reward and emotional processing differentiate among adults with a history of childhood maltreatment: Implications for substance use behaviors. Emotion, 21(8), 1625–1636. https://doi.org/10.1037/emo0000979
Bress, J. N., & Hajcak, G. (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. https://doi.org/10.1111/psyp.12053
Burkhouse, K. L., Gorka, S. M., Afshar, K., & Phan, K. L. (2017). Neural reactivity to reward and internalizing symptom dimensions. Journal of Affective Disorders, 217, 73–79. https://doi.org/10.1016/j.jad.2017.03.061
Craske, M. G., Meuret, A. E., Ritz, T., Treanor, M., Dour, H., & Rosenfield, D. (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457–471. https://doi.org/10.1037/ccp0000396
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. https://doi.org/10.1016/S0301-0511(99)00044-7
Da Silva, S., Apatsidou, A., Saperia, S., Siddiqui, I., Jeffay, E., Voineskos, A. N., Daskalakis, Z. J., Remington, G., Zakzanis, K. K., & Foussias, G. (2018). An examination of the multi-faceted motivation system in healthy young adults. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00191
Ethridge, P., Kujawa, A., Dirks, M. A., Arfer, K. B., Kessel, E. M., Klein, D. N., & Weinberg, A. (2017). Neural responses to social and monetary reward in early adolescence and emerging adulthood. Psychophysiology, 54(12), 1786–1799. https://doi.org/10.1111/psyp.12957
Ethridge, P., & Weinberg, A. (2018). Psychometric properties of neural responses to monetary and social rewards across development. International Journal of Psychophysiology, 132, 311–322. https://doi.org/10.1016/j.ijpsycho.2018.01.011
Feldmann, L., Landes, I., Kohls, G., Bakos, S., Bartling, J., Schulte-Körne, G., & Greimel, E. (2021). Neural processes of reward and punishment processing in childhood and adolescence: An event-related potential study on age differences. Developmental Cognitive Neuroscience, 47, 100896. https://doi.org/10.1016/j.dcn.2020.100896
Ferguson, S. L., Moore, E. W. G., & Hull, D. M. (2020). Finding latent groups in observed data: A primer on latent profile analysis in Mplus for applied researchers. International Journal of Behavioral Development, 44(5), 458–468. https://doi.org/10.1177/0165025419881721
Franken, I. H., Stam, C. J., Hendriks, V. M., & van den Brink, W. (2003). Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology, 170, 205–212. https://doi.org/10.1007/s00213-003-1542-7
Freeman, C., Dirks, M., & Weinberg, A. (2020). Neural response to rewards predicts risk-taking in late but not early adolescent females. Developmental Cognitive Neuroscience, 45, 100808–100808. https://doi.org/10.1016/j.dcn.2020.100808
Freeman, C., Ethridge, P., Banica, I., Sandre, A., Dirks, M. A., Kujawa, A., & Weinberg, A. (2022). Neural response to rewarding social feedback in never-depressed adolescent girls and their mothers with remitted depression: Associations with multiple risk indices. Journal of Psychopathology and Clinical Science, 131(2), 141–151. https://doi.org/10.1037/abn0000728
Freeman, C., Panier, L., Schaffer, J., & Weinberg, A. (2023). Neural response to social, but not monetary, reward predicts increases in depressive symptoms across the COVID-19 pandemic. Psychophysiology, 60(4), e14206. https://doi.org/10.1111/psyp.14206
Fussner, L. M., Mancini, K. J., & Luebbe, A. M. (2018). Depression and approach motivation: Differential relations to monetary, social, and food Reward. Journal of Psychopathology and Behavioral Assessment, 40, 117–129. https://doi.org/10.1007/s10862-017-9620-z
Gard, D. E., Gard, M. G., Kring, A. M., & John, O. P. (2006). Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality, 40(6), 1086–1102. https://doi.org/10.1016/j.jrp.2005.11.001
Gibb, B. E., Tsypes, A., Israel, E., & Owens, M. (2022). Age differences in neural response to reward and loss in children. Psychophysiology, 59(8), 1–12. https://doi.org/10.1111/psyp.14039
Gilman, R., Carter-Sowell, A., DeWall, C. N., Adams, R. E., & Carboni, I. (2013). Validation of the ostracism experience scale for adolescents. Psychological Assessment, 25, 319–330. https://doi.org/10.1037/a0030913
Hajcak, G., Dunning, J. P., & Foti, D. (2007). Neural response to emotional pictures is unaffected by concurrent task difficulty: An event-related potential study. Behavioral Neuroscience, 121(6), 1156–1162. https://doi.org/10.1037/0735-7044.121.6.1156
Hajcak, G., Dunning, J. P., & Foti, D. (2009). Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology, 120(3), 505–510. https://doi.org/10.1016/j.clinph.2008.11.028
Huggins, A. A., Weinberg, A., Gorka, S. M., & Shankman, S. A. (2019). Blunted neural response to gains versus losses associated with both risk-prone and risk-averse behavior in a clinically diverse sample. Psychophysiology, 56(6), e13342. https://doi.org/10.1111/psyp.13342
Joyner, K. J., Bowyer, C. B., Yancey, J. R., Venables, N. C., Foell, J., Worthy, D. A., Hajcak, G., Bartholow, B. D., & Patrick, C. J. (2019). Blunted reward sensitivity and trait disinhibition interact to predict substance use problems. Clinical Psychological Science, 7(5), 1109–1124. https://doi.org/10.1177/2167702619838480
Khazanov, G. K., Ruscio, A. M., & Forbes, C. N. (2020). The Positive Valence Systems Scale: Development and Validation. Assessment, 27(5), 1045–1069. https://doi.org/10.1177/1073191119869836
Klawohn, J., Brush, C. J., & Hajcak, G. (2021a). Neural responses to reward and pleasant pictures prospectively predict remission from depression. Journal of Abnormal Psychology, 130(7), 702–712. https://doi.org/10.1037/abn0000696
Klawohn, J., Burani, K., Bruchnak, A., Santopetro, N., & Hajcak, G. (2021b). Reduced neural response to reward and pleasant pictures independently relate to depression. Psychological Medicine, 51(5), 741–749. https://doi.org/10.1017/S0033291719003659
Kujawa, A., Arfer, K. B., Klein, D. N., & Proudfit, G. H. (2014). Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–147. https://doi.org/10.1016/j.dcn.2014.08.008
Kujawa, A., Kessel, E. M., Carroll, A., Arfer, K. B., & Klein, D. N. (2017). Social processing in early adolescence: Associations between neurophysiological, self-report, and behavioral measures. Biological Psychology, 128, 55–62. https://doi.org/10.1016/j.biopsycho.2017.07.001
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 1(39-58), 3.
Levinson, A. R., Speed, B. C., & Hajcak, G. (2018). Neural response to pleasant pictures moderates prospective relationship between stress and depressive symptoms in adolescent girls. Journal of Clinical, Child & Adolescent Psychology, 48(4), 643–655. https://doi.org/10.1080/15374416.2018.1426004
Liu, Y., Huang, H., McGinnis-Deweese, M., Keil, A., & Ding, M. (2012). Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience, 32(42), 14563–14572. https://doi.org/10.1523/JNEUROSCI.3109-12.2012
Luking, K. R., Nelson, B. D., Infantolino, Z. P., Sauder, C. L., & Hajcak, G. (2017). Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(3), 289–297. https://doi.org/10.1016/j.bpsc.2016.12.004
Masyn, K. E. (2013). Latent Class Analysis and Finite Mixture Modeling. In T. D. Little (Ed.), The Oxford Handbook of Quantitative Methods: Statistical Analysis (Vol. 2, pp. 551–611). Oxford University Press.
Meyer, A., Lerner, M. D., De Los Reyes, A., Laird, R. D., & Hajcak, G. (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. https://doi.org/10.1111/psyp.12664
Miller, G. A., Gratton, G., & Yee, C. M. (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology, 25(2), 241–243. https://doi.org/10.1111/j.1469-8986.1988.tb00999.x
Morgan, J. K., Shaw, D. S., & Forbes, E. E. (2015). Fearfulness moderates the link between childhood social withdrawal and adolescent reward response. Social Cognitive and Affective Neuroscience, 10(6), 761–768. https://doi.org/10.1093/scan/nsu113
Muthén, L. K., & Muthén, B. O. (1998-2017). Mplus User’s Guide (Eighth ed.). Muthén & Muthén.
Nelson, B. D., & Jarcho, J. M. (2021). Neural response to monetary and social feedback demonstrates differential associations with depression and social anxiety. Social Cognitive and Affective Neuroscience, 16(10), 1048–1056. https://doi.org/10.1093/scan/nsab055
Nusslock, R., & Alloy, L. B. (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. https://doi.org/10.1016/j.jad.2017.02.001
Nylund, K. L., Asparouhov, T., & Muthén, B. O. (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. https://doi.org/10.1080/10705510701575396
Nylund-Gibson, K., & Choi, A. Y. (2018). Ten frequently asked questions about latent class analysis. Translational Issues in Psychological Science, 4(4), 440–461. https://doi.org/10.1037/tps0000176
Park, J. & Naragon-Gainey, K. (revise and resubmit). Positive and Negative Emotion Regulation Ability Profiles: Links with Strategies, Goals, and Internalizing Symptoms.
Pegg, S., Arfer, K. B., & Kujawa, A. (2021). Altered reward responsiveness and depressive symptoms: An examination of social and monetary reward domains and interactions with rejection sensitivity. Journal of Affective Disorders, 282, 717–725. https://doi.org/10.1016/j.jad.2020.12.093
Pegg, S., Ethridge, P., Shields, G. S., Slavich, G. M., Weinberg, A., & Kujawa, A. (2019). Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms. Frontiers in Behavioral Neuroscience, 13, 178. https://doi.org/10.3389/fnbeh.2019.00178
Prause, N., Steele, V. R., Staley, C., & Sabatinelli, D. (2014). Late positive potential to explicit sexual images associated with the number of sexual intercourse partners. Social Cognitive and Affective Neuroscience, 10(1), 93–100. https://doi.org/10.1093/scan/nsu024
Proudfit, G. H. (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. https://doi.org/10.1111/psyp.12370
Rappaport, B. I., Hennefield, L., Kujawa, A., Arfer, K. B., Kelly, D., Kappenman, E. S., Luby, J. L., & Barch, D. M. (2019). Peer victimization and dysfunctional reward processing: ERP and behavioral responses to social and monetary rewards. Frontiers in Behavioral Neuroscience, 13, 120. https://doi.org/10.3389/fnbeh.2019.00120
Sandre, A., Bagot, R. C., & Weinberg, A. (2019). Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biological Psychology, 145, 31–41. https://doi.org/10.1016/j.biopsycho.2019.04.001
Sandre, A., Ethridge, P., Kim, I., & Weinberg, A. (2018). Childhood maltreatment is associated with increased neural response to ambiguous threatening facial expressions in adulthood: evidence from the late positive potential. Cognitive, Affective, & Behavioral Neuroscience, 18(1), 143–154. https://doi.org/10.3758/s13415-017-0559-z
Schell, S. E., Banica, I., Weinberg, A., & Racine, S. E. (2021). Hunger games: Associations between core eating disorder symptoms and responses to rejection by peers during competition. International Journal of Eating Disorders, 54(5), 802–811. https://doi.org/10.1002/eat.23487
Shankman, S. A., Katz, A. C., DeLizza, A. A., Sarapas, C., Gorka, S. M., & Campbell, M. L. (2014). The Different Facets of Anhedonia and Their Associations with Different Psychopathologies. In M. S. Ritsner (Ed.), Anhedonia: A Comprehensive Handbook Volume I: Conceptual Issues And Neurobiological Advances (pp. 3–22). Springer. https://doi.org/10.1007/978-94-017-8591-4_1
Sherdell, L., Waugh, C. E., & Gotlib, I. H. (2012). Anticipatory pleasure predicts motivation for reward in major depression. Journal of Abnormal Psychology, 121(1), 51–60. https://doi.org/10.1037/a0024945
Speed, B. C., Nelson, B. D., Perlman, G., Klein, D. N., Kotov, R., & Hajcak, G. (2015). Personality and emotional processing: A relationship between extraversion and the late positive potential in adolescence. Psychophysiology, 52(8), 1039–1047. https://doi.org/10.1111/psyp.12436
Spurk, D., Hirschi, A., Wang, M., Valero, D., & Kauffeld, S. (2020). Latent profile analysis: A review and “how to” guide of its application within vocational behavior research. Journal of Vocational Behavior, 120, 103445. https://doi.org/10.1016/j.jvb.2020.103445
Taylor, C. T., Lyubomirsky, S., & Stein, M. B. (2017). Upregulating the positive affect system in anxiety and depression: Outcomes of a positive activity intervention. Depression and Anxiety, 34(3), 267–280. https://doi.org/10.1002/da.22593
Tein, J.-Y., Coxe, S., & Cham, H. (2013). Statistical power to detect the correct number of classes in latent profile analysis. Structural Equation Modeling, 20(4), 640–657. https://doi.org/10.1080/10705511.2013.824781
Treadway, M. T., & Zald, D. H. (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35(3), 537–555. https://doi.org/10.1016/j.neubiorev.2010.06.006
van de Laar, M. C., Licht, R., Franken, I. H., & Hendriks, V. M. (2004). Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology, 177(1–2), 121–129. https://doi.org/10.1007/s00213-004-1928-1
Van den Berg, I., Franken, I. H., & Muris, P. (2010). A new scale for measuring reward responsiveness. Frontiers in Psychology, 1, 239. https://doi.org/10.3389/fpsyg.2010.00239
Wardenaar, K. J., van Veen, T., Giltay, E. J., de Beurs, E., Penninx, B. W., & Zitman, F. G. (2010). Development and validation of a 30-item short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ). Psychiatry Research, 179(1), 101–106. https://doi.org/10.1016/j.psychres.2009.03.005
Weber, E. U., Blais, A. R., & Betz, N. E. (2002). A domain-specific risk-attitude scale: Measuring risk perceptions and risk behaviors. Journal of Behavioral Decision Making, 15(4), 263–290. https://doi.org/10.1002/bdm.414
Weinberg, A. (2022). Pathways to depression: Dynamic associations between neural responses to appetitive cues in the environment, stress, and the development of illness. Psychophysiology, e14193. Advance online publication. https://doi.org/10.1111/psyp.14193
Weinberg, A., Correa, K. A., Stevens, E. S., & Shankman, S. A. (2021a). The emotion-elicited late positive potential is stable across five testing sessions. Psychophysiology, 58(11), e13904. https://doi.org/10.1111/psyp.13904
Weinberg, A., Ethridge, P., Pegg, S., Freeman, C., Kujawa, A., & Dirks, M. A. (2021b). Neural responses to social acceptance predict behavioral adjustments following peer feedback in the context of a real-time social interaction task. Psychophysiology, 58(11), e13904. https://doi.org/10.1111/psyp.13748
Weinberg, A., & Hajcak, G. (2010). Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion., 10(6), 767–782. https://doi.org/10.1037/a0020242
Weinberg, A., Liu, H., Hajcak, G., & Shankman, S. A. (2015a). Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. Journal of Abnormal Psychology, 124(4), 878–889. https://doi.org/10.1037/abn0000081
Weinberg, A., Perlman, G., Kotov, R., & Hajcak, G. (2016). Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology, 125(1), 26–39. https://doi.org/10.1037/abn0000118
Weinberg, A., Riesel, A., & Kujawa, A. (2022). Neural responses to threat and reward as indices of differential stress susceptibility. Current Directions in Psychological Science, 31(2), 115–123. https://doi.org/10.1177/09637214211049228
Weinberg, A., Riesel, A., & Proudfit, G. H. (2014). Show me the money: The impact of actual rewards and losses on the feedback negativity. Brain and Cognition, 87, 134–139. https://doi.org/10.1016/j.bandc.2014.03.015
Weinberg, A., Venables, N. C., Proudfit, G. H., & Patrick, C. J. (2015b). Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social, Cognitive and Affective Neuroscience, 10(3), 424–434. https://doi.org/10.1093/scan/nsu059
Winterstein, B. P., Silvia, P. J., Kwapil, T. R., Kaufman, J. C., Reiter-Palmon, R., & Wigert, B. (2011). Brief assessment of schizotypy: Developing short forms of the Wisconsin Schizotypy Scales. Personality and Individual Differences, 51, 920–924. https://doi.org/10.1016/j.paid.2011.07.027
Wilson, K. A., & MacNamara, A. (2021). Savor the moment: Willful increase in positive emotion and the persistence of this effect across time. Psychophysiology, 58(3). https://doi.org/10.1111/psyp.13754
Code availability
The Mplus syntaxes used in the analyses are available on the Open Science Framework at https://osf.io/gvhnp/.
Funding
This study was funded by the Canada Research Chairs Program awarded to Dr. Anna Weinberg.
Author information
Authors and Affiliations
Contributions
JP and AW developed the study concept, and IB and AW developed the experimental paradigm. JP performed the data analysis and interpretation with assistance from IB. JP and IB drafted the paper, and AW provided critical revisions. All authors approved the final version of the paper for submission.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have no conflicts of interest/competing interests to declare that are relevant to the content of this article.
Ethical approval
The McGill University Research Ethics Board approved all study procedures.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Open Practices Statement: The dataset generated and/or analyzed during the current study and a list of all measures included in the current study are available on the Open Science Framework at https://osf.io/gvhnp/. The current study was not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 204 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, J., Banica, I. & Weinberg, A. Parsing patterns of reward responsiveness: Initial evidence from latent profile analysis. Cogn Affect Behav Neurosci 23, 1384–1400 (2023). https://doi.org/10.3758/s13415-023-01110-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-023-01110-7