Abstract
Life stress increases risk for multiple forms of psychopathology, in part by altering neural processes involved in performance monitoring. However, the ways in which these stress-cognition effects are influenced by the specific timing and types of life stressors experienced remains poorly understood. To address this gap, we examined how different social-psychological characteristics and developmental timing of stressors are related to the error-related negativity (ERN), a negative-going deflection in the event-related potential (ERP) waveform that is observed from 0 to 100 ms following error commission. A sample of 203 emerging adults performed an ERN-eliciting arrow flanker task and completed an interview-based measure of lifetime stress exposure. Adjusting for stress severity during other developmental periods, there was a small-to-medium effect of stress on performance monitoring, such that more severe total stress exposure, as well as more severe social-evaluative stress in particular, experienced during early adolescence significantly predicted an enhanced ERN. These results suggest that early adolescence may be a sensitive developmental period during which stress exposure may result in lasting adaptations to neural networks implicated in performance monitoring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major life stressors are a key risk factor for a wide range of health problems, including multiple forms of psychopathology (Brown & Harris, 1978; Ingram & Luxton, 2005; Kendler et al., 2002; Kendler et al., 2003; Mazure, 1998). One proposed manner in which stress can affect mental health outcomes is by promoting adaptations in brain structure and function that are beneficial during times of stress but that also may represent latent vulnerabilities for future dysfunction if persistent (Lupien et al., 2009; Masten & Cicchetti, 2010; McCrory et al., 2010; McCrory et al., 2017; McEwen, 1998). In fact, a wealth of evidence shows that individuals who have experienced heightened life stress exhibit volumetric and functional alterations in multiple brain regions, including the anterior cingulate cortex (ACC; Cohen et al., 2006; Treadway et al., 2009) and prefrontal cortex (PFC; De Bellis et al., 2002; Hanson et al., 2010; McLaughlin et al., 2014; Tomoda et al., 2009), which are involved in adaptive performance monitoring and cognitive control (Carter & van Veen, 2007; Miller & Cohen, 2001; Shenhav et al., 2013; Stemmer et al., 2004). Such individuals also demonstrate increased risk for multiple forms of psychopathology (Bae et al., 2006; Botteron et al., 2002; Dell'Osso et al., 2015; Kujawa et al., 2016b; Murrough et al., 2016; Pizzagalli et al., 2018).
Life stress, however, is not a monolithic construct (Epel et al., 2018; Hammen, 2005; Monroe & Roberts, 1990; Slavich, 2019). For example, stressors can occur in multiple domains (e.g., interpersonal conflict, physical danger, financial difficulties) and can be episodic (e.g., serious car accident) or chronic (e.g., ongoing physical abuse; McEwen, 1998; Slavich, 2016; Shields & Slavich, 2017). Consistent with this specificity, emerging research suggests that these different stressor characteristics can exert varying effects on neural development (Andersen et al., 2008; Gollier-Briant et al., 2016; Humphreys et al., 2019; Teicher et al., 2018). Moreover, although many studies have primarily been concerned with differentiating the effects of early life stress from adulthood or more proximal experiences of stress, there are periods across childhood and adolescence when susceptibility to the remodeling effects of stress might be especially high (Heim & Binder, 2012; Lupien et al., 2009; Steinberg, 2005), in part because of functional changes in physiological systems involved in the body’s stress response across development (Gunnar & Donzella, 2002; Gunnar, Talge, & Herrera, 2009a; Gunnar et al., 2009b; Lupien et al., 2009).
Additionally, given that different regions of the brain mature at different rates, it is likely that stress-susceptible regions of the brain have differing sensitive periods (Andersen et al., 2008; Andersen & Teicher, 2008; Cohen et al., 2006; De Bellis et al., 2000; Lim et al., 2015; Luby et al., 2019; Lupien et al., 2009; McCrory et al., 2017; Mueller et al., 2010; Tottenham & Galván, 2016). For instance, brain regions involved in perception and sensory processing show significant development in childhood, before approximately age 7 years (Gogtay et al., 2004; Shaw et al., 2008). Plasticity in neural regions involved in cognitive control is also seen in infancy and early childhood (Guyer et al., 2018; Inguaggiato et al., 2017). Adolescence (approximately ages 8 to 18) is another important time during which neural systems involved in performance monitoring and cognitive control undergo substantial change (Giedd, 2004; Gogtay et al., 2004; Kelly et al., 2008; Segalowitz & Davies, 2004; Sowell et al., 1999; Sowell et al., 2003; Steinberg, 2005), and this change is accompanied by increased cognitive control over behaviors by higher-order prefrontal regions (Griffin, 2017; Luna & Sweeney, 2004).
Plasticity in regions involved in cognitive control appears particularly pronounced during the pubertal transition to adolescence, between approximately ages 8 and 12 years (Dahl & Gunnar, 2009; Shaw et al., 2008; Sowell et al., 2004; Tottenham & Galván, 2016). Importantly, this developmental period is also characterized by reorganization of the hypothalamic–pituitary–adrenal (HPA) axis, a key part of the body’s stress response system (Doom & Gunnar, 2013; Gunnar & Vazquez, 2006). Heightened reactivity to stressors and increased neural sensitivity to the effects of cortisol is also seen during early adolescence, making this a period of heightened vulnerability (Gunnar & Donzella, 2002; Gunnar & Quevedo, 2007; Gunnar, Talge, & Herrera, 2009a; Gunnar et al., 2009b; Lupien et al., 2009).
Combined, these data suggest that it will be beneficial to clarify associations between characteristics and timing of stressors and the activity of neural systems involved in performance monitoring and cognitive control. Effective goal-directed behaviour requires the ability to monitor the outcomes of our actions, which allows us to adapt our behaviour to changing and demanding environments (Ridderinkhof et al., 2004; Ullsperger et al., 2014). The ability to detect errors in one’s performance is a crucial step in recognizing that behaviour should change (Falkenstein et al., 2000; Holroyd & Coles, 2002). The error-related negativity (ERN), an event-related potential (ERP) component involved in error detection, measures one aspect of performance monitoring. The ERN is a fronto-centrally maximal negative deflection in the ERP waveform that differentiates erroneous from correct responses within 100 ms of response onset (Falkenstein et al., 1991; Gehring et al., 1995). It functions as an early alarm signal in an action monitoring network that indicates the need to adjust behaviour and increase executive control to remediate mistakes (Botvinick et al., 2001; Gehring et al., 1993; Holroyd & Coles, 2002).
Although the bulk of existing data implicates the ACC as the primary neural generator of the ERN, the ACC has dense interconnections to both limbic and prefrontal areas (Bush et al., 2000), which also modulate ERN magnitude (de Bruijn et al., 2004; Gehring & Knight, 2000; Manoach & Agam, 2013). The amplitude of the ERN appears to increase across adolescence (Davies et al., 2004; Meyer et al., 2012; Tamnes et al., 2013; Weinberg et al., 2016), an effect that is hypothesized to be due in part to increased activity in ventral-frontal cortical regions involved in error monitoring (Buzzell et al., 2017a). This is consistent with evidence for continued maturation of the ACC and PFC from childhood through young adulthood (Caballero et al., 2016; Lichenstein et al., 2016; Petanjek et al., 2011; Sturman & Moghaddam, 2011; Velanova et al., 2008).
The ERN has also been shown to be sensitive to the effects of a diverse array of naturalistic and experimental stressors, as well as cumulative effects of different stressors (Brooker, 2018; Kessel et al., 2019). For instance, neural response to errors is enhanced under threatening or dangerous conditions: an enhanced ERN is seen under threat of shock (Meyer & Gawlowska, 2017), and when errors are punished by noxious sounds (Riesel et al., 2012), an effect that persists up to 24 hours after the cessation of punishment (Riesel et al., 2019). Naturalistic studies also bear out the results of these laboratory findings. For instance, adolescents who experienced high levels of trauma (Lackner et al., 2018) and veterans with greater exposure to combat (Khan et al., 2018) exhibit an enhanced ERN. It may be adaptive to monitor performance more carefully under conditions of danger and threat – and, indeed, errors potentiate defensive reflexes that are also activated in response to stressful or dangerous situations, such as exposure to threatening stimuli (Bradley et al., 2006; Grillon et al., 1993; Riesel et al., 2012). Neural responses to errors may result in the downstream activation of such defensive systems to avoid physical harm (Riesel et al., 2012). However, other studies have found no associations between posttraumatic stress disorder (PTSD) diagnosis and ERN magnitude in veterans (Gorka et al., 2016; Rabinak et al., 2013; Swick et al., 2015), suggesting that threats to physical integrity may not always lead to increased error monitoring. Moreover, there is to date limited research examining whether the timing of threats to physical integrity is important in understanding associations with the ERN.
Nor does this effect appear to be unique to life-threatening stressors. ERN magnitude, for example, is also enhanced under conditions of social-evaluative stress, such as when participants are told that their performance is being observed (Barker et al., 2015; Buzzell et al., 2017b; Hajcak et al., 2005; Kim et al., 2005; Schillinger et al., 2016; Van Meel & Van Heijningen, 2010). Furthermore, experiencing harsh and controlling parenting has been associated with a larger ERN in children (Brooker & Buss, 2014; Meyer et al., 2015b; Meyer et al., 2019), an association that persists into early adulthood (Banica et al., 2019). The magnitude of the ERN also appears to be particularly susceptible to social stress in early adolescence: young (approximately aged 9 to 12 years), but not older (approximately aged 15 to 18 years), adolescents display an enhanced ERN in social situations compared to nonsocial contexts (Barker et al., 2018), suggesting that the influence of social evaluation on the ERN changes across adolescence. Further, a study by Moor et al. (2012) suggests that early adolescents (aged 10 to 12 years) show increased activity in response to social rejection in the ACC compared to mid-adolescent teenagers (aged 14 to 16 years) and emerging adults (aged 19 to 21 years). These data are consistent with evidence that early adolescence is a period of social reorientation toward peers (Parker et al., 2015) and subsequently heightened sensitivity to social stress (e.g., peer evaluation and exclusion; Bolling et al., 2011; Silk et al., 2013).
