Abstract
The regulation of motor resonance processes in daily life is indispensable. The automatic imitation task is an experimental model of those daily-life motor resonance processes. Recent research suggests that both self-other distinction and cognitive control processes may be involved in interference control during automatic imitation. Yet, we lack a clear understanding of the chronological sequence of interacting processes. To this end, this study used event-related potentials (ERPs) to investigate the time course underlying interference control during automatic imitation. We moreover aimed to extend previous results by investigating its modulation by social context. Cognitive conflict/action monitoring was assessed with the N2, in an exploratory manner the N450, and the CRN components. The Pre-Motor Positivity (PMP), associated with movement initiation, was suggested as a possible correlate of the successful resolution of self-other distinction. The cognitive control/action monitoring ERP components were influenced by the social context manipulation and partly by congruency, while PMP amplitudes were only sensitive to congruency. In addition, the exploratorily investigated N450 component predicted response times on incongruent relative to congruent trials in the different social contexts. This suggested that cognitive control/action monitoring processes, reflected in the N450, are guiding behavioral outcomes. Overall, interference control may primarily be guided by processes of cognitive control/action monitoring, whilst being modulated by social context demands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interactive alignment of motor behavior is a prominent phenomenon in our daily social lives. This includes synchrony, such as dancing (Tarr, Launay, & Dunbar, 2016), marching (Hove & Risen, 2009), or singing together (Pearce, Launay, & Dunbar, 2015), and also mimicry. Mimicry is the automatic alignment of postures to one’s interaction partner (Chartrand & Bargh, 1999). These phenomena may rely on a direct matching of the visual representation of a perceived action mapped onto the motor representations of the same action (direct matching hypothesis, Iacoboni et al., 1999; Rizzolatti, Fogassi, & Gallese, 2001). This shared representation between perception and execution of an action may evoke motor resonance in the observer’s motor system. For example, even when we just see someone lifting a finger, the motor representation of such a finger lifting movement is activated in our motor system. This link between the perception and execution of an action (i.e., perception-action link) may be related to mirror neurons originally discovered in monkey’s ventral premotor cortex (F5), firing when observing as well as when executing a specific action (Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004). A human homologue of the mirror neuron system has been suggested (Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Hilt et al., 2017; Mukamel, Ekstrom, Kaplan, Iacoboni, & Fried, 2010; Rizzolatti et al., 2001; Rizzolatti & Craighero, 2004). Indeed, single-cell recordings in, amongst others, the supplementary motor area (SMA) show human mirror neuron activity (Mukamel et al., 2010).
The observation of an action may interfere with one’s own action agenda: the perception of a movement may facilitate one’s own if both match, yet may require inhibition if they do not. This phenomenon has been termed automatic imitation (Heyes, 2011). It is usually investigated using the automatic imitation task, which is a stimulus-response compatibility (SRC) paradigm, employing the fact that observation of a movement interferes with task execution of the same or a different movement (Brass, Bekkering, Wohlschläger, & Prinz, 2000). In the automatic imitation task, participants are asked to perform a simple action, such as lifting an index or a middle finger, when a number cue is shown. Yet, simultaneously, and irrelevant to the task at hand, a matching or mismatching finger lifting movement is present on the screen. Motor resonance with the concurrent on-screen movement, while irrelevant, requires interference control for successful task execution. A match between the presented movement and target response (e.g., middle finger lifting) facilitates participants’ motor responses in congruent trials (i.e., perception-action match). This speeds up response times. In contrast, a mismatch between concurrent on-screen movement (e.g., an index finger lifting) and task execution (e.g., middle finger lifting) (i.e., perception-action mismatch) requires inhibition of the respective motor resonance with the task-irrelevant movement for successful task execution in incongruent trials. This slows down reaction times. The mismatch is caused by an overlap between externally activated and internally generated motor representation (i.e., motoric self-other overlap). This motoric overlap may rely on shared representations of the perception of another’s action when executing the same action (Chartrand & Bargh, 1999). According to ideomotor theory, motoric behavior may be represented as an image of its produced sensory feedback (Brass, Derrfuss, Matthes-von Cramon, & von Cramon, 2003; Brass, Ruby, & Spengler, 2009; Brass, Derrfuss, & von Cramon, 2005; Prinz, 1990, 1997, 2005). Repeated coupling between the sensory image of an action and the execution of the same, may evoke a sense of motoric self-other overlap between one’s own movements and movement intentions and the other’s movements, via strengthened shared motor representations (Brass et al., 2009; Prinz, 2005). As such, the direct matching hypothesis between perception of an action and the activation of its internal motor representation (Iacoboni et al., 1999) suggests imitation as a prepotent response tendency (Brass et al., 2005). This may interfere with response execution as mentioned above. To counteract this prepotent imitation, we need so-called interference control mechanisms to distinguish between internally generated and externally activated motor representations. As one of those mechanisms, self-other distinction (SOD) has been suggested (Brass et al., 2005; Brass et al., 2009). Supporting evidence comes from a neuropsychological study showing that patients with frontal lesions exhibit problems inhibiting imitative response tendencies (Brass et al., 2003). This finding is corroborated by brain imaging studies, pointing to a role of the temporoparietal junction (TPJ) during interference control of overlapping action perception and execution, specifically the inhibition of imitation. It has been suggested that the TPJ may play a crucial role in SOD processes (Brass et al., 2005, 2009).
More recently, it has been suggested that interference control may involve an interaction between SOD and general cognitive control mechanisms (Cross, Torrisi, Losin, & Iacoboni, 2013). Cognitive control refers to the ability to configure and monitor thoughts and actions for task performance (Botvinick, Cohen, & Carter, 2004). One fundamental aspect of cognitive and motor control is the ability to detect and resolve conflict in the face of competing task-irrelevant stimuli and response execution (Miller & Cohen, 2001). This may require the implementation of regulative and evaluative control processes (Larson, Clayson, & Clawson, 2014). Regulative processes refer to the implementation of top-down control for behavioral adjustment, thus successful task execution. The anterior cingulate cortex (ACC), as one core brain hub for top-down control of cognitive conflict (Botvinick et al., 2004), has been attributed an essential role in evaluative control processes. This reflects cognitive/action monitoring processes, which signal the demand for implementing cognitive or motor control in line with task demands (Larson et al., 2014).
We took advantage of the high temporal resolution of event-related potentials (ERP) to describe the time course of interference control during the automatic imitation task. We assume interference control during automatic imitation to be constituted via an interplay between SOD and cognitive conflict/action monitoring. Specifically, we plan to extend this research further by investigating the modulation of this interplay by social context, which might facilitate disentangling the two. Findings from a behavioral study suggest that interference control is modulated by contextual factors such as group membership and emotional facial expressions (Rauchbauer, Majdandžić, Stieger, & Lamm, 2016).
To this end, we applied the Social-Affective Mimicry Task (SAMT) while measuring brain activation via electroencephalography (EEG). The SAMT is based on the established automatic imitation paradigm by Brass et al. (2000) and adds task-irrelevant social and affective context information by presenting happy and angry ethnically diverse in-group (white) and out-group (black) faces and hand stimuli (see Methods for details) (Rauchbauer et al., 2016; Rauchbauer, Majdandžić, Hummer, Windischberger, & Lamm, 2015). Determining the time course of the social modulation of automatic imitation can give insights into successful interference control. This can furthermore inform potential difficulties in the modulation of motor resonance in social contexts, such as, for example, in individuals with autism spectrum disorder (ASD) (Cook & Bird, 2012).