Taken together, these findings suggest that the ERN is enhanced under stressful circumstances—particularly those involving threats to physical integrity or social standing—in which errors may be more consequential. However, most studies that have examined these associations have only looked at a single type of stress exposure. Consequently, it is not clear whether all types of stress show similar associations with the ERN, nor whether there might be cumulative effects of multiple stressors (Dunn et al., 2019; Evans et al., 2013). Critical to this investigation, it is also not clear whether the developmental timing of stressors differentially affects associations with the ERN. For instance, in contrast to the work cited above, some studies have found that early childhood psychosocial deprivation has been associated with a blunted ERN (in 11- and 12-year-old children; Loman et al., 2013; Troller-Renfree et al., 2016), whereas others have found no such association (in 8-year-old children; McDermott et al., 2013). Additionally, there is some evidence that early adolescents who experienced institutional stress for longer during childhood appear to display later blunted error processing compared to early adolescents with less exposure during infancy (McDermott et al., 2012). This highlights the potential importance of identifying the type and timing of stress to understand its association with neural response to errors.
The present study sought to address this gap by investigating associations between the ERN and both specific and cumulative life stress during distinct developmental periods as retrospectively reported by an emerging adult sample. We tested the following two hypotheses: (1) experiencing socially evaluative and life-threatening stressors would be associated with a heightened ERN; and (2) the developmental timing of exposure to these forms of adversity would influence their associations with the ERN, with stronger associations being observed for stressors occurring earlier in development. In particular, we predicted that early adolescent stress would be most strongly associated with an enhanced ERN magnitude because of prior research indicating that young adolescents exhibit an enhanced ERN under stressful social-evaluative conditions (Barker et al., 2018) and that plasticity in neural regions involved in higher-order cognitive functioning is particularly heightened during the transition into puberty (Tottenham & Galván, 2016), as opposed to mid- and late-adolescence. Finally, we conducted exploratory analyses with cumulative lifetime stress exposure across different developmental periods in order to understand the specificity of our hypothesized effects.
Method
Participants
Two hundred forty-five participants were recruited from McGill University’s psychology human participant pool, flyers posted around the McGill campus, online advertisements, and in-class advertisements. To participate in the study, which is a part of an ongoing longitudinal project (Banica et al., 2020), participants were required to be at least 18 years old and in the first semester of their first year of their undergraduate degree. Participants received course credit or monetary compensation for their time. All participants provided informed, written consent after reviewing the protocol, and all procedures were approved by the McGill University Research Ethics Board.
Two participants were excluded due to excessive noise in their EEG data, one participant was excluded for not completing the stress assessment, 11 participants were excluded because they were currently taking psychotropic medication (e.g., antidepressant or antianxiety medication; De Bruijn et al., 2004; Zirnheld et al., 2004), and one participant was excluded because their correct-related brain activity was more than three standard deviations from the sample mean. Because six or more errors trials are required to elicit a reliable ERN (Meyer et al., 2013; Olvet & Hajcak, 2009), 27 participants were excluded for committing fewer than six errors.Footnote 1 The final sample for the ERN and stress analyses thus included 203 participants. Six participants were removed from the regression analysis involving life-threatening situations variables because their stress severity scores were more than three standard deviations from the sample mean, leaving a sample of 197 for these analyses. Seven participants were removed from the regression analysis involving the social-evaluative stress variables because their stress severity scores were more than three standard deviations from the sample mean, leaving a sample of 196 for these analyses. Finally, four participants were removed from the regression analysis involving the total stress variables because their stress severity scores were more than three standard deviations from the sample mean, leaving 199 participants in these analyses.Footnote 2,Footnote 3
The average age of the 203-person sample was 18.14 (SD = 0.39) years. Of these participants, 75.4% were female and 24.6% were male; 51.7% of participants were Caucasian, 22.7% were Chinese, 4.4% were South Asian, 2.5% were Arab/West Asian, 2.5% were Hispanic, 1.0% were South East Asian, 1.0% were Caribbean, 2.0% were Korean, 9.4% indicated they were of another ethnicity, and 2.5% did not indicate their ethnicity. The median family income was between $90,000 and $99,999 CAD (range between less than $10,000 and more than $250,000).
Measures
All participants completed the Stress and Adversity Inventory for Adults (Adult STRAIN; Slavich & Shields, 2018), an online interview that assesses individuals’ exposure to major stressors over the entire lifespan. The STRAIN has demonstrated excellent test-retest reliability; good concurrent, predictive, incremental, and discriminant validity; and does not appear to be influenced by participants’ social desirability or personality characteristics (Slavich & Shields, 2018; Sturmbauer et al., 2019). Participants respond to questions probing 55 types of acute life events and chronic difficulties, and for each stressor that is endorsed, follow-up questions are asked about its severity, frequency, timing, and duration. Up to 115 summary scores indicating overall count and severity of total, acute, and chronic stress experienced in multiple domains, such as life-threatening situations, humiliation, interpersonal loss, marital/partner, and housing, can be computed for each participant from the raw life stress data (Slavich & Shields, 2018). For persons experiencing multiple instances of the same stressor, the stress severity scores reflect severity of the worst (i.e., most severe) instance of each stressor. Participants rated severity using the following response options: Very slightly or not at all, a little, moderately, quite a bit, or extremely stressful or threatening. The severity ratings for the stressors occurring in the same life stress domain are then summed together to yield a total life domain severity score for the time window specified (e.g., early life, adulthood).
Stressor timing and duration data also allow for the investigation of stressor severity during different life periods, ranging from infancy to the past year. To that end, we created severity variables for different types of life stress exposure occurring during different developmental periods. Consistent with prior research (Dahl, 2004), childhood was defined as 7 years or younger; early adolescence was 8 to 12 years; and mid-adolescence was 13 to 15 years. Given naturalistic data showing that recent life stress impacts psychological functioning (Kendler et al., 1998; Kendler et al., 2003) and experimental data demonstrating an effect of proximal stressors on ERN magnitude (Meyer & Gawlowska, 2017; Riesel et al., 2012; Riesel et al., 2019), we also calculated participants’ total stress severity during the past year, defined as the 365-day period prior to completing the STRAIN. Because our participants were nearly uniformly 18 years old, this 365-day period included age 17 for most (N = 242) but not all (N = 44) participants. For these reasons, we could not reliably calculate stress severity scores for all participants between the ages of 16 to 17 years that were distinct from the past-year variables we created.
Severity of life-threatening stressors during different developmental periods was calculated by summing severity scores of stressors in the following life domains that occurred during each period: ongoing sexual abuse; accident experienced by a close other; having one’s life threatened; being robbed; being jailed; physical sexual attack; and/or physical abuse. This yielded separate sum scores of the severity of life-threatening stressors in childhood, early adolescence, mid-adolescence, and the past year. Similarly, severity of social stressors at different time points was calculated by summing severity scores of stressors in the domains of experiencing harsh discipline, losing a job, dropping out of school, having an unfaithful romantic partner, experiencing emotional abuse, and experiencing bullying, during each separate time period. Lastly, severity of total stress was calculated by adding together severity scores across all stress domains during each of the developmental periods.
Task and materials
Using an Intel Core i7 computer, an arrow version of the flanker task (Eriksen & Eriksen, 1974) was administered to participants using Presentation software (Neurobehavioral Systems, Inc., Albany, CA) to control the timing and presentation of task stimuli. All stimuli were displayed on a 19-inch (48.3 cm) computer monitor. Five horizontally aligned arrowheads were presented on every trial, and the center arrow was always the target. Participants used the computer mouse to indicate the target arrow’s direction (i.e., pressing the left mouse button if the center arrow points to the left). Approximately 50% of the trials were congruent (“>>>>>” or “<<<<<”) and approximately 50% were incongruent (“<<><<” or “>><>>”). The order of incongruent and congruent trials was random. Arrow stimuli were presented for 200 ms, followed by a period of a maximum of 1,800 ms, which ended once a response was provided. An intertrial interval ranging randomly from 1,000 to 2,000 ms was then presented. Participants were presented with a black screen with a white cross in the center during response and intertrial periods.
Procedure
Participants were first given a brief description of the study. Next, they completed the STRAIN. Electroencephalograph (EEG) sensors were then attached, and participants were provided with more detailed flanker task instructions. Participants were instructed to indicate the central arrowhead’s direction using the right or left mouse button. Participants first did a 6-trial practice block and were told to be both as fast and accurate as possible. The actual task consisted of five blocks of 30 trials (150 trials total), and each block was initiated by the participant. To encourage both accurate and fast responding, participants were provided with feedback at the end of each block, based on their performance. If participants got 75% or less of trials correct, the message “Please try to be more accurate” was displayed; if participant performance was above 80% correct, they received the message “Please try to respond faster”; otherwise, if participant performance was between 76% and 79% accurate, the message “You're doing a great job” was displayed. Participants performed several additional tasks during the experiment: a monetary reward task; a social feedback task (both described in Ethridge & Weinberg, 2018); and an emotional picture viewing task (described in Sandre et al., 2019). The task order was counterbalanced across participants.
Electroencephalographic recording and data processing
Continuous EEG was recorded with a 32-electrode cap and a BrainVision actiCHamp system (Brain Products, Munich, Germany) with a ground electrode at Fpz and based on the standard 10/20 layout. The electrooculogram (EOG) generated from blinks and eye movements was recorded using facial electrodes placed around 1 cm below and above one eye (VEO) and 1 cm to the left and right of both eyes (HEO). A sampling rate of 1,000 Hz was used to record data.
BrainVision Analyzer software (Brain Products, Munich, Germany) was used to conduct offline analysis. Unsegmented data were band-pass filtered with low and high cutoffs of 0.01 and 30 Hz, respectively, with a Butterworth zero phase filter with a 24 db/octave roll-off. For each trial, the EEG was segmented into 1,500 ms windows starting 500 ms before each response onset and continuing for 1,000 ms post-response. Data were then referenced to the average of the left (TP9) and right (TP10) mastoids. Ocular and eye-blink corrections were conducted using HEO and VEO using a modification of the formula defined in Miller et al. (1988).
Detecting and rejecting non-encephalic artifacts (e.g., slow-wave activity, muscle movements) was conducted using a semi-automatic procedure. The criteria applied were a maximum voltage difference of 175 μV within 400-ms intervals, a minimum voltage difference of less than 0.50 μV within 100-ms intervals, and a voltage step of more than 50.0 μV between sample points. These intervals were rejected in each trial from individual channels. To detect and reject remaining artifacts, visual inspection of the data was then conducted. Following artifact rejection, the minimum number of correct trials at Cz for our sample was 79, the maximum was 144, and the average was 132.56. The minimum number of error trials at Cz was 6, the maximum was 60, and the average was 14.94.
Error and correct trials were then averaged separately.Footnote 4 The mean voltage in the 200-ms window from −500 to −300 ms before response onset served as a baseline and was subtracted from each data point (Sandre et al., 2020). Consistent with prior research (Banica et al., 2019; Riesel et al., 2013; Weinberg et al., 2010; Weinberg et al., 2012; Weinberg & Hajcak, 2011), and based on visual inspection of the data, the ERN was quantified on error trials as the average activity from 0 to 100 ms at electrode site Cz, where brain activity following errors was maximal across all participants. In addition, the correct response negativity (CRN)—a negative deflection in the ERP typically present following both correct and error trials (Burle et al., 2008), which appears to index generic response monitoring (Simons, 2010)—was evaluated in the same time window and sites on correct trials. Throughout the manuscript, “conditional ERN” refers to average electrical activity (in microvolts) between 0 and 100 ms following erroneous responses; “conditional CRN” refers to average electrical activity between 0 and 100 ms following correct responses; and “residual ERN”/“ERNresid” refer to average electrical activity between 0 and 100 ms following erroneous responses after controlling for the conditional CRN.
The residual method of calculating the ERN creates scores unrelated to activity following correct responses, identifying brain activity that indexes error processing specifically and providing a more reliable measure than subtraction-based methods (Meyer et al., 2017b). A regression-based procedure (Meyer et al., 2017b; Weinberg et al., 2015) was thus used to compute a standardized ERN residual score to quantify error-specific neural activity. To calculate the residual ERN (ERNresid), participants’ conditional CRN was entered as the predictor, and the conditional ERN was entered as the dependent variable; the standardized residual scores from this regression were saved and used as the ERNresid.
The conditional ERN and CRN displayed good internal consistency (Clayson & Miller, 2017), determined using split-half reliability analyses examining associations between even and odd trials. The Spearman-Brown coefficient for the conditional ERN was r = 0.81. For the conditional CRN, the coefficient was r = 0.98. For the residual ERN, the Spearman-Brown coefficient was r = 0.67.
Data analysis
All statistical analyses were conducted using IBM SPSS Statistics software (Version 24). Paired samples t-tests were used to compare the magnitude of correct- and error-related brain activity. Stress severity scores were z-scored before analysis to facilitate comparison of severity ratings for different types of stress and developmental periods. Pearson’s correlations were used to report the magnitude of bivariate associations between the ERNresid and stress severity variables. To investigate associations between the ERNresid and stressor severity during different developmental periods, we conducted simultaneous multiple regressions with severity of stressors during childhood, early adolescence, mid-adolescence, and past year as independent variables, and the residual ERN as the dependent variable. One regression was conducted for life-threatening stressors and one for social-evaluative stressors in order to investigate unique effects of different stressor characteristics. To further investigate the cumulative effects of stress, we also conducted a simultaneous multiple regression that included total stressor severity during each developmental period as the predictors. Prior research indicates that there are gender differences in both ERN magnitude and its relationship with individual difference variables (Larson et al., 2011; Moran et al., 2012; Moser et al., 2016); therefore, gender was included as a covariate in each regression.
Results
Error-related brain activity
Figure 1a displays waveforms of all participants’ average ERN, CRN, and difference between them (conditional ERN values minus conditional CRN values), and a topographical map displaying activity post-error minus post-correct. A paired-samples t-test indicated that participants’ average brain activity between 0 and 100 ms following erroneous responses was significantly more negative (i.e., larger) than activity following correct responses, t(202) = −17.77, p < 0.001. A repeated-measures ANOVA comparing ΔERN values (error-related neural activity minus correct-related neural activity) at electrode sites Fz, Cz, and Pz confirmed that brain activity following errors was maximal at Cz, F(1.54, 308.49) = 81.79, p < 0.001. Given that Mauchly’s test indicated a violation of sphericity, χ2(2)= 70.02, p < 0.001, we report results with a Greenhouse-Geisser correction. Descriptive statistics, as well as bivariate associations between time-limited stress severity variables and the residual ERN, are presented in Table 1.
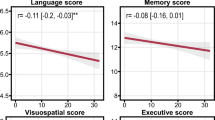
(a) Response-locked ERP average waveforms after erroneous and correct responses, and the difference (conditional ERN values minus conditional CRN values), at electrode site Cz, and a topographical map displaying the error minus correct neural response difference (in μV) for all participants (N = 203). (b) A partial regression plot depicting the association between life-threatening stress severity during mid-adolescence and the residual ERN, adjusting for gender and stress severity during other time periods. (c) Partial regression plot depicting the association between social-evaluative stress severity during early adolescence and the residual ERN, adjusting for gender and stress severity during other time periods. (d) Partial regression plot depicting the association between total stress severity during early adolescence and the residual ERN, adjusting for gender and stress severity during other time periods
Life stress exposure
Means, standards deviations, and ranges of number and severity of cumulative, life-threatening, and social-evaluative stressors are displayed in Table 2. The total sample experienced an average of 11.49 stressors over their lives (SD = 7.05; range = 0-34), with an average total severity of 26.47 (SD = 17.46; range = 0-87), which corresponds to a severity rating of “moderately” stressful.
Flanker task behavioural data
Descriptive statistics for flanker task behavioural variables (number of errors committed, reaction time on error trials, reaction time on correct trials, and post-error slowing), as well as bivariate associations between behavioural variables, stress variables, and ERNresid, are reported in the supplemental material (Table S3). A paired samples t-test indicated that participants were significantly more accurate on congruent trials than incongruent trials, t(198) = −27.61, p < 0.001, and significantly faster on congruent than incongruent trials, t(198) = −37.16, p < 0.001.
Stress and the ERN
Results of the three multiple regressions predicting ERNresid magnitude from severity of life-threatening, social-evaluative, and cumulative life stress exposure experienced during childhood, early adolescence, mid-adolescence, and the year prior to completing the stress questionnaire are presented in Table 3.
We first investigated the effects of timing of life-threatening stressors. Although the overall model predicted a significant amount of variance in the ERNresid, after adjusting for the effects of gender and severity of life-threatening situations during all other time periods, only severity of life-threatening situations experienced in mid-adolescence was uniquely significantly associated with the ERNresid, β = 0.17, p = 0.02, with more severity predicting a less negative (i.e., smaller) ERN (Fig. 1b). We did not observe significant associations with either more proximal (i.e., past-year) or more distal (i.e., childhood or early adolescence) stressors.
Next, we examined the effects of timing of social-evaluative stressors. As above, the overall model accounted for a significant portion of variance in the ERNresid. However, after adjusting for effects of other predictors, only the severity of social-evaluative stressors occurring during early adolescence was significantly associated with magnitude of the ERNresid, β = −0.22, p = 0.007. Specifically, more severe social-evaluative stress occurring during this time period was associated with a more negative (i.e., larger) ERN (Fig. 1c).
The final regression was conducted to estimate the effects of cumulative stress exposure occurring during each developmental period. The results indicated that the ERNresid was also significantly negatively associated with severity of total stress experienced during early adolescence, β = −0.23, p = 0.006, with more severe life stress exposure occurring across all life domains predicting a larger ERN (Fig. 1d). Because this effect was similar in magnitude to that observed for social-evaluative stress, we calculated a new total severity score of stress in early adolescence excluding severity ratings for social-evaluative stress. This new total severity score was also significantly correlated with the ERNresid, r = −0.17, p = 0.02.
Discussion
Although prior studies have examined associations between life stress and the ERN, this body of research has not thoroughly explored the important question of how such effects might differ as a function of the specific timing or type of stressors experienced. To address this gap, we investigated how type of life stress experienced (social-evaluative, life-threatening, and cumulative) and timing of stress exposure (from childhood to the past year) was associated with participants’ neural response to errors. We found that more severe total stress exposure occurring during early adolescence (8 to 12 years old) was associated with an enhanced ERN. These findings are consistent with studies indicating that stress experienced during early adolescence is associated with alterations in the function and structure of the ACC and PFC (De Bellis et al., 2000; Lupien et al., 2009; Tottenham & Galván, 2016), which are involved in generating the ERN (Brázdil et al., 2005; de Bruijn et al., 2004; Dehaene et al., 1994; Gehring & Knight, 2000). In particular, prior studies have found that extreme threat or deprivation occurring during childhood and adolescence are associated with increased activation of the ACC (Lim et al., 2015; McCrory et al., 2017; Mueller et al., 2010). The present study extends these findings by demonstrating that even common forms of stress, such as bullying, may be associated with increased engagement of a performance monitoring network when they occur during certain developmental periods.
In particular, the data suggest that more severe social-evaluative stress experienced during early adolescence is associated with a larger ERN. This finding is consistent with prior research indicating that social stressors, such as punitive parenting and peer evaluation, are related to enhanced error monitoring (Barker et al., 2015; Banica et al., 2019; Brooker & Buss, 2014; Buzzell et al., 2017b; Hajcak et al., 2005; Kim et al., 2005; Meyer et al., 2015b; Meyer et al., 2019; Schillinger et al., 2016; Van Meel & Van Heijningen, 2010). Importantly, consistent with Barker et al. (2018), who found that young (but not older) teenagers show enhanced error monitoring in social contexts, our results indicate that social stress experienced during early adolescence is particularly strongly associated with a larger ERN in emerging adulthood. Sensitivity to social stress is heightened in adolescence (Bolling et al., 2011; Silk et al., 2013), which is a developmental period characterized by increased emphasis on peer relationships and social feedback (Parker et al., 2015). This sensitivity appears particularly pronounced in early adolescence (Moor et al., 2012). Therefore, the present results suggest that increased social stress, which is especially salient during this time, may result in lasting adaptations in neural networks involved in performance monitoring.
These adaptations may be functional in the context of stress. In social settings, for example, errors may pose a threat to safety or social standing, and better performance monitoring in times of social stress may help individuals to avoid mistakes that could result in negative evaluation (Banica et al., 2019; Brooker & Buss, 2014; Hajcak, 2012; Kujawa et al., 2016a; Lim et al., 2015; Meyer et al., 2015b; Meyer et al., 2019). Yet such adaptations may become maladaptive in other circumstances. For instance, an enhanced ERN has been observed in individuals with social anxiety (Endrass et al., 2014; Kujawa et al., 2016a), who, due in part to past social stress, find social situations threatening and closely monitor their performance to prevent mistakes (Clark & Wells, 1995). This heightened self-monitoring can then interfere with social performance (Clark & Wells, 1995). An enhanced ERN may thus be one pathway through which social interactions become even more stressful. More specifically, social stress in early adolescence may sensitize neural performance monitoring networks to errors, leading to enhanced performance monitoring and increased neural reactivity to mistakes in social settings, as well as poorer social skills. Longitudinal studies examining associations between social stress, the ERN, social functioning, and social anxiety will be necessary to explore this possibility.
We also examined the possibility that variability in the magnitude of the ERN might be better predicted by cumulative stress (Brooker, 2018; Kessel et al., 2019), both in terms of cumulative effects over time for each distinct type of stress across all developmental periods (regressions one and two) and in terms of cumulative effects of multiple stressors within distinct developmental periods (regression three). Our results are consistent with evidence for a cumulative effect of multiple stressors within distinct developmental periods in that the data indicated that more severe total stress exposure occurring during early adolescence was associated with an enhanced ERN magnitude, even when adjusting for social stress severity. These findings are in line with broader neuroscience literature demonstrating that alterations in ACC and PFC volume and function, as well as the magnitude of the ERN, are associated with many different types of stress (Banica et al., 2019; Barker et al., 2015; Buzzell et al., 2017b; Cohen et al., 2006; De Bellis et al., 2002; Hanson et al., 2010; Kim et al., 2005; McLaughlin et al., 2014; Meyer et al., 2015b; Meyer & Gawlowska, 2017; Riesel et al., 2019; Tomoda et al., 2009; Treadway et al., 2009). Moreover, they suggest that cumulative effects of stress—particularly during early adolescence, when neural plasticity is increased—are also related to alterations in performance monitoring networks. Future studies with larger samples that are better powered to examine interactions between stressor types and timing will be useful for exploring such cumulative effects more thoroughly.
Extending prior research, our results suggest some specificity in terms of the effects of the timing of stressors and associations with the ERN. For instance, although we did not observe a significant association with life-threatening stressors experienced in early adolescence, our results did indicate that increased severity of life-threatening stressors experienced during mid-adolescence was associated with a smaller ERN magnitude. This is contrary to our hypotheses and to literature indicating that shock and noxious sounds (Meyer & Gawlowska, 2017; Riesel et al., 2012; Riesel et al., 2019) and trauma (Khan et al., 2018; Lackner et al., 2018) are related to an enhanced ERN, as well as research suggesting that neural systems that generate the ERN may activate defensive systems designed to protect oneself from harm (Riesel et al., 2012). Furthermore, it should be noted that the effect size is small and changed in magnitude when including outlier participants in the regression analyses (see supplemental material). Thus, we are hesitant to speculate about why mid-adolescent exposure to stress related to threats to physical integrity may be associated with decreased performance monitoring. Future studies should further investigate this research question.
However, there is also research showing that war veterans with PTSD display a similar ERN magnitude as control participants without PTSD (Gorka et al., 2016; Rabinak et al., 2013; Swick et al., 2015), indicating that individuals exposed to life-threatening stressors do not always display enhanced performance monitoring. In our study, life-threatening stress was measured by asking participants about the severity of situations, such as physical and sexual abuse, being robbed, and living in a war zone, which may exert different effects on performance monitoring than threats in a controlled laboratory setting. However, another possibility is that the relatively restricted range of reported severity of life-threatening situations limited our ability to detect the expected associations with the ERN. This range is also small compared with the range of the social-evaluative stress and total stress severity variables—stressors that were more common in our sample of college students. Future studies should investigate this question in samples with a greater number and more severe dangerous stressors.
Contrary to research demonstrating that childhood is a vulnerable period during which environmental influences may have a particularly strong effect on neural development (Andersen, 2003; Crews et al., 2007; De Bellis, 2005; Hart & Rubia, 2012), and that stress during childhood is associated with alterations in neural regions involved in performance monitoring (Cohen et al., 2006; Tomoda et al., 2009; Treadway et al., 2009), the present analyses did not reveal significant associations between childhood stress exposure and ERN magnitude. In our sample, however, relatively few participants reported severe stressors during childhood. Further, we were not able to separate stress severity experienced in later childhood from that experienced in infancy, which has previously shown different associations with ERN magnitude (Loman et al., 2013; McDermott et al., 2013; Troller-Renfree et al., 2016). Future studies are required to clarify associations between ERN magnitude in adulthood and more severe stress occurring early in development. Similarly, our results did not identify significant associations between ERN magnitude and stress experienced during the past year, despite prior research suggesting that there are proximal effects of stress on performance monitoring (e.g., Meyer & Gawlowska, 2017; Riesel et al., 2012; Riesel et al., 2019). However, in this study, proximal stress included stressors experienced over the preceding 12 months, whereas other studies investigated effects of same-day or previous-day stress, which may explain the observed differences. Future experimental research examining the duration of the effects of laboratory stressors will be helpful to clarify the nature of these associations.
Limitations of this study point to directions for future research. First, our sample consisted of relatively few male participants, which did not allow us to investigate whether there are different stress-susceptible developmental periods for males versus females. Given prior research indicating possible sex differences in rates of neural maturation (Andersen, 2003; Lenroot et al., 2007; Lenroot & Giedd, 2010), future research should look closely at sex-specific sensitive periods. In particular, adolescence is thought to begin with puberty onset (Blakemore et al., 2010), which tends to be between the ages of 8 and 12 for females (Hayward, 2003). However, hormonal events involved in puberty typically occur 1 or 2 years later for males (Blakemore et al., 2010). Our stress severity time intervals classified early adolescence as 8 to 12 years old, which may be accurate for denoting the period of transition to puberty for females (Hayward, 2003) but may not effectively capture male transition to puberty (Blakemore et al., 2010). Additionally, a sample that consisted of 75% female participants may not have provided adequate statistical power to detect gender effects, or potential moderating effects of gender.
Second, our sample consisted of undergraduate students at the beginning of their college studies, who endorsed fewer, and less severe, stressors compared with other studies that have employed the STRAIN (Cazassa et al., 2020; Slavich & Shields, 2018; Sturmbauer et al., 2019). Our results may reflect experiences unique to those with higher-than-average socioeconomic status and education, limiting their generalizability. Indeed, prior research has found different patterns of error monitoring alterations among individuals reporting more extreme forms of stress—for instance, a blunted, as opposed to enhanced, ERN in early adolescents who experienced prolonged early institutionalization (Loman et al., 2013; Troller-Renfree et al., 2016). At a broader level, relative to most of the global population, our sample was Western, Educated, Industrialized, Rich, and Democratic (i.e., WEIRD; Henrich et al., 2010a), and findings in WEIRD samples do not always replicate in non-WEIRD samples (Henrich et al., 2010b). Future research should thus aim to replicate our findings in a more racially and socioeconomically diverse sample reporting more, and more severe, stressors. However, research has shown that experiencing even one major social stressor may substantially impact health (Slavich et al., 2009; Slavich et al., 2014), and consistent with such findings, we observed significant associations between social-evaluative stress exposure and the ERN even with a relatively limited range of stress severity. Furthermore, investigating effects of stressors in the mild-moderate range is important for elucidating how different levels of stress severity—not just the presence and absence of severe stress—may impact development (McLaughlin et al., 2020).
Third, although prior research indicates that social desirability and personality characteristics do not influence responding on the STRAIN (e.g., Slavich & Shields, 2018), we cannot rule out the possible influence of such biases in this study. Furthermore, as is the case with all retrospective self-report measures, recall of stressful events and severity may not have been completely accurate. For example, participant reports of childhood stress may not be as accurate as their reports on stressors that occurred more recently (Maughan & Rutter, 1997). However, we note that the STRAIN focuses on stressors that are moderate to severe in nature, which prior research has shown can be reliably recalled (Brown & Harris, 1978; Reuben et al., 2016). Moreover, validation studies using the STRAIN have shown very high test-retest reliability over time, suggesting that individuals are recalling the same stressors at different time points (Cazassa et al., 2020; Slavich & Shields, 2018). Additionally, because of the correlational nature of the study, it is also possible that individuals with a larger ERN have more biased recall. Yet a negative recall bias should result in global biases reflected across all stressor types and timing, and we did not find associations between ERN magnitude and stress experienced in every domain or developmental period, again suggesting minimal impact of recall biases. Nevertheless, future prospective studies documenting stress across time will be necessary to validate the results reported here, and, in particular, the present study should be replicated using contemporaneous assessments of life stress exposure.
Fourth, unlike traditional interview-based measure of life stress, such as the UCLA Life Stress Interview (Hammen, 1991) and Life Events and Difficulties Schedule (Brown & Harris, 1978), the STRAIN does not generate interviewer-rated life stress exposure or severity scores. A wealth of research has shown that perceptions of stress exposure and severity are strongly tied to biological and clinical health (Epel et al., 2018; Slavich & Cole, 2013), suggesting that it is important to investigate individuals’ stressor appraisals and not just objective stress severity. However, future research should certainly employ other interview-based measures of life stress to examine the robustness of the effects described here.
Fifth, given the present study design, we cannot be certain whether the effects observed are due to adolescent-limited stress versus chronic stress that began in childhood and perhaps peaked in adolescence (Tottenham & Galván, 2016). Research suggests that interpersonal stress is highly continuous (Chapell et al., 2006), and the social stress reported by participants in the present sample during adolescence may have started earlier and continued past early adolescence. However, the results of our multiple regression analysis—in which stress across other periods was controlled for—are suggestive in this regard, in that social stress during early adolescence showed unique associations with the ERN. Nonetheless, prospective studies would be helpful for further disentangling the effects of stress experienced during different developmental periods on the ERN.
Finally, the effect sizes observed in the present sample are small to medium in magnitude (Cohen, 1988), and should be interpreted with caution. However, self-report data and psychophysiological variables share no method variance and thus are expected to moderately correlate with one another (Campbell & Fiske, 1959; Patrick et al., 2013). Consistent with this, similar effect sizes are common in the literature investigating associations between the ERN and important individual difference variables (Cavanagh & Shackman, 2015; Meyer et al., 2015a; Moser et al., 2013; Weinberg et al., 2016) and can have meaningful implications for real-world outcomes (Hajcak et al., 2019; Meyer et al., 2017a). For instance, the ERN has demonstrated incremental predictive ability for the later development of anxiety disorders over and above other common risk factors (Meyer et al., 2015a; Meyer et al., 2018) and appears to predict adolescents’ tobacco use initiation (Anokhin & Golosheykin, 2015), effects that were in the small to medium range. These studies suggest that even relatively modest effects can improve prediction of important health-related outcomes.
Conclusions
The present findings indicate that greater total and social-evaluative stress severity during early adolescence is associated with increased error monitoring in emerging adulthood. These results provide support for the notion that early adolescence is a sensitive period during which stress may more significantly influence neural networks involved in performance monitoring. In a notable extension of prior work, the present results also indicate that it is important to consider both the developmental timing and type of stressors experienced over the lifespan. Specially, we found that social-evaluative stress occurring during early adolescence was associated with a larger ERN, whereas life-threatening stress severity during mid-adolescence was related to a smaller ERN. However, total stress severity in early adolescence was also associated with enhanced error monitoring, which suggests that there are both cumulative and specific effects of stressor type on performance monitoring across development. This research therefore lays the groundwork for future prospective studies seeking to understand the long-term effects of stress-induced neural adaptations to the environment.
Notes
Participants excluded for having fewer than six errors did not differ on life-threatening stressor count, t(274) = −0.83, p = 0.41, social-evaluative stressor count, t(274) = 0.43, p = 0.67, or total stressor count, t(274) = 0.91, p = 0.37, compared with participants who had six or more errors. They also did not differ on the stressor severity timing variables, all ps > 0.14.
Participants excluded based on stress severity scores (N = 14) did not significantly differ on conditional ERN magnitude, t(201) = −1.75, p = 0.08, or conditional CRN values, t(201) = −1.73, p = 0.09, compared with participants included in the analyses.
For results of the regressions with these outliers included, see Table S1 in supplemental analyses.
We collapsed across congruent and incongruent error and correct trials. See Table S2 in the supplemental material for results of the main regression analyses using residual ERN activity following incongruent trials only.
References
Andersen, S. L. (2003). Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews, 27(1-2), 3-18. https://doi.org/10.1016/S0149-7634(03)00005-8
Andersen, S. L., & Teicher, M. H. (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31(4), 183-191. https://doi.org/10.1016/j.tins.2008.01.004
Andersen, S. L., Tomada, A., Vincow, E. S., Valente, E., Polcari, A., & Teicher, M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 292-301.
Anokhin, A. P., & Golosheykin, S. (2015). Neural correlates of error monitoring in adolescents prospectively predict initiation of tobacco use. Developmental Cognitive Neuroscience, 16, 166-173. https://doi.org/10.1016/j.dcn.2015.08.001
Bae, J. N., MacFall, J. R., Krishnan, K. R. R., Payne, M. E., Steffens, D. C., & Taylor, W. D. (2006). Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry, 60(12), 1356-1363. https://doi.org/10.1016/j.biopsych.2006.03.052
Banica, I., Sandre, A., Shields, G. S., Slavich, G. M., & Weinberg, A. (2020). The error-related negativity (ERN) moderates the association between interpersonal stress and anxiety symptoms six months later. International Journal of Psychophysiology, 153, 27-36. https://doi.org/10.1016/j.ijpsycho.2020.03.006.
Banica, I., Sandre, A., & Weinberg, A. (2019). Overprotective/authoritarian maternal parenting is associated with an enhanced error-related negativity (ERN) in young adult females. International Journal of Psychophysiology, 137, 12 – 20. https://doi.org/10.1016/j.ijpsycho.2018.12.013
Barker, T. V., Troller-Renfree, S. V., Bowman, L. C., Pine, D. S., & Fox, N. A. (2018). Social influences of error monitoring in adolescent girls. Psychophysiology, e13089. https://doi.org/10.1111/psyp.13089
Barker, T. V., Troller-Renfree, S., Pine, D. S., & Fox, N. A. (2015). Individual differences in social anxiety affect the salience of errors in social contexts. Cognitive, Affective, & Behavioral Neuroscience, 15(4), 723-735. https://doi.org/10.3758/s13415-015-0360-9
Blakemore, S. J., Burnett, S., & Dahl, R. E. (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926-933. https://doi.org/10.1002/hbm.21052
Bradley, M. M., Codispoti, M., & Lang, P. J. (2006). A multi-process account of startle modulation during affective perception. Psychophysiology, 43(5), 486-497. https://doi.org/10.1111/j.1469-8986.2006.00412.x
Brázdil, M., Roman, R., Daniel, P., & Rektor, I. (2005). Intracerebral error-related negativity in a simple Go/NoGo task. Journal of Psychophysiology, 19(4), 244–255. https://doi.org/10.1027/0269-8803.19.4.244
Brooker, R. J. (2018). Maternal behavior and socioeconomic status predict longitudinal changes in error-related negativity in preschoolers. Child Development, 89(3), 725-733. https://doi.org/10.1111/cdev.13066
Brooker, R. J., & Buss, K. A. (2014). Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience, 9, 148-159. https://doi.org/10.1016/j.dcn.2014.03.001
Bolling, D. Z., Pitskel, N. B., Deen, B., Crowley, M. J., Mayes, L. C., & Pelphrey, K. A. (2011). Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science, 14(6), 1431-1444. https://doi.org/10.1111/j.1467-7687.2011.01087.x
Botteron, K. N., Raichle, M. E., Drevets, W. C., Heath, A. C., & Todd, R. D. (2002). Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry, 51(4), 342-344. https://doi.org/10.1016/S0006-3223(01)01280-X
Botvinick, M. M., Braver, T. S., Carter, C. S., Barch, D. M., & Cohen, J. D. (2001). Evaluating the demand for control: Anterior cingulate cortex and crosstalk monitoring. Psychological Review, 108, 624-652. https://doi.org/10.1037/0033-295X.108.3.624
Brown, G.W., & Harris, T.O. (1978). Social origins of depression: A study of psychiatric disorders in women. Tavistock Publications Limited.
Burle, B., Roger, C., Allain, S., Vidal, F., & Hasbroucq, T. (2008). Error negativity does not reflect conflict: A reappraisal of conflict monitoring and anterior cingulate cortex activity. Journal of Cognitive Neuroscience, 20(9), 1637-1655. https://doi.org/10.1162/jocn.2008.20110
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215-222. https://doi.org/10.1016/S1364-6613(00)01483-2
Buzzell, G. A., Richards, J. E., White, L. K., Barker, T. V., Pine, D. S., & Fox, N. A. (2017a). Development of the error-monitoring system from ages 9–35: Unique insight provided by MRI-constrained source localization of EEG. NeuroImage, 157, 13-26. https://doi.org/10.1016/j.neuroimage.2017.05.045
Buzzell, G. A., Troller-Renfree, S. V., Barker, T. V., Bowman, L. C., Chronis-Tuscano, A., Henderson, H. A., … Fox, N. A. (2017b). A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child & Adolescent Psychiatry, 56(12), 1097-1105. https://doi.org/10.1016/j.jaac.2017.10.007
Caballero, A., Granberg, R., & Tseng, K. Y. (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews, 70, 4-12. https://doi.org/10.1016/j.neubiorev.2016.05.013
Campbell, D. T., & Fiske, D. W. (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin, 56(2), 81–105. https://doi.org/10.1037/h0046016
Carter, C. S., & van Veen, V. (2007). Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience, 7(4), 367-379. https://doi.org/10.3758/CABN.7.4.367
Cavanagh, J. F., & Shackman, A. J. (2015). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology-Paris, 109(1-3), 3-15. https://doi.org/10.1016/j.jphysparis.2014.04.003
Cazassa, M. J., Oliveira, M. D. S., Spahr, C. M., Shields, G. S., & Slavich, G. M. (2020). The Stress and Adversity Inventory for Adults (Adult STRAIN) in Brazilian Portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Frontiers in Psychology, 10, 3083. https://doi.org/10.3389/fpsyg.2019.03083
Chapell, M. S., Hasselman, S. L., Kitchin, T., Lomon, S. N., MacIver, K. W., & Sarullo, P. L. (2006). Bullying in elementary school, high school, and college. Adolescence, 41(164), 633-649.
Clark, D. M. and Wells, A. (1995). A cognitive model of social phobia. In R. G. Heimberg, M. R. Liebowitz, D. A. Hope and F. R. Schneier (Eds.), Social Phobia: diagnosis, assessment and treatment (pp. 69–93). Guilford Press.
Clayson, P. E., & Miller, G. A. (2017). Psychometric considerations in the measurement of event-related brain potentials: Guidelines for measurement and reporting. International Journal of Psychophysiology, 111, 57-67. https://doi.org/10.1016/j.ijpsycho.2016.09.005
Cohen, J. (1988). Statistical power of Analysis for the Behavioral Sciences, 2nd Edn. Lawrence Erlbaum Associates, Inc.
Cohen, R. A., Grieve, S., Hoth, K. F., Paul, R. H., Sweet, L., Tate, D., … Williams, L. M. (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry, 59(10), 975-982. https://doi.org/10.1016/j.biopsych.2005.12.016
Crews, F., He, J., & Hodge, C. (2007). Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology Biochemistry and Behavior, 86(2), 189-199. https://doi.org/10.1016/j.pbb.2006.12.001
Dahl, R. E. (2004). Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021(1), 1-22. https://doi.org/10.1196/annals.1308.001
Dahl, R. E., & Gunnar, M. R. (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and Psychopathology, 21(1), 1-6. https://doi.org/10.1017/S0954579409000017
Davies, P. L., Segalowitz, S. J., & Gavin, W. J. (2004). Development of error-monitoring event-related potentials in adolescents. Annals of the New York Academy of Sciences, 1021(1), 324-328. https://doi.org/10.1196/annals.1308.039
De Bellis, M. D. (2005). The psychobiology of neglect. Child Maltreatment, 10(2), 150-172. https://doi.org/10.1177/1077559505275116
De Bellis, M. D., Keshavan, M. S., Spencer, S., & Hall, J. (2000). N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. American Journal of Psychiatry, 157(7), 1175-1177. https://doi.org/10.1176/appi.ajp.157.7.1175
De Bellis, M. D., Keshavan, M. S., Shifflett, H., Iyengar, S., Beers, S. R., Hall, J., & Moritz, G. (2002). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: A sociodemographically matched study. Biological Psychiatry, 52(11), 1066-1078. https://doi.org/10.1016/S0006-3223(02)01459-2
De Bruijn, E. R., Hulstijn, W., Verkes, R. J., Ruigt, G. S., & Sabbe, B. G. (2004). Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology, 177(1-2), 151-160. https://doi.org/10.1007/s00213-004-1915-6
Dehaene, S., Posner, M. I., & Tucker, D. M. (1994). Localization of a neural system for error detection and compensation. Psychological Science, 5(5), 303-305. https://doi.org/10.1111/j.1467-9280.1994.tb00630.x
Dell'Osso, B., Cinnante, C., Di Giorgio, A., Cremaschi, L., Palazzo, M. C., Cristoffanini, M., … Bertolino, A. (2015). Altered prefrontal cortex activity during working memory task in bipolar disorder: A functional magnetic resonance imaging study in euthymic bipolar I and II patients. Journal of Affective Disorders, 184, 116-122. https://doi.org/10.1016/j.jad.2015.05.026
Doom, J. R., & Gunnar, M. R. (2013). Stress physiology and developmental psychopathology: Past, present, and future. Development and Psychopathology, 25(4pt2), 1359-1373. https://doi.org/10.1017/S0954579413000667
Dunn, E. C., Soare, T. W., Zhu, Y., Simpkin, A. J., Suderman, M. J., Klengel, T., … Relton, C. L. (2019). Sensitive periods for the effect of childhood adversity on DNA methylation: Results from a prospective, longitudinal study. Biological Psychiatry, 85(10), 838-849. https://doi.org/10.1016/j.biopsych.2018.12.023
Endrass, T., Riesel, A., Kathmann, N., & Buhlmann, U. (2014). Performance monitoring in obsessive–compulsive disorder and social anxiety disorder. Journal of Abnormal Psychology, 123(4), 705-714. https://doi.org/10.1037/abn0000012
Epel, E. S., Crosswell, A. D., Mayer, S. E., Prather, A. A., Slavich, G. M., Puterman, E., & Mendes, W. B. (2018). More than a feeling: A unified view of stress measurement for population science. Frontiers in Neuroendocrinology, 49, 146-169. https://doi.org/10.1016/j.yfrne.2018.03.001
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a non-search task. Perception & Psychophysics, 16(1), 143-149. https://doi.org/10.3758/BF03203267
Ethridge, P., & Weinberg, A. (2018). Psychometric properties of neural responses to monetary and social rewards across development. International Journal of Psychophysiology, 132, 311–322. https://doi.org/10.1016/j.ijpsycho.2018.01.011
Evans, G. W., Li, D., & Whipple, S. S. (2013). Cumulative risk and child development. Psychological Bulletin, 139(6), 1342-1396. https://doi.org/10.1037/a0031808
Falkenstein, M., Hoormann, J., Christ, S., & Hohnsbein, J. (2000). ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology, 51(2-3), 87-107. https://doi.org/10.1016/S0301-0511(99)00031-9
Falkenstein, M., Hohnsbein, J., Hoormann, J., & Blanke, L. (1991). Effects of crossmodal divided attention on ERP components: Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447-455. https://doi.org/10.1016/0013-4694(91)90061-8
Gehring, W. J., Coles, M. G., Meyer, D. E., & Donchin, E. (1995). A brain potential manifestation of error-related processing. Electroencephalography and Clinical Neurophysiology, 44, 261-272.
Gehring, W. J., Goss, B., Coles, M. G., Meyer, D. E., & Donchin, E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385-390. https://doi.org/10.1111/j.1467-9280.1993.tb00586.x
Gehring, W. J., & Knight, R. T. (2000). Prefrontal–cingulate interactions in action monitoring. Nature Neuroscience, 3(5), 516-520. https://doi.org/10.1038/74899
Giedd, J. N. (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021(1), 77-85. https://doi.org/10.1196/annals.1308.009
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., … Rapoport, J. L. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, 101(21), 8174-8179. https://doi.org/10.1073/pnas.0402680101
Gollier-Briant, F., Paillere-Martinot, M. L., Lemaitre, H., Miranda, R., Vulser, H., Goodman, R., … Poustka, L. (2016). Neural correlates of three types of negative life events during angry face processing in adolescents. Social Cognitive and Affective Neuroscience, 11(12), 1961-1969. https://doi.org/10.1093/scan/nsw100
Gorka, S. M., MacNamara, A., Aase, D. M., Proescher, E., Greenstein, J. E., Walters, R., … DiGangi, J. A. (2016). Impact of alcohol use disorder comorbidity on defensive reactivity to errors in veterans with posttraumatic stress disorder. Psychology of Addictive Behaviors, 30(7), 733-742. https://doi.org/10.1037/adb0000196
Griffin, A. (2017). Adolescent neurological development and implications for health and well-being. Healthcare, 5(4), 62-69. https://doi.org/10.3390/healthcare5040062
Grillon, C., Ameli, R., Merikangas, K., Woods, S. W., & Davis, M. (1993). Measuring the time course of anticipatory anxiety using the fear-potentiated startle reflex. Psychophysiology, 30(4), 340-346. https://doi.org/10.1111/j.1469-8986.1993.tb02055.x
Gunnar, M. R., & Donzella, B. (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1-2), 199-220. https://doi.org/10.1016/S0306-4530(01)00045-2
Gunnar, M., & Quevedo, K. (2007). The neurobiology of stress and development. Annu. Rev. Psychol., 58, 145-173. https://doi.org/10.1146/annurev.psych.58.110405.085605
Gunnar, M. R., Talge, N. M., & Herrera, A. (2009a). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34(7), 953-967. https://doi.org/10.1016/j.psyneuen.2009.02.010
Gunnar, M. R., & Vazquez, D. (2006). Stress neurobiology and developmental psychopathology. In D. Cicchetti, & D. J. Cohen (Eds.), Developmental Psychopathology: Developmental Neuroscience (pp. 533 -577). Wiley.
Gunnar, M. R., Wewerka, S., Frenn, K., Long, J. D., & Griggs, C. (2009b). Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology, 21(1), 69-85. https://doi.org/10.1017/S0954579409000054
Guyer, A. E., Pérez-Edgar, K., & Crone, E. A. (2018). Opportunities for neurodevelopmental plasticity from infancy through early adulthood. Child Development, 89(3), 687-697. https://doi.org/10.1111/cdev.13073
Hajcak, G. (2012). What we’ve learned from mistakes: Insights from error-related brain activity. Current Directions in Psychological Science, 21(2), 101-106. https://doi.org/10.1177/0963721412436809
Hajcak, G., Klawohn, J., & Meyer, A. (2019). The utility of event-related potentials in clinical psychology. Annual Review of Clinical Psychology, 15, 71-95. https://doi.org/10.1146/annurev-clinpsy-050718-095457
Hajcak, G., Moser, J. S., Yeung, N., & Simons, R. F. (2005). On the ERN and the significance of errors. Psychophysiology, 42(2), 151-160. https://doi.org/10.1111/j.1469-8986.2005.00270.x
Hammen, C. (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology, 100(4), 555-561. https://doi.org/10.1037/0021-843X.100.4.555
Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1, 293-319. https://doi.org/10.1146/annurev.clinpsy.1.102803.143938
Hanson, J. L., Chung, M. K., Avants, B. B., Shirtcliff, E. A., Gee, J. C., Davidson, R. J., & Pollak, S. D. (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30(22), 7466-7472. https://doi.org/10.1523/JNEUROSCI.0859-10.2010
Hart, H., & Rubia, K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 1-24. https://doi.org/10.3389/fnhum.2012.00052
Hayward, C. (Ed.). (2003). Gender differences at puberty. Cambridge University Press.
Heim, C., & Binder, E. B. (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology, 233(1), 102-111. https://doi.org/10.1016/j.expneurol.2011.10.032
Henrich, J., Heine, S. J., & Norenzayan, A. (2010a). The weirdest people in the world? Behavioral and Brain Sciences, 33(2-3), 61-83. https://doi.org/10.1017/S0140525X0999152X
Henrich, J., Heine, S. J., & Norenzayan, A. (2010b). Most people are not WEIRD. Nature, 466(7302), 29.
Holroyd, C. B., & Coles, M. G. (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679-709. https://doi.org/10.1037/0033-295X.109.4.679
Humphreys, K. L., King, L. S., Sacchet, M. D., Camacho, M. C., Colich, N. L., Ordaz, S. J., … Gotlib, I. H. (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Developmental Science, 22(3), e12775. https://doi.org/10.1111/desc.12775
Ingram, R. E., & Luxton, D. D. (2005). Vulnerability-stress models. In B.L. Hankin & J.R.Z. Abela (Eds.), Development of psychopathology: A vulnerability-stress perspective (pp. 32-46). Sage Publications, Inc.
Inguaggiato, E., Sgandurra, G., & Cioni, G. (2017). Brain plasticity and early development: Implications for early intervention in neurodevelopmental disorders. Neuropsychiatrie de l'Enfance et de l'Adolescence, 65(5), 299-306. https://doi.org/10.1016/j.neurenf.2017.03.009
Kelly, A. C., Di Martino, A., Uddin, L. Q., Shehzad, Z., Gee, D. G., Reiss, P. T., … Milham, M. P. (2008). Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex, 19(3), 640-657. https://doi.org/10.1093/cercor/bhn117
Kendler, K. S., Gardner, C. O., & Prescott, C. A. (2002). Toward a comprehensive developmental model for major depression in women. American Journal of Psychiatry, 159(7), 1133-1197. https://doi.org/10.1176/appi.ajp.159.7.1133
Kendler, K. S., Hettema, J. M., Butera, F., Gardner, C. O., & Prescott, C. A. (2003). Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Archives of General Psychiatry, 60(8), 789-796. https://doi.org/10.1001/archpsyc.60.8.789
Kendler, K. S., Karkowski, L. M., & Prescott, C. A. (1998). Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. The Journal of Nervous and Mental Disease, 186(11), 661-669. https://doi.org/10.1097/00005053-199811000-00001
Kessel, E. M., Nelson, B. D., Finsaas, M., Kujawa, A., Meyer, A., Bromet, E., … Klein, D. N. (2019). Parenting style moderates the effects of exposure to natural disaster-related stress on the neural development of reactivity to threat and reward in children. Development and Psychopathology, 31(4), 1589-1598. https://doi.org/10.1017/S0954579418001347
Khan, N. I., Burkhouse, K. L., Lieberman, L., Gorka, S. M., DiGangi, J. A., Schroth, C., … Proescher, E. (2018). Individual differences in combat experiences and error-related brain activity in OEF/OIF/OND veterans. International Journal of Psychophysiology, 129, 52-57. https://doi.org/10.1016/j.ijpsycho.2018.04.011
Kim, E. Y., Iwaki, N., Uno, H., & Fujita, T. (2005). Error-related negativity in children: Effect of an observer. Developmental Neuropsychology, 28(3), 871-883. https://doi.org/10.1207/s15326942dn2803_7
Kujawa, A., Weinberg, A., Bunford, N., Fitzgerald, K. D., Hanna, G. L., Monk, C. S., … Phan, K. L. (2016a). Error-related brain activity in youth and young adults before and after treatment for generalized or social anxiety disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 71, 162-168. https://doi.org/10.1016/j.pnpbp.2016.07.010
Kujawa, A., Wu, M., Klumpp, H., Pine, D. S., Swain, J. E., Fitzgerald, K. D., … Phan, K. L. (2016b). Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(4), 345-352. https://doi.org/10.1016/j.bpsc.2016.01.006
Lackner, C. L., Santesso, D. L., Dywan, J., O’Leary, D. D., Wade, T. J., & Segalowitz, S. J. (2018). Adverse childhood experiences are associated with self-regulation and the magnitude of the error-related negativity difference. Biological Psychology, 132, 244-251. https://doi.org/10.1016/j.biopsycho.2018.01.006
Larson, M. J., South, M., & Clayson, P. E. (2011). Sex differences in error-related performance monitoring. Neuroreport, 22(1), 44-48. https://doi.org/10.1097/WNR.0b013e3283427403
Lenroot, R. K., & Giedd, J. N. (2010). Sex differences in the adolescent brain. Brain and Cognition, 72(1), 46-55. https://doi.org/10.1016/j.bandc.2009.10.008
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., … Thompson, P. M. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage, 36(4), 1065-1073. https://doi.org/10.1016/j.neuroimage.2007.03.053
Lichenstein, S. D., Verstynen, T., & Forbes, E. E. (2016). Adolescent brain development and depression: A case for the importance of connectivity of the anterior cingulate cortex. Neuroscience & Biobehavioral Reviews, 70, 271-287. https://doi.org/10.1016/j.neubiorev.2016.07.024
Lim, L., Hart, H., Mehta, M. A., Simmons, A., Mirza, K., & Rubia, K. (2015). Neural correlates of error processing in young people with a history of severe childhood abuse: An fMRI study. American Journal of Psychiatry, 172(9), 892-900. https://doi.org/10.1176/appi.ajp.2015.14081042
Loman, M. M., Johnson, A. E., Westerlund, A., Pollak, S. D., Nelson, C. A., & Gunnar, M. R. (2013). The effect of early deprivation on executive attention in middle childhood. Journal of Child Psychology and Psychiatry, 54(1), 37-45. https://doi.org/10.1111/j.1469-7610.2012.02602.x
Luby, J. L., Tillman, R., & Barch, D. M. (2019). Association of timing of adverse childhood experiences and caregiver support with regionally specific brain development in adolescents. JAMA Network Open, 2(9), e1911426-e1911426. https://doi.org/10.1001/jamanetworkopen.2019.11426
Luna, B., & Sweeney, J. A. (2004). The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Annals of the New York Academy of Sciences, 1021(1), 296-309. https://doi.org/10.1196/annals.1308.035
Lupien, S. J., McEwen, B. S., Gunnar, M. R., & Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434-445. https://doi.org/10.1038/nrn2639
Manoach, D. S., & Agam, Y. (2013). Neural markers of errors as endophenotypes in neuropsychiatric disorders. Frontiers in Human Neuroscience, 7:350. https://doi.org/10.3389/fnhum.2013.00350
Masten, A. S., & Cicchetti, D. (2010). Developmental cascades. Development and Psychopathology, 22(3), 491-495. https://doi.org/10.1017/S0954579410000222
Maughan, B., & Rutter, M. (1997). Retrospective reporting of childhood adversity: Issues in assessing long-term recall. Journal of Personality Disorders, 11(1), 19-33.
Mazure, C. M. (1998). Life stressors as risk factors in depression. Clinical Psychology: Science and Practice, 5(3), 291-313. https://doi.org/10.1111/j.1468-2850.1998.tb00151.x
McCrory, E., De Brito, S. A., & Viding, E. (2010). Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry, 51(10), 1079-1095. https://doi.org/10.1111/j.1469-7610.2010.02271.x
McCrory, E. J., Gerin, M. I., & Viding, E. (2017). Annual research review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry–the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry, 58(4), 338-357. https://doi.org/10.1111/jcpp.12713
McDermott, J. M., Troller-Renfree, S. V., Vanderwert, R., Nelson, C. A., Zeanah, C. H., & Fox, N. (2013). Psychosocial deprivation, executive functions, and the emergence of socio-emotional behavior problems. Frontiers in Human Neuroscience, 7, 167. https://doi.org/10.3389/fnhum.2013.00167
McDermott, J. M., Westerlund, A., Zeanah, C. H., Nelson, C. A., & Fox, N. A. (2012). Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience, 2, S59-S66. https://doi.org/10.1016/j.dcn.2011.09.008
McEwen, B. S. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33-44. https://doi.org/10.1111/j.1749-6632.1998.tb09546.x
McLaughlin, K. A., Sheridan, M., Humphreys, K., Belsky, J., & Ellis, B. J. (2020). The Value of Dimensional Models of Early Experience: Thinking Clearly about Concepts and Categories. PsyArXiv Preprints. https://doi.org/10.31234/osf.io/29fmt
McLaughlin, K. A., Sheridan, M. A., & Lambert, H. K. (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578-591. https://doi.org/10.1016/j.neubiorev.2014.10.012
Meyer, A., Carlton, C., Chong, L. J., & Wissemann, K. (2019). The presence of a controlling parent is related to an increase in the error-related negativity in 5–7-year-old children. Journal of Abnormal Child Psychology, 47(6), 935-945. https://doi.org/10.1007/s10802-018-0503-x
Meyer, A., Danielson, C. K., Danzig, A. P., Bhatia, V., Black, S. R., Bromet, E., … Klein, D. N. (2017a). Neural biomarker and early temperament predict increased internalizing symptoms after a natural disaster. Journal of the American Academy of Child & Adolescent Psychiatry, 56(5), 410-416. https://doi.org/10.1016/j.jaac.2017.02.005
Meyer, A., & Gawlowska, M. (2017). Evidence for specificity of the impact of punishment on error-related brain activity in high versus low trait anxious individuals. International Journal of Psychophysiology, 120, 157-163. https://doi.org/10.1016/j.ijpsycho.2017.08.001
Meyer, A., Hajcak, G., Torpey-Newman, D. C., Kujawa, A., & Klein, D. N. (2015a). Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology, 124(2), 266-274. https://doi.org/10.1037/abn0000044
Meyer, A., Lerner, M. D., De Los Reyes, A., Laird, R. D., & Hajcak, G. (2017b). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114-122. https://doi.org/10.1111/psyp.12664
Meyer, A., Nelson, B., Perlman, G., Klein, D. N., & Kotov, R. (2018). A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. Journal of Child Psychology and Psychiatry, 59(11), 1162-1170. https://doi.org/10.1111/jcpp.12922
Meyer, A., Proudfit, G. H., Bufferd, S. J., Kujawa, A. J., Laptook, R. S., Torpey, D. C., & Klein, D. N. (2015b). Self-reported and observed punitive parenting prospectively predicts increased error-related brain activity in six-year-old children. Journal of Abnormal Child Psychology, 43(5), 821-829. https://doi.org/10.1007/s10802-014-9918-1
Meyer, A., Riesel, A., & Hajcak Proudfit, G. (2013). Reliability of the ERN across multiple tasks as a function of increasing errors. Psychophysiology, 50(12), 1220-1225. https://doi.org/10.1111/psyp.12132
Meyer, A., Weinberg, A., Klein, D. N., & Hajcak, G. (2012). The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8- to 13-year-olds. Developmental Cognitive Neuroscience, 2(1), 152-161. https://doi.org/10.1016/j.dcn.2011.09.005
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167-202. https://doi.org/10.1146/annurev.neuro.24.1.167
Miller, G. A., Gration, G., & Yee, C. M. (1988). Generalized implementation of an eye movement correction procedure. Psychophysiology, 25(2), 241-243. https://doi.org/10.1111/j.1469-8986.1988.tb00999.x
Monroe, S. M., & Roberts, J. E. (1990). Conceptualizing and measuring life stress: Problems, principles, procedures, progress. Stress Medicine, 6(3), 209-216. https://doi.org/10.1002/smi.2460060306
Moor, B. G., Güroğlu, B., de Macks, Z. A. O., Rombouts, S. A., Van der Molen, M. W., & Crone, E. A. (2012). Social exclusion and punishment of excluders: Neural correlates and developmental trajectories. Neuroimage, 59(1), 708-717. https://doi.org/10.1016/j.neuroimage.2011.07.028
Moran, T. P., Taylor, D., & Moser, J. S. (2012). Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. International Journal of Psychophysiology, 85(2), 188-194. https://doi.org/10.1016/j.ijpsycho.2012.05.005
Moser, J. S., Moran, T. P., Kneip, C., Schroder, H. S., & Larson, M. J. (2016). Sex moderates the association between symptoms of anxiety, but not obsessive-compulsive disorder, and error-monitoring brain activity: A meta-analytic review. Psychophysiology, 53(1), 21-29. https://doi.org/10.1111/psyp.12509
Moser, J., Moran, T., Schroder, H., Donnellan, B., & Yeung, N. (2013). On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7, 466. https://doi.org/10.3389/fnhum.2013.00466
Mueller, S. C., Maheu, F. S., Dozier, M., Peloso, E., Mandell, D., Leibenluft, E., … Ernst, M. (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia, 48(10), 3037-3044. https://doi.org/10.1016/j.neuropsychologia.2010.06.013
Murrough, J. W., Abdallah, C. G., Anticevic, A., Collins, K. A., Geha, P., Averill, L. A., … Wong, E. (2016). Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Human Brain Mapping, 37(9), 3214-3223. https://doi.org/10.1002/hbm.23235
Olvet, D. M., & Hajcak, G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology, 46(5), 957-961. https://doi.org/10.1111/j.1469-8986.2009.00848.x
Parker, J. G., Rubin, K. H., Erath, S. A., Wojslawowicz, J. C., & Buskirk, A. A. (2015). Peer relationships, child development, and adjustment: A developmental psychopathology perspective. In D. Cicchetti & D. J. Cohen (Eds.), Developmental psychopathology: Theory and method (pp. 419–493). John Wiley & Sons Inc. https://doi.org/10.1002/9780470939383.ch12
Patrick, C. J., Venables, N. C., Yancey, J. R., Hicks, B. M., Nelson, L. D., & Kramer, M. D. (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902-916. https://doi.org/10.1037/a0032807
Petanjek, Z., Judaš, M., Šimić, G., Rašin, M. R., Uylings, H. B., Rakic, P., & Kostović, I. (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 108(32), 13281-13286. https://doi.org/10.1073/pnas.1105108108
Pizzagalli, D. A., Webb, C. A., Dillon, D. G., Tenke, C. E., Kayser, J., Goer, F., … Adams, P. (2018). Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry, 75(6), 547-554. https://doi.org/10.1001/jamapsychiatry.2018.0252
Rabinak, C. A., Holman, A., Angstadt, M., Kennedy, A. E., Hajcak, G., & Phan, K. L. (2013). Neural response to errors in combat-exposed returning veterans with and without post-traumatic stress disorder: A preliminary event-related potential study. Psychiatry Research: Neuroimaging, 213(1), 71-78. https://doi.org/10.1016/j.pscychresns.2013.01.002
Reuben, A., Moffitt, T. E., Caspi, A., Belsky, D. W., Harrington, H., Schroeder, F., … Danese, A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103-1112. https://doi.org/10.1111/jcpp.12621
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443-447. https://doi.org/10.1126/science.1100301
Riesel, A., Kathmann, N., Wuellhorst, V., Banica, I., & Weinberg, A. (2019). Punishment has a persistent effect on error-related brain activity in highly anxious individuals twenty-four hours after conditioning. International Journal of Psychophysiology, 146, 63-73. https://doi.org/10.1016/j.ijpsycho.2019.09.014
Riesel, A., Weinberg, A., Endrass, T., Kathmann, N., & Hajcak, G. (2012). Punishment has a lasting impact on error-related brain activity. Psychophysiology, 49(2), 239-247. https://doi.org/10.1111/j.1469-8986.2011.01298.x
Riesel, A., Weinberg, A., Endrass, T., Meyer, A., & Hajcak, G. (2013). The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology, 93(3), 377-385. https://doi.org/10.1016/j.biopsycho.2013.04.007
Sandre, A., Bagot, R. C., & Weinberg, A. (2019). Blunted neural response to appetitive images prospectively predicts symptoms of depression, and not anxiety, during the transition to university. Biological Psychology, 145, 31–41. https://doi.org/10.1016/j.biopsycho.2019.04.001
Sandre, A., Banica, I., Riesel, A., Flake, J., Klawohn, J., & Weinberg, A. (2020). Comparing the effects of different methodological decisions on the error-related negativity and its association with behaviour and gender. International Journal of Psychophysiology, 156, 18-39. https://doi.org/10.1016/j.ijpsycho.2020.06.016
Schillinger, F. L., De Smedt, B., & Grabner, R. H. (2016). When errors count: An EEG study on numerical error monitoring under performance pressure. ZDM, 48(3), 351-363. https://doi.org/10.1007/s11858-015-0746-8
Segalowitz, S. J., & Davies, P. L. (2004). Charting the maturation of the frontal lobe: An electrophysiological strategy. Brain and Cognition, 55(1), 116-133. https://doi.org/10.1016/S0278-2626(03)00283-5
Shaw, P., Kabani, N. J., Lerch, J. P., Eckstrand, K., Lenroot, R., Gogtay, N., … Giedd, J. N. (2008). Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience, 28(14), 3586-3594. https://doi.org/10.1523/JNEUROSCI.5309-07.2008
Shenhav, A., Botvinick, M. M., & Cohen, J. D. (2013). The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217-240. https://doi.org/10.1016/j.neuron.2013.07.007
Shields, G. S., & Slavich, G. M. (2017). Lifetime stress exposure and health: A review of contemporary assessment methods and biological mechanisms. Social and Personality Psychology Compass, 11(8), e12335. https://doi.org/10.1111/spc3.12335
Silk, J. S., Siegle, G. J., Lee, K. H., Nelson, E. E., Stroud, L. R., & Dahl, R. E. (2013). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798-1807. https://doi.org/10.1093/scan/nst175
Simons, R.F. (2010). The way of our errors: Theme and variations. Psychophysiology, 47, 1-14. https://doi.org/10.1111/j.1469-8986.2009.00929.x
Slavich, G. M. (2016). Life stress and health: A review of conceptual issues and recent findings. Teaching of Psychology, 43(4), 346-355. https://doi.org/10.1177/0098628316662768
Slavich, G. M. (2019). Stressnology: The primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain, Behavior, and Immunity, 75, 3-5. https://doi.org/10.1016/j.bbi.2018.08.011
Slavich, G. M., & Cole, S. W. (2013). The emerging field of human social genomics. Clinical Psychological Science, 1, 331-348. https://doi.org/10.1177/2167702613478594
Slavich, G. M., & Shields, G. S. (2018). Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17-27. https://doi.org/10.1097/PSY.0000000000000534
Slavich, G. M., Tartter, M. A., Brennan, P. A., & Hammen, C. L. (2014). Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology, 49, 141-149. https://doi.org/10.1016/j.psyneuen.2014.07.009
Slavich, G. M., Thornton, T., Torres, L. D., Monroe, S. M., & Gotlib, I. H. (2009). Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology, 28(2), 223-243. https://doi.org/10.1521/jscp.2009.28.2.223
Sowell, E. R., Peterson, B. S., Thompson, P. M., Welcome, S. E., Henkenius, A. L., & Toga, A. W. (2003). Mapping cortical change across the human life span. Nature Neuroscience, 6(3), 309-315. https://doi.org/10.1038/nn1008
Sowell, E. R., Thompson, P. M., Leonard, C. M., Welcome, S. E., Kan, E., & Toga, A. W. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24(38), 8223-8231. https://doi.org/10.1523/JNEUROSCI.1798-04.2004
Sowell, E. R., Thompson, P. M., Holmes, C. J., Batth, R., Jernigan, T. L., & Toga, A. W. (1999). Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage, 9(6), 587-597. https://doi.org/10.1006/nimg.1999.0436
Steinberg, L. (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9(2), 69-74. https://doi.org/10.1016/j.tics.2004.12.005
Stemmer, B., Segalowitz, S. J., Witzke, W., & Schönle, P. W. (2004). Error detection in patients with lesions to the medial prefrontal cortex: An ERP study. Neuropsychologia, 42(1), 118-130. https://doi.org/10.1016/S0028-3932(03)00121-0
Sturman, D. A., & Moghaddam, B. (2011). The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neuroscience & Biobehavioral Reviews, 35(8), 1704-1712. https://doi.org/10.1016/j.neubiorev.2011.04.003
Sturmbauer, S. C., Shields, G. S., Hetzel, E. L., Rohleder, N., & Slavich, G. M. (2019). The Stress and Adversity Inventory for Adults (Adult STRAIN) in German: An overview and initial validation. PloS One, 14(5), e0216419. https://doi.org/10.1371/journal.pone.0216419
Swick, D., Honzel, N., & Turken, U. (2015). Intact error monitoring in combat Veterans with post-traumatic stress disorder. Psychiatry Research: Neuroimaging, 234(2), 227-238. https://doi.org/10.1016/j.pscychresns.2015.09.016
Tamnes, C. K., Walhovd, K. B., Torstveit, M., Sells, V. T., & Fjell, A. M. (2013). Performance monitoring in children and adolescents: A review of developmental changes in the error-related negativity and brain maturation. Developmental Cognitive Neuroscience, 6, 1-13. https://doi.org/10.1016/j.dcn.2013.05.001
Teicher, M. H., Anderson, C. M., Ohashi, K., Khan, A., McGreenery, C. E., Bolger, E. A., … Vitaliano, G. D. (2018). Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. NeuroImage, 169, 443-452. https://doi.org/10.1016/j.neuroimage.2017.12.055
Tomoda, A., Suzuki, H., Rabi, K., Sheu, Y-S., Polcari, A., & Teicher, M. H. (2009). Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. Neuroimage, 47, T66–T71. https://doi.org/10.1016/j.neuroimage.2009.03.005
Tottenham, N., & Galván, A. (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews, 70, 217-227. https://doi.org/10.1016/j.neubiorev.2016.07.030
Treadway, M. T., Grant, M. M., Ding, Z., Hollon, S. D., Gore, J. C., & Shelton, R. C. (2009). Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PloS One, 4(3), e4887. https://doi.org/10.1371/journal.pone.0004887
Troller-Renfree, S., Nelson, C. A., Zeanah, C. H., & Fox, N. A. (2016). Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. Journal of Child Psychology and Psychiatry, 57(10), 1145-1153. https://doi.org/10.1111/jcpp.12604
Ullsperger, M., Danielmeier, C., & Jocham, G. (2014). Neurophysiology of performance monitoring and adaptive behavior. Physiological Reviews, 94(1), 35-79. https://doi.org/10.1152/physrev.00041.2012
Weinberg, A., & Hajcak, G. (2011). Longer term test–retest reliability of error-related brain activity. Psychophysiology, 48(10), 1420-1425. https://doi.org/10.1111/j.1469-8986.2011.01206.x
Weinberg, A., Klein, D. N., & Hajcak, G. (2012). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology, 121(4), 885-896. https://doi.org/10.1037/a0028270
Weinberg, A., Kotov, R., & Hajcak Proudfit, G. (2015). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology, 124(1), 172-185. https://doi.org/10.1037/abn0000019
Weinberg, A., Meyer, A., Hale-Rude, E., Perlman, G., Kotov, R., Klein, D. N., & Hajcak, G. (2016). Error-related negativity (ERN) and sustained threat: Conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372-385. https://doi.org/10.1111/psyp.12538
Weinberg, A., Olvet, D. M., & Hajcak, G. (2010). Increased error-related brain activity in generalized anxiety disorder. Biological Psychology, 85(3), 472-480. https://doi.org/10.1016/j.biopsycho.2010.09.011
Van Meel, C. S., & Van Heijningen, C. A. (2010). The effect of interpersonal competition on monitoring internal and external error feedback. Psychophysiology, 47(2), 213-222. https://doi.org/10.1111/j.1469-8986.2009.00944.x
Velanova, K., Wheeler, M. E., & Luna, B. (2008). Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex, 18(11), 2505-2522. https://doi.org/10.1093/cercor/bhn012
Zirnheld, P. J., Carroll, C. A., Kieffaber, P. D., O’donnell, B. F., Shekhar, A., & Hetrick, W. P. (2004). Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience, 16(6), 1098-1112. https://doi.org/10.1162/0898929041502779
Open practices statement
The experiment and analyses reported in this manuscript were not pre-registered. The data and SPSS syntax for the primary analyses reported are available on the Open Science Framework (OSF) website at the following links:
Data: https://mfr.ca-1.osf.io/render?url=https://osf.io/53k7q/?action=download%26mode=render
Funding
This work was supported by the Canada Research Chairs Program awarded to Dr. Anna Weinberg, and a Canada Graduate Scholarships—Master’s Program scholarship awarded to Iulia Banica. George Slavich was supported by a Society in Science—Branco Weiss Fellowship, NARSAD Young Investigator Grant #23958 from the Brain & Behavior Research Foundation, and National Institutes of Health grant K08 MH103443.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declarations of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 28.7 kb)
Rights and permissions
About this article
Cite this article
Banica, I., Sandre, A., Shields, G.S. et al. Associations between lifetime stress exposure and the error-related negativity (ERN) differ based on stressor characteristics and exposure timing in young adults. Cogn Affect Behav Neurosci 22, 672–689 (2022). https://doi.org/10.3758/s13415-021-00883-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00883-z