Cognitive conflict/action monitoring is prominently investigated by examining amplitude variations of the stimulus-locked N2 component and two response-locked components, the error-related negativity (ERN) on erroneous, and the correct-response negativity (CRN) on correct trials. Moreover, we investigated the N450 component concerning the proposed function in an exploratory post-hoc fashion, as suggested by a reviewer, to examine control-related processes in complex task settings. These components have been associated with monitoring and resolution of cognitive conflict and behavioral adaptation (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Folstein & Van Petten, 2008; Pailing & Segalowitz, 2004).
Cognitive conflict/action monitoring studies usually assess the N2 component when they apply simple cognitive conflict tasks. The N2 is a negative amplitude deflection within 200–300 ms after the onset of stimulus presentation at frontocentral electrodes. In SRC paradigms, it varies according to stimulus congruency, with more negative amplitudes for incongruent than congruent trials (Folstein & Van Petten, 2008; Larson et al., 2014; Nieuwenhuis, Yeung, & Cohen, 2004; Yeung, Botvinick, & Cohen, 2004). It may have a functional significance representing strategic monitoring and action control (see for review Folstein & Van Petten, 2008). Importantly, N2 amplitudes are also sensitive to social and emotional manipulations, revealing for example an “own-race effect” with more negative N2 amplitudes for in-group compared with out-group faces (Ito & Bartholow, 2009; Ito & Urland, 2003, 2005; Kubota & Ito, 2007; Tanaka, Kiefer, & Bukach, 2004). In addition, more negative N2 amplitudes were reported for happy compared with angry facial expressions (Kubota & Ito, 2007). This has been suggested to reflect deeper processing due to perceptual individuation of faces beneficial to the participants (e.g., happy and in-group faces; Ito & Bartholow, 2009; Kubota & Ito, 2007).
Moreover, in an exploratory approach we also investigated the later occurring N450 component. The N450 may account for the complex set-up by which cognitive conflict is induced in the SAMT compared with the N2, which may account for conflict in less complex task set-ups. The N450 is a negative amplitude deflection within 400–550 ms after the onset of stimulus presentation at central electrodes. In complex SCR tasks, more negative amplitudes are observed for incongruent compared to congruent trials (Liotti, Woldorff, Perez III, & Mayberg, 2000; West & Alain, 1999). While its function is still be under debate (Larson et al., 2014, but see Galashan, Siemann, & Herrmann, 2019), it has been strongly associated with cognitive control and conflict detection processes (Hanslmayr et al., 2008; Larson et al., 2014; West, Bowry, & McConville, 2004; West, Jakubek, Wymbs, Perry, & Moore, 2005).
The response-locked component of choice in this study was the CRN, reflecting successful interference control on correct trials. We focused on the CRN variation because error rates were extremely low in our previous SAMT studies (Rauchbauer et al., 2015, 2016), leaving not enough trials for a reliable investigation of the ERN component (Olvet & Hajcak, 2009). The CRN component is a negative deflection around response execution elicited by correct trials at frontocentral electrodes and is related to the ERN component (Allain, Carbonnell, Falkenstein, Burle, & Vidal, 2004; Bartholow et al., 2005; Coles, Scheffers, & Holroyd, 2001; Vidal, Burle, Bonnet, Grapperon, & Hasbroucq, 2003; Vidal, Hasbroucq, Grapperon, & Bonnet, 2000). More negative CRN amplitudes were reported following correct responses in incongruent than congruent trials and moreover when the current trial type violated expectations (Bartholow et al., 2005). Its amplitude is further inversely related to error rates (Allain et al., 2004). These findings suggest that CRN amplitudes index strategic use of information to guide adaptive responses (Bartholow et al., 2005). Simons (2010) further proposed that CRN enhancement might have a protective function against error commission and indicates the degree of engagement of conflict/action monitoring during correct trials. CRN amplitude enhancement has also been shown in response to successful stereotype control related to ethnic group membership (Amodio, 2008; Amodio, Devine, & Harmon-Jones, 2008).
We further investigated the Pre-Motor Positivity (PMP), which has been related to motor commands for movement initiation (Bortoletto, Sarlo, Poli, & Stegagno, 2006; Deecke, Grözinger, & Kornhuber, 1976; Deecke, Scheid, & Kornhuber, 1969). It can be observed before the onset of motor execution (Deecke et al., 1969) and may be related to the inhibition of imitative actions (Shibasaki & Kato, 1975). The PMP is observed as a positive amplitude shift, which occurs at the end of the readiness potential (RP), a motor preparation ERP for ipsilateral finger movements (Deecke et al., 1969; Kornhuber & Deecke, 1965). The SMA has been suggested as one potential generator of the PMP (Deecke, Kornhuber, Lang, Lang, & Schreiber, 1985) and also has been ascribed mirror neuron functions (Mukamel et al., 2010).
Recently, we observed that the PMP component not only distinguished between movement congruency, with more positive amplitudes in congruent than incongruent trials. We also observed that PMP amplitude variation in incongruent relative to congruent trials predicted the respective response times of incongruent versus congruent trials. We attempted to replicate this finding, which we had interpreted as the PMP serving as a proxy for successfully resolved self-other distinction (Rauchbauer, Pfabigan, & Lamm, 2018). The process of self-other distinction most likely unfolds and operates during the whole time-course of stimulus processing and action selection and is supported by cognitive conflict/action monitoring. Despite our proposal that this might be reflected by the PMP component, no other ERP components have been described to reflect self-other distinction processes yet. This awaits further targeted experiments.
We expected more negative amplitudes on incongruent compared with congruent trials for the N2, the exploratory analysis of the N450, and the CRN, reflecting increased cognitive control/action monitoring demands. According to the above-mentioned literature, we expected the N2 to be most prominent for happy in-group faces. In line with results from a previous behavioral study (Rauchbauer et al., 2016), we specifically expected CRN amplitudes, and in an exploratory manner also N450 amplitudes, to be most negative for angry out-group trials compared with angry in-group trials. The angry out-group condition may be behaviorally most relevant and thus may require enhanced behavioral adaptation. In line with this, we expected the CRN and also the N450 to predict response times especially in an angry out-group context, with more negative amplitudes predicting longer response times on incongruent trials. Based on our previous results (Rauchbauer et al., 2018), we furthermore expected more positive PMP amplitudes on congruent than incongruent trials. Specifically, we expected more positive-going PMP amplitudes for happy in-group compared with happy out-group congruent trials due to increased self-other overlap. We furthermore expected that PMP amplitudes predict response times, which would replicate our previous results (Rauchbauer et al., 2018). We expected this to be most evident for happy in-group congruent trials, due to the hypothesized increased self-other distinction demands, with more positive amplitudes predicting faster response times. For behavioral results, we expected to replicate our previous results, demonstrating enhanced regulation of automatic imitation in response to angry out-group compared with angry in-group stimuli (Rauchbauer et al., 2016).
Methods
Participants
Thirty white participants took part in this EEG experiment. They received a financial compensation of 25 Euro. An initial data check identified two participants as outliers in the Attitudes toward black (ATB) scale with scores on the lower end of the scale, thus showing a very positive attitude toward the out-group compared with the rest of the sample (mean scores lower than the 25th percentile minus 1.5 times the interquartile range). Data from these participants were not included in any further data analysis to ensure a homogenous sample. The final sample consisted of 28 individuals (19 women, mean age: 24.58 years, standard deviation [SD] = 3.55 years). Participants were right-handed as assessed with the questionnaire by Oldfield (1971) and reported no prior medical history of neurological, cardiovascular, or psychiatric disorders and had normal/corrected to normal vision. Participants all signed an informed consent statement before the experimental task.
The present study was approved by the ethics committee of the University of Vienna and conducted in accordance with the Declaration of Helsinki (and its revision, 2013). Based on previous studies with a similar setup, a sample of 30 healthy volunteers was recruited. Please refer to the Supplementary Materials for details on statistical power.
Social-Affective Mimicry Task (SAMT)
Stimuli and experimental design
Participants performed the Social-Affective Mimicry Task (SAMT) (Rauchbauer et al., 2015, 2016), which is a modified version of the imitation-inhibition task by Brass et al. (2000). The simultaneous perception of a task-irrelevant action leads to interference (e.g., facilitation or inhibition) with a target movement (Brass et al., 2000). Matching motor perception and target action (e.g. presented and target movement are the same) constitutes congruent trials, which facilitate action execution and speed up response times. An action-perception mismatch (between presented and target movement) constitutes incongruent trials, which result in slower reaction times for movement execution. This is due to a required inhibition of the automatic action-perception link for the sake of correct response execution (Brass et al., 2000). On baseline trials, the task-irrelevant hand does not move. Regulation of automatic imitation is measured as the mean response time difference between incongruent and congruent trials (Rauchbauer et al., 2015). Baseline trials were not included in EEG analysis, due to the differing visual input of movement on (in-) congruent and no movement in these trials.
In the SAMT, task-irrelevant, female facial stimuli are simultaneously presented above the hand stimulus (with a white or black wrist; Fig. 1), depicting white in-group, or black out-group members, with happy or angry facial expression (face stimuli taken from NimStim Set of Facial Expressions (Tottenham et al., 2009). The factors Group (In-group, Out-group), Emotion (Happy, Angry) and Congruency (Congruent, Incongruent, Baseline) constituted a 2x2x3 factorial within-subjects design.
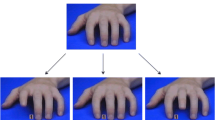
Experimental setup of SAMT. (A) Timeline of Social-Affective Mimicry Task (SAMT). Example of one trial; Face stimuli taken from the NimStim Set of Facial Expressions (Tottenham et al., 2009). (B) Ethnic (black and white) hand stimuli: upper row shows an example of an in-group congruent trial (task relevant cue indexing index finger movement, simultaneous task-irrelevant index finger lifting movement); lower row shows an example of an out-group incongruent trial (task relevant cue indexing index finger movement, simultaneous task-irrelevant middle finger lifting movement). Hand pictures by BR.
The same in- or out-group face model was used for both happy and angry emotional expressions. The concurrently presented task-irrelevant hand stimulus depicted a frontal shot of a left hand, mirroring participant’s right hand. This hand was either white or black, in accordance with the simultaneously presented face. The hand (wearing a beige glove, ethnicity visible at the wrist) performed a simultaneous finger lifting movements. Gloves were used to ensure that a potential regulation of automatic imitation was due to the task-irrelevant presentation of ethnically diverse emotional stimuli and not confounded by low-level attentional or perceptual influences caused by color differences of the hands and the target cue. The target cues were represented by the numbers “1” and “2” and displayed in black font in a grey square between the index and middle finger of the on-screen hand stimuli. Participants were asked to keep the number “1” on the keyboard pressed with their index and the number “2” with their middle finger. They were asked to lift their index finger whenever a “1” and the middle finger whenever a “2” appeared on the screen and to disregard any other stimuli presented on the screen. This aimed to ensure that potential regulation of automatic imitation by the ethnically and emotionally diverse stimuli occurred implicitly.
Task-irrelevant stimuli (i.e., face and hand stimuli) were matched for luminance across conditions using the SHINE (Spectrum, Histogram, and Intensity Normalization and Equalization) – toolbox (Willenbockel et al., 2010) for Matlab 8.3 (TheMathworks, Inc., MA).
Stimuli were presented centrally (visual angle approx. 2.4° × 5.1°) in two consecutive frames simulating finger lifting movements. Two instead of four consecutive frames were used in this experiment (compared with previous studies (Rauchbauer et al., 2015, 2016) to minimize the influence of overlapping ERP components caused by rapidly changing multiple frames (see also Rauchbauer et al., 2018). Both frames were displayed for 1,534 ms: the first frame presented only the task-irrelevant face and hand stimuli, the second frame presented the target cue and the task-irrelevant finger-lifting movement. Trials started with the presentation of a white fixation cross on black background for 1,000 ms, followed by the image presentation (in total 3,068 ms) and a variable intertrial interval with a random duration of 1,200-1,800 ms, again presenting the fixation cross.
Procedure
We used an EEG cap with 58 equidistantly mounted Ag/AgCl electrodes (model M10, EASYCAP, GmbH, Herrsching, Germany). Two electrodes were placed one cm above and below the right eye to record vertical eye-movements (electrooculogram [EOG]). Participants were seated in a sound-attenuated and electrically shielded chamber for EEG-recording. Distance to the computer screen was held constant at about 70 cm in front of a 19” cathode ray tube (CRT) monitor (Sony GDM-F520; 85 Hz refresh rate). Stimuli were presented using E-Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA); display resolution was 1,280 x 1,024 pixels (300 dpi).
A DC-amplifier setup (NeuroPrax, neuroConn GmbH, Ilmenau, Germany) recorded EEG signals within a frequency range of DC to 500 Hz and sampled it at 1,000 Hz for digital storage. Out of the scope of the present study and thus not presented in the article, four facial electromyographic electrodes also were attached. Electrode impedances were ensured to be below 4 kΩ before recording using a skin-scratching procedure (Picton & Hillyard, 1972).
Detailed instructions were presented at the beginning of the experiment and ten practice trials were performed. The task consisted of 50 trials per condition (i.e., 25 trials per index and middle finger) in a 2×2×3 within-subjects design: Group (In-group, Out-group), Emotion (Happy, Angry), and Congruency (Congruent, Incongruent, Baseline), resulting in a total of 600 randomly presented trials. After every 100 trials, participants were given short self-paced breaks. Duration of EEG data acquisition was approximately 60 minutes.
Post-experimental questionnaire
Attitudes towards blacks scale (ATB)
The Attitudes towards blacks scale (Brigham, 1993) measured explicit ethnic bias of our participants. Participants rated 20 statements on attitudes towards black individuals on a seven-point scale, indicating strong disagreement (“1”) to strong agreement (“7”) with the statement. A low mean score would imply a positive, whereas a high score a negative explicit attitude towards black individuals. This allowed identifying participants exhibiting either extremely favorable or unfavorable explicit attitudes towards black individuals (see “Participants” section).
Threat/security implicit association test (threat IAT)
We administered an adapted version of the Implicit Association Test (IAT) (Greenwald, McGhee, & Schwartz, 1998; see also Rauchbauer et al., 2016) to assess implicit ethnic bias of our participants. The threat-IAT assessed the strength of participants’ implicit associations between the target concepts of white in-group and black out-group with the attributes Security and Threat. The Security and Threat categories each consisted of five nouns matched for word length and valence in German (for Threat: fear, threat, violence, attack, danger; for Security: peace, protection, calmness, shelter, security). For more details on the threat-IAT see Supplementary Material (Section 2.1).
Statistical analysis
Statistical analyses of repeated measures ANOVA were performed in JASP 0.12.1 (JASP Team, 2020); https://jasp-stats.org/), jamovi 1.2.8. (The jamovi project, 2020; https://www.jamovi.org/), and Statistica 6.0 (StatSoft Inc., Tulsa, OK). Effect sizes of significant ANOVA results are described with partial eta squared (partial η2) (Kirk, 1996); the alpha-level was set at p < 0.05 in both behavioral and EEG data analyses. We further performed exploratory Spearman correlations with: IAT and ATB scores; ERP components and ATB, IAT and reaction times; ERP components showing a significant effect of congruency with each other; and ERP components showing a significant effect of congruency and individual RTs per condition – see Supplementary Materials (Section 2.5).
Statistical analysis of behavioral data
SAMT
Only data from correct trials entered data analysis. Participants’ individual mean response times (RT) were winsorized per condition and target cue to account for outliers before further statistical analyses (Wilcox, 2011). Mean RTs higher than the 75th percentile plus 1.5 times the interquartile range, and mean RTs lower than the 25th percentile minus 1.5 times the interquartile range of conditions and target cue were replaced with the corresponding maximum, respectively, the minimum RT.
First, participants’ mean RTs and percentage of error trials were analysed with a three-way repeated measurement ANOVA with factors Group (In-group, Out-group), Emotion (Happy, Angry), and Congruency (Congruent, Incongruent, and Baseline). Furthermore, we calculated the automatic imitation effect (i.e., the difference of participants’ mean RT on congruent minus incongruent trials per condition) to resolve a potential three-way interaction. Paired t-test will be used as post-hoc tests for the automatic imitation effect.
Threat IAT
The IAT score was calculated as the D-measure (Greenwald et al., 1998; Greenwald, Poehlman, Uhlmann, & Banaji, 2009): High D-measure values represent a strong implicit association of black out-group with Threat and white in-group with Security, whereas low D-measure values represent a low association of black out-group with Threat and white in-group with Security.
Obtained reaction times were winsorized to correct for outliers. Participants with more than 30% errors in one of the blocks will be excluded from analysis. The D-measure was calculated by dividing the RT (ms) difference of the fifth and third task by the latency standard deviation (SD) of RT of the combined fifth and the third task.
Preprocessing and statistical analysis of EEG data
Procedure
EEG data analysis was performed offline using EEGLAB 13.3.2b (Delorme & Makeig, 2004) implemented in Matlab 8.3 (The Mathworks, Inc., MA). First, data were down-sampled to 500 Hz, high (0.1 Hz) and low-pass filtered (30 Hz), and re-referenced to linked mastoids. Linked mastoid were chosen as offline reference sites as these electrodes have a similar noise level as the electrodes of interest, and their symmetric set-up guarantees a comparable weighing of left- and right-hemispheric activation (Luck, 2005; Nunez & Srinivasan, 2006). Additionally, we wanted to guarantee comparability with our previous study (Rauchbauer et al., 2018). Eye movement-related artefacts were detected applying extended infomax independent components analysis (ICA) (Bell & Sejnowski, 1995; Lee, Girolami, & Sejnowski, 1999) and subsequently removed. Data were segmented based on the factors Group (In-group, Out-group), Emotion (Happy, Angry), and Congruency (Congruent, Incongruent) during the presentation of the second frame. This resulted in eight conditions: In-group Happy Congruent, In-group Happy Incongruent, In-group Angry Congruent, In-group Angry Incongruent, Out-group Happy Congruent, Out-group Happy Incongruent, Out-group Angry Congruent, and Out-group Angry Incongruent.Footnote 1 For stimulus-locked data analysis, data segments were extracted 200 ms before target cue onset until 1,534 ms post-target cue onset, the first 200 ms served as baseline interval. For response-locked data analysis, data segments were extracted from −400 before to 600 ms after response execution. The mean voltage of the whole epoch served as baseline per trial, following Luck’s (2005) suggestion for response-locked data. Semiautomatic artefact correction eliminated trials that exceeded voltage values of ±75 μV or showed voltage drifts of >50 μV. Additional visual inspection was applied to exclude marked trials by the EEGLAB algorithms if artefact affliction was confirmed. Artefact-free segments were averaged per participant and condition: stimulus-locked on average 40.37 trials (SD = 5.007) and response-locked on average 33.88 trials (SD = 6.026).
Time windows and electrode locations were determined by literature and visual data inspection, which yielded overlapping results. In detail, we calculated global field power (Hamburger & vd Burgt, 1991; Murray, Brunet, & Michel, 2008; Skrandies, 1990) to specify respective time windows suggested by previous studies via assessing peak latency over all conditions per ERP. Subsequently, we inspected grand means and topographical maps of these time windows to define electrode clusters of interest. To keep the statistical model simple and to increase ERP signal-to-noise ratio, we assessed all ERPs at clusters of several merged electrodes applying a region of interest approach (Luck & Gaspelin, 2017). The N2 was analyzed by extracting mean amplitudes 200–300 ms after stimulus onset at an electrode cluster centred on FCz (Clayson & Larson, 2013); the same cluster as for the CRN was used here. In an exploratory approach, the N450 was analyzed by extracting mean amplitudes 400-550 ms after stimulus onset at an electrode cluster comprising Cz, CPz, and two electrodes in between (Tillman & Wiens, 2011). CRN amplitudes were extracted as peak-to-peak measure (CRN minus the preceding positive peak) to account for visible differences in the positive ERP peak before the CRN (which were, however, not significantly affected by the experimental manipulation, all p-values > 0.141). Negative-going CRN peaks and preceding positive peaks were assessed in a time-window of [−100; 100] ms around response execution at the frontocentral cluster centered on FCz (Pailing & Segalowitz, 2004). Mean PMP amplitudes were extracted in a time-window of 80 to 20 ms before response execution at a parietal cluster comprising three electrodes, which showed reliable activation across all conditions; see Figure S1 in Supplementary Materials for a visual depiction of the current electrode clusters.
First, to assess the modulation of interference control during automatic imitation by social context, three-way repeated measures ANOVAs with the within-subject factors Group (In-group, Out-group), Emotion (Happy, Angry), and Congruency (Congruent, Incongruent) were conducted for all ERP components. In case of significant three-way interactions, a congruency difference wave of the respective ERP (i.e., incongruent minus congruent trials per condition) will be calculated according to the logic of the difference measure of automatic imitation in the behavioral data analysis to resolve the interaction effect. Subsequently, these values will be subjected to a two-way repeated measures ANOVA with Group and Emotion. In line with our previous study (Rauchbauer et al., 2018), paired t-tests will be used as post-hoc tests. For all other interactions, Tukey post-hoc tests will be conducted.
Second, to investigate the prediction of reaction times by ERPs sensitive to congruency (i.e., showing an automatic imitation effect), we conducted linear mixed models in R (Version 1.3.1093, RStudio Team, 2020) with the lme4 package (Bates, Maechler, & Bolker, 2015). For this, we extracted single-trial reaction times for correctly answered trials participant- and condition-wise. Trials with reaction times faster than 3 standard deviations (SD) from the condition mean and slower than 3 SD from the condition mean were excluded from further analyses. Concerning ERPs, we extracted single-trial mean amplitudes in the respective time windows for N450 and PMP components (N450 [400; 550]; PMP [−80; −20]) participant- and condition-wise for correctly answered trials. For the CRN component, we extracted single-trial mean amplitudes ±20 ms around individual peaks (averaged across all conditions) of the CRN and the preceding positivity, which were then subtracted from each other on a single-trial basis (analogue to the peak-to-peak approach used for the main analysis). These single-trial difference mean amplitudes were extracted participant- and condition-wise. Single-trial reaction time data were modelled as a function of Group, Emotion, and Congruency (effect-coded: Group: Black: 1; White: −1; Emotion: angry: 1; happy: −1; Congruency: congruent: 1; incongruent: −1), and the continuous trial-wise mean-centered N450, PMP, and CRN amplitudes as fixed effects, as well as their individual interactions with the three-way full factorial model Group x Emotion x Congruency. The random effects structure included the three-way interaction of Group x Emotion x Congruency, estimating a random intercept and a random slope for each participant. We applied a restricted maximum likelihood estimation. Test statistics (from Wald chi-square tests) were calculated using the car package (Fox & Weisberg, 2019) with orthogonal sum contrasts and Type 3 sum of squares. To resolve significant interactions, we used the emmeans package (Searle, Speed, & Milliken, 1980) for the factor combinations of interest with the continuous ERPs of interest and pairwise comparisons among them. Figures were created using ggplot2 (Wickham, 2016). Asymptotic approximation of degrees of freedom was used.
Results
Social context modulation during automatic imitation in the SAMT: Behavior
On average across participants and conditions, 96% of trials were correct and entered subsequent analyses. The ANOVA on accuracy rates showed, by trend, an interaction of Group x Congruency with slightly less errors for Congruent out- than in-group trials (F(2, 27) = 2.969, p = 0.060, partial η2 = 0.10; Congruent: out-group: M = 1.16; SE = 0.11, in-group: M = 1.45, SE = 0.09). The ANOVA on reaction times (correct trials only) revealed a significant main effect of Group with faster reaction times on out- than in-group trials (F(1, 27) = 4.201, p = 0.050, partial η2 = 0.135; out-group: M = 512.35, SE = 15.69, in-group: M = 517.12, SE = 16.46). We observed a main effect of Congruency in the expected direction of longer RTs for incongruent than baseline and congruent trials (F(2,27) = 66.426, p < 0.001, partial η2 = 0.77; Congruent: M = 481.69, SE = 14.50; Baseline: M = 519.99, SE = 16.30, Incongruent: M = 542.52, SD = 17.99). Furthermore, a trend was observed for the three-way interaction (F(2,27) = 3.076, p = 0.054, partial η2 = 0.10). No significant difference between conditions was found for baseline trials (all p-values ≥ 0.154). Consequently, we calculated the automatic imitation effect as the mean difference of incongruent minus congruent trials. A repeated measures ANOVA showed again a trend result for the interaction GROUP x EMOTION (F(1,27) = 3.911, p = 0.058, partial η2 = 0.127), in the absence of significant main effects (both p-values ≥ 0.231, partial η2’s ≤ 0.053; main effect of Group). Post-hoc comparisons revealed a larger automatic imitation effect for Happy in-group versus Happy out-group stimuli, (t(27) = 1.918, p = 0.023; Happy: in-group: M = 68.98, SE = 9.07; out-group: M = 57.91, SE = 5.43). Furthermore, another trend was found for a larger automatic imitation effect for Angry versus Happy out-group faces (t(27) = 2.418, p = 0.066; Out-group: Angry: M = 62.26, SE = 7.54; Happy: M = 54.14, SE = 6.69). No further significant differences for pairwise comparisons were found (all p-values ≥ 0.166; Table 1; Fig. 2).
Automatic imitation effect in social context. Mean reaction time (RT) difference of incongruent minus congruent trials (in ms) of the four social context conditions is presented in box plots depicting the median and quartiles (whiskers demonstrating 1.5 times the interquartile range). The violin plots around illustrate kernel probability density (i.e., its width demonstrates the proportion of data located there); individual values are plotted within the violin plots. *p = 0.058 (for detail of interaction see text).
Attitudes towards Black Scale (ATB) and Threat-Implicit Association Test (IAT)
Data of two participants was missing for the ATB and the IAT due to the same technical error. Two participants were identified as outliers in the ATB because of their extremely favorable attitude towards Blacks and were thus excluded from all subsequent analysis. Participants exhibited a neutral to favorable explicit attitude towards the out-group in the ATB (N = 26; M = 4.10, SD = 0.31). In the IAT only, data from two additional participants were excluded from analysis due to an error percentage > 30%. The Threat-IAT revealed a large effect of the out-group being more associated with threat, whereas the in-group being more associated with security (t(23) = 3.713, p = 0.001, d = 0.76; Block 3 compatible: M = 840.91, SE = 47.97; Block 5 incompatible: M = 958.78, SE = 36.61).
EEG
Social context modulation during automatic imitation in the SAMT: ERPs
N2
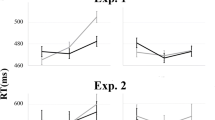
The N2 mean amplitudes ANOVA revealed a significant interaction of Group x Emotion (F(1,27) = 7.233, p = 0.012, partial η2 = 0.211). No further significant effects were found (all p-values ≥ 0.113, partial η2 = 0.091). Tukey post-hoc tests showed a trend for more pronounced N2 amplitudes for Angry out-group stimuli compared with Angry in-group stimuli (p = 0.077; Angry out-group: M = 0.158, SE = 0.585; Angry in-group: M = 0.734, SE = 0.480; all other p-values > 0.127; Figure 3; Figure S2 in Supplementary Materials).
Grand average of stimulus-locked N2. Right panel depicts N2 amplitude courses for happy; left panel for angry faces at a frontocentral electrode cluster centered on FCz. SRC = stimulus-response compatibility. Negative amplitudes are drawn upwards by convention. Stimulus presentation started at 0, indicated by a ticked vertical line. For demonstrational purposes, only the first 800 ms of the frames are depicted.
N450
The N450 mean amplitudes ANOVA revealed a significant interaction effect of GROUP x CONGRUENCY (F(1,27) = 8.882, p = 0.006, partial η2 = 0.25). No other significant effects were observed (all p-values ≥ 0.322, partial η2 = 0.04). More negative N450 amplitudes for incongruent compared to congruent trials were observed for out-group stimuli (p = 0.005) but not for in-group stimuli (p = 0.967). Moreover, congruent in-group stimuli elicited more negative N450 amplitudes than congruent out-group stimuli (p = 0.018; Fig. 4).
Grand average of stimulus-locked N450. Right panel depicts N450 amplitude courses for happy; left panel for angry faces at a central electrode cluster. SRC = stimulus-response compatibility. Negative amplitudes are drawn upwards by convention. Stimulus presentation started at 0, indicated by a ticked vertical line. For demonstrational purposes, only the first 800 ms of the frames are depicted.
CRN
The CRN peak-to-peak amplitudes ANOVA revealed a significant main effect of Congruency, with more negative CRN amplitudes for incongruent than congruent trials (F(1,27) = 7.250, p = 0.012, partial η2 = 0.21; Congruent: M = −5.033, SE = 0.461; Incongruent: M = −5.660, SE = 0.548), and a significant three-way interaction (F(1,27) = 4.865, p = 0.036, partial η2 = 0.15), which was resolved in the subsequent difference wave analysis (Figure 5).
Grand average of response-locked CRN. Right panel depicts CRN amplitude courses for happy; left panel for angry faces at a frontocentral electrode cluster centered on FCz. SRC = stimulus-response compatibility. Negative amplitudes are drawn upwards by convention. Response execution occurred at time point 0, indicated by a ticked vertical line.
CRN congruency difference waves
The CRN difference wave ANOVA revealed a significant interaction effect (F(1,27) = 4.865, p = 0.036, partial η2 = 0.15). Post-hoc comparisons showed by trend larger congruency differences for Happy out-group compared with Angry out-group stimuli (t(27) = −1.994, p = 0.056; Out-group: Happy: M = −1.264, SE = 0.534; Angry: M = 0.086, SE = 0.431).
PMP
The PMP mean amplitudes ANOVA revealed a significant main effect of Congruency with more positive PMP amplitudes for congruent than incongruent trials (F(1,27) = 6.176, p = 0.019, partial η2 = 0.19; Congruent: M = 7.048, SE = 0.607; Incongruent: M = 6.289, SE = 0.639). No other significant effects were found (all p-values > 0.108, partial η2 = 0.09; Fig. 6), Table 2.
Prediction of reaction times by ERPs sensitive to congruency (linear mixed model)
The linear mixed model predicting reaction times showed main effects of Congruency and the N450 component (Table 3). Furthermore, a significant interaction effect of Group x Emotion x Congruency x mean N450 amplitudes predicted reaction times (Table 3; Fig. 7; Figures S6 and S7 in Supplementary Material) with more negative N450 amplitudes predicting longer RTs.
Linear mixed model prediction of single-trial reaction time data of the four-way interaction (Congruency x Emotion x Group x N450), plotted for congruent and incongruent trials separately. Congruency: Con = congruent (in red); Incon = incongruent (in turquoise). The shaded area around the slopes denotes the standard error of the mean.
To resolve the significant interaction effect, we conducted pairwise post-hoc tests. The comparisons between Emotion x Congruency per Group (all Emotion x Congruency comparisons calculated separately for in-group and out-group trials, Table S3) and Group x Congruency per Emotion (all Group x Congruency comparisons calculated separately for Angry and Happy trials; Table S4) showed significant differences for the N450 predicting reaction times only in comparisons between incongruent and congruent trials, with more negative N450 amplitudes predicting longer RTs (all b > −60.85 (SE = 6.30), all z’s > −9.67, all p < 0.001; Fig. 7). Pairwise comparisons between Emotion x Group by Congruency (all Emotion x Group comparisons calculated separately for Incongruent and Congruent trials) did not reveal any significant differences. Thus, the post-hoc pairwise comparisons were not able to explain the origin of the observed interaction effect. However, when further plotting the interaction effect, we observed that the slopes in Figures S6 and S7 point towards potential interactions; on the one hand for incongruent Happy in-group and Happy out-group trials (Figure S6), and on the other hand for incongruent Happy and Angry out-group trials (Figure S7). This suggests that the four-way interaction may be driven by social context effects in incongruent trials; yet this remains entirely speculative, because these did not reach statistical significance.
Discussion
The present study investigated interference control during the automatic imitation task as an interplay between self-other distinction (SOD) and cognitive conflict/action monitoring, based on the assumption that the social context manipulation may further aid in disentangling these two. We hypothesized that this interplay would be reflected in amplitude variation of the N2, the CRN, as well as the PMP, and in an exploratory manner, the N450 components. These ERPs were furthermore hypothesized to jointly predict individual behavioral responses given they are sensitive to congruency. PMP, N450, and CRN amplitudes were sensitive to congruency, albeit this applied only to out-group trials for the N450 component. The social context manipulation (a variation of emotional expression and group membership) affected cognitive control/action monitoring ERPs only (N2, CRN, N450). Reaction times were predicted by N450 amplitudes for incongruent relative to congruent trials across all conditions.
On the behavioral level, as expected, we observed faster reaction times on congruent than on baseline and incongruent trials, replicating the established automatic imitation effect. By trend, the automatic imitation effect was affected by emotional expressions and group membership. Results of post-hoc comparisons showed a larger automatic imitation effect for happy in-group than out-group stimuli. Also, we found, by trend, a larger automatic imitation effect for angry compared with happy out-group stimuli. We did not replicate previous behavioral results of enhanced regulation of automatic imitation for angry out-group compared with angry in-group faces (Rauchbauer et al., 2016). The post-hoc comparisons should be taken with caution, because the interaction was only a trend result. Not replicating our previous behavioral results might be due to the smaller sample size compared with our previous studies. In general, neural correlates seemed to be better suited to pick up the influence of social context effects than our behavioral measures. Behavioral correlates may capture a compound of several cognitive processes, explaining why its effect sizes are smaller than those of ERPs (see also Pfabigan, Holzner, & Lamm, 2016). Behavioral results furthermore showed that black individuals were implicitly more associated with threat and white individuals more with security, whereas participants concurrently exhibited a neutral explicit attitude towards black individuals.
On the neural level, N2 amplitudes on angry out-group trials were by trend more pronounced than on angry in-group trials. That is, N2 amplitude variation was by trend sensitive to social context information, which could be due to a modulation of top-down processes. Our N2 trend result of more negative amplitudes for angry out-group than angry in-group trials mirrored previous behavioral results in the SAMT (Rauchbauer et al., 2016). Our N2 results may seem in contrast to the “own-race effect,” which constitutes of more negative N2 amplitudes in response to in-group compared with out-group faces (Ito & Bartholow, 2009; Ito & Urland, 2003, 2005; Kubota & Ito, 2007; Tanaka et al., 2004), and more negative N2 amplitudes for happy than angry facial expressions (Kubota & Ito, 2007). Yet, recent accounts suggest that the “own race effect” (Ito & Bartholow, 2009; Kubota & Ito, 2007) and the allocation of attentional resources according to group membership are context sensitive (Derks, Stedehouder, & Ito, 2014; Willadsen-Jensen & Ito, 2008). Asian participants, for example, attend to the most prominent ethnic group (e.g., white) and not necessarily their own (Willadsen-Jensen & Ito, 2008). Also, high identity threat in response to the in-group resulted in enhanced N2 amplitudes to out-group stimuli, potentially through enhanced attentional resource allocation (Derks et al., 2014). Relating the current N2 findings to the IAT results, N2 variation may reflect enhanced attentional resource allocation to the potentially as most salient experienced out-group face before subsequent movements are initiated. Potentially, top-down motivational effects of the social context influence early neural information processing for adaptive response execution or affect regulation (Amodio, 2013; Amodio, Harmon-Jones, & Devine, 2003; Derks et al., 2014; Ratner & Amodio, 2013). In the case of the present study, the categorization of the most salient social context stimuli (i.e., angry out-group faces) may specifically highlight the need to adapt behavior accordingly.
In the present experiment, N2 amplitudes were not sensitive to congruency information, which is in line with a recent study administering an automatic imitation task (Rauchbauer et al., 2018; see Section 2.4 in Supplementary Materials). Nevertheless, N2 amplitude variation according to stimulus congruency has been found in other SRC tasks (for review, see Folstein & Van Petten, 2008). Given the simultaneous processing of social context information in the SAMT, the processing of congruency might be temporally shifted.
Our exploratory N450 results support the suggestion that congruency between the motor command and the task-irrelevant movement is processed after the N2 time window. Previous studies reported more negative N450 amplitudes for incongruent than congruent trials as a sign for enhanced monitoring (Liotti et al., 2000; West & Alain, 1999). This established finding was observed for out-group trials in the current study, which is in line with the literature, suggesting that the N450 reflects cognitive control/action monitoring. However, no such congruency effect was found for in-group trials. Surprisingly, congruent in-group trials elicited N450 amplitudes on the same level as incongruent in-group and out-group trials, suggesting that our participants deployed enhanced conflict monitoring during all in-group trials. However, this monitoring was not adaptive but rather detrimental, as task performance did not benefit (by trend more errors in congruent in-group than out-group trials despite longer reaction times in in-group trials).
Importantly, the N450 is not only tied to cognitive conflict/action monitoring, but has also been associated with congruency processing during action observation (e.g., whether gestures and a given context match or not, Wu & Coulson, 2005), and unexpected socially inappropriate actions (Proverbio & Riva, 2009). More negative N450 amplitudes were reported for incongruent set-ups and unexpected actions (Proverbio & Riva, 2009; Wu & Coulson, 2005; see also Amoruso et al., 2013). Interpreting the current findings in light of these studies, we could suggest that the in-group setting interfered with the differentiation as to whether the observed action and the respective context matched. It might be possible that implicit in-group biases were at play here (see Supplementary Materials 1.5, for a potential pointer, as a more positive attitude towards blacks was associated with more negative N450 amplitudes in in-group angry congruent trials). Avoiding any further speculation, the current result awaits future investigation with larger sample sizes and more diverse samples.
Replicating previous results, CRN amplitudes were enhanced in incongruent than congruent trials (Bartholow et al., 2005), suggesting enhanced task engagement in these trials to successfully avoid erroneous responses. Moreover, CRN difference waves as an indicator of task engagement were by trend more pronounced for happy than angry out-group stimuli. This finding suggests that enhanced cognitive conflict/action monitoring was required for happy outgroup trials to avoid errors. Supporting this interpretation, also previous studies have related CRN amplitude variation to emotional processes (Luu, Collins, & Tucker, 2000; Vidal et al., 2000; Vidal et al., 2003). The current findings suggest that the CRN component is sensitive to socioemotional information and informs required behavioral adaptation of the current social context. The present results suggest that, early on, attention may be preferably allocated to the most salient contextual cue at the time of the N2 component (angry out-group condition). Later, the CRN component may subsequently inform strategic behavioral adaptation (Bartholow et al., 2005) according to contextual cues that might require a change of contextual priorities (happy out-group condition). This social context condition may require enhanced behavioral adaptation.
PMP amplitude variation distinguished between congruency regardless of social context, which corroborates the results of our previous study (Rauchbauer et al., 2018). As such, it could reflect the degree of motoric overlap between self- and other-generated movements at the moment of response execution. Yet in the current study, the PMP component did not predict response times. Our results rather suggest that preparation of the behavioral response may already be under way during stimulus processing, potentially reflecting cognitive conflict/action monitoring processes. The PMP component has been suggested to reflect a command for movement initiation (Bortoletto et al., 2006; Deecke et al., 1969; Deecke et al., 1976) and may depend on strategies for movement initiation (Bortoletto et al., 2006). As a kind of “go-signal” for the motor system (Bortoletto et al., 2006; Burwell, Makeig, Iacono, & Malone, 2019; Deecke et al., 1985), the PMP showed more positive amplitudes on correct than incorrect trials, suggesting that it precedes appropriate motor actions (Burwell et al., 2019). Our data could be in line with this, as more positive PMP amplitudes on congruent trials may reflect a potential go-signal on easy-to-resolve congruent trials compared to incongruent ones. It could be suggested that the PMP as a go-signal on congruent trials may reflect response facilitation, due to the motoric overlap of one’s own executed action and the movement of the task-irrelevant hand. The generator of the PMP has been suggested to be located in the SMA (Deecke et al., 1985), which has also been ascribed human mirror neuron functions (Mukamel et al., 2010). Future studies should try to further disentangle response execution processes from those related to the comparison of internal and external motor representations and their neural manifestations.
Linking neural and behavioral data, we observed that the N450 component predicted reaction times for incongruent versus congruent trials in all conditions, with more negative amplitudes for longer RTs, as expected. However, while the LMM revealed a significant interaction of the N450 for the factor combinations of Emotion, Group and Congruency, pairwise comparisons did not show any significant differences between the different social context conditions (all Emotion and Group comparisons calculated separately for congruent and for incongruent trials). They only showed significant differences between congruent/incongruent factor combinations (Figure 7). Visual inspection of the data (Figures S6 and S7 in the Supplementary Material) may suggest that the interaction was driven by Emotion x Group x N450 effects in incongruent trials, specifically for the N450 on Happy in-group versus out-group trials (Figure S6) and on Happy and Angry out-group trials (Figure S7). Yet this is only a descriptive interpretation and should be taken with caution. Nevertheless, the results seem to further underline the role of the N450 for cognitive conflict/action monitoring to resolve motoric (in)congruency.
The present study thus partly extends our previous results (Rauchbauer et al., 2018) as it suggests that primarily processes of cognitive conflict/action monitoring are guiding our behavioral responses. The present results also point towards the importance of social context in regulating automatic imitation at the stimulus processing level before response execution. Thus, interference control may primarily be guided by processes of cognitive control/action monitoring, whilst being modulated by social context demands.
We acknowledge a limitation in terms of statistical power in the present article. While the current sample size had enough power to detect the classic automatic imitation effect on the behavioral level as well as congruency effects on the neural level, we have to acknowledge that it was underpowered in terms of interaction effects that are based on smaller effect sizes. Our interaction results should therefore be considered exploratory.
Nonetheless, our results could inform processes underlying atypical modulation of automatic imitation in, for example, individuals with autism spectrum disorder (ASD). While high-functioning ASD individuals do not show modulation of automatic imitation (Cook & Bird, 2012), hyper-imitation can be observed with increased ASD symptom severity (Bird, Leighton, Press, & Heyes, 2007; Sowden, Koehne, Catmur, Dziobek, & Bird, 2016; Spengler, Bird, & Brass, 2010). Our results could be used to investigate impaired neurocognitive processes underlying the absent or hyper-modulation of automatic imitation in ASD individuals. Supporting this suggestion, PMP amplitude variation has recently been proposed to reflect clinical implications (Burwell et al., 2019). Thus, this might be an interesting avenue to pursue.
Conclusions
Our findings suggest that interference control during automatic imitation is predominantly driven by cognitive control/action monitoring. These processes are malleable by social context. Our results can be useful in identifying potentially maladaptive processes during motor resonance. Interactive motor alignment in everyday life predominantly engages cognitive conflict/action monitoring processes to inform orchestration of behavior during perception-action mismatch.
Notes
Baseline trials were included during the experiment for maximal comparability of the paradigm to previous behavioral studies (Rauchbauer et al., 2016), but were not used for EEG data analyses, because they did not depict any movement and thus no cognitive conflict/action monitoring or self-other distinction were required (see also Rauchbauer et al., 2018).
References
Allain, S., Carbonnell, L., Falkenstein, M., Burle, B., & Vidal, F. (2004). The modulation of the Ne-like wave on correct responses foreshadows errors. Neuroscience Letters, 372(1–2), 161–166.
Amodio, D. M. (2008). The social neuroscience of intergroup relations. European Review of Social Psychology, 19(1), 1–54.
Amodio, D. M. (2013). Implicit prejudice and the regulation of intergroup responses: theoretical contributions of the social neuroscience approach. In Neuroscience of prejudice and intergroup relations (pp. 167–189). Taylor and Francis.
Amodio, D. M., Devine, P. G., & Harmon-Jones, E. (2008). Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. Journal of Personality and Social Psychology, 94(1), 60.
Amodio, D. M., Harmon-Jones, E., & Devine, P. G. (2003). Individual differences in the activation and control of affective race bias as assessed by startle eyeblink response and self-report. Journal of Personality and Social Psychology, 84(4), 738.
Amoruso, L., Gelormini, C., Aboitiz, F., Alvarez González Miguel, Manes, F., Cardona, J., & Ibanez, A. (2013). N400 ERPs for actions: building meaning in context. Frontiers in Human Neuroscience, 7, 57.
Bartholow, B. D., Pearson, M. A., Dickter, C. L., Sher, K. J., Fabiani, M., & Gratton, G. (2005). Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology, 42(1), 33–42.
Bates, D., Maechler, M., & Bolker, B. (2015). Walker., S. Fitting linear mixed-effects models using lme4. J Stat Softw, 67(1), 1–48.
Bell, A. J., & Sejnowski, T. J. (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159.
Bird, G., Leighton, J., Press, C., & Heyes, C. (2007). Intact automatic imitation of human and robot actions in autism spectrum disorders. Proceedings of the Royal Society B: Biological Sciences, 274(1628), 3027–3031.
Bortoletto, M., Sarlo, M., Poli, S., & Stegagno, L. (2006). Pre-motion positivity during self-paced movements of finger and mouth. NeuroReport, 17(9), 883–886.
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences, 8(12), 539–546.
Brass, M., Bekkering, H., Wohlschläger, A., & Prinz, W. (2000). Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain and Cognition, 44(2), 124–143.
Brass, M., Derrfuss, J., Matthes-von Cramon, G., & von Cramon, D. Y. (2003). Imitative response tendencies in patients with frontal brain lesions. Neuropsychology, 17(2), 265.
Brass, M., Derrfuss, J., & von Cramon, D. Y. (2005). The inhibition of imitative and overlearned responses: a functional double dissociation. Neuropsychologia, 43(1), 89–98.
Brass, M., Ruby, P., & Spengler, S. (2009). Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2359–2367.
Brigham, J. C. (1993). College students’ racial attitudes. Journal of Applied Social Psychology, 23(23), 1933–1967.
Burwell, S. J., Makeig, S., Iacono, W. G., & Malone, S. M. (2019). Reduced premovement positivity during the stimulus-response interval precedes errors: Using single-trial and regression ERPs to understand performance deficits in ADHD. Psychophysiology, 56(9), e13392.
Chartrand, T. L., & Bargh, J. A. (1999). The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology, 76(6), 893.
Clayson, P. E., & Larson, M. J. (2013). Psychometric properties of conflict monitoring and conflict adaptation indices: Response time and conflict N2 event-related potentials. Psychophysiology, 50(12), 1209–1219. https://doi.org/10.1111/psyp.12138
Coles, M. G. H., Scheffers, M. K., & Holroyd, C. B. (2001). Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biological Psychology, 56(3), 173–189.
Cook, J. L., & Bird, G. (2012). Atypical social modulation of imitation in autism spectrum conditions. Journal of Autism and Developmental Disorders, 42(6), 1045–1051.
Cross, K. A., Torrisi, S., Losin, E. A. R., & Iacoboni, M. (2013). Controlling automatic imitative tendencies: interactions between mirror neuron and cognitive control systems. Neuroimage, 83, 493–504.
Deecke, L., Grözinger, B., & Kornhuber, H. H. (1976). Voluntary finger movement in man: cerebral potentials and theory. Biological Cybernetics, 23(2), 99–119.
Deecke, L., Kornhuber, H. H., Lang, W., Lang, M., & Schreiber, H. (1985). Timing function of the frontal cortex in sequential motor and learning tasks. Human Neurobiology, 4(3), 143–154.
Deecke, L., Scheid, P., & Kornhuber, H. H. (1969). Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Experimental Brain Research, 7(2), 158–168.
Delorme, A., & Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Derks, B., Stedehouder, J., & Ito, T. A. (2014). Social identity modifies face perception: An ERP study of social categorization. Social Cognitive and Affective Neuroscience, 10(5), 672–679.
Falkenstein, M., Hoormann, J., Christ, S., & Hohnsbein, J. (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology, 51(2–3), 87–107.
Folstein, J. R., & Van Petten, C. (2008). Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology, 45(1), 152–170.
Fox, J., & Weisberg, S. (2019). An R companion to applied regression (Third). Thousand Oaks CA: Sage.
Galashan, D., Siemann, J., & Herrmann, M. (2019). Top-down feature-based cueing modulates conflict-specific ERP components in a Stroop-like task with equiprobable conditions. BioRxiv, 807412.
Gallese, V., Fadiga, L., Fogassi, L., & Rizzolatti, G. (1996). Action recognition in the premotor cortex. Brain, 119(2), 593–609.
Greenwald, A. G., McGhee, D. E., & Schwartz, J. L. K. (1998). Measuring individual differences in implicit cognition: the implicit association test. Journal of Personality and Social Psychology, 74(6), 1464.
Greenwald, A. G., Poehlman, T. A., Uhlmann, E. L., & Banaji, M. R. (2009). Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. Journal of Personality and Social Psychology, 97(1), 17.
Hamburger, H. L., & vd Burgt, M. A. G. (1991). Global field power measurement versus classical method in the determination of the latency of evoked potential components. Brain Topography, 3(3), 391–396.
Hanslmayr, S., Pastötter, B., Bäuml, K.-H., Gruber, S., Wimber, M., & Klimesch, W. (2008). The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience, 20(2), 215–225.
Heyes, C. (2011). Automatic Imitation. Psychological Bulletin, 137(3), 463–483. https://doi.org/10.1037/a0022288
Hilt, P. M., Bartoli, E., Ferrari, E., Jacono, M., Fadiga, L., & D’Ausilio, A. (2017). Action observation effects reflect the modular organization of the human motor system. Cortex, 95, 104–118.
Hove, M. J., & Risen, J. L. (2009). It’s all in the timing: Interpersonal synchrony increases affiliation. Social Cognition, 27(6), 949–960.
Iacoboni, M., Woods, R. P., Brass, M., Bekkering, H., Mazziotta, J. C., & Rizzolatti, G. (1999). Cortical mechanisms of human imitation. Science, 286(5449), 2526–2528.
Ito, T. A., & Bartholow, B. D. (2009). The neural correlates of race. Trends in Cognitive Sciences, 13(12), 524–531.
Ito, T. A., & Urland, G. R. (2003). Race and gender on the brain: electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology, 85(4), 616.
Ito, T. A., & Urland, G. R. (2005). The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience, 5(1), 21–36.
JASP Team. (2020). JASP (Version 0.12.1) [Computer software].
Kirk, R. E. (1996). Practical significance: A concept whose time has come. Educational and Psychological Measurement, 56(5), 746–759.
Kornhuber, H.-H., & Deecke, L. (1965). Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflugers Archiv Fur Die Gesamte Physiologie Des Menschen Und Der Tiere, 284, 1–17.
Kubota, J. T., & Ito, T. A. (2007). Multiple cues in social perception: The time course of processing race and facial expression. Journal of Experimental Social Psychology, 43(5), 738–752.
Larson, M. J., Clayson, P. E., & Clawson, A. (2014). Making sense of all the conflict: a theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology, 93(3), 283–297.
Lee, T.-W., Girolami, M., & Sejnowski, T. J. (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computation, 11(2), 417–441.
Liotti, M., Woldorff, M. G., Perez III, R., & Mayberg, H. S. (2000). An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia, 38(5), 701–711.
Luck, S. J. (2005). An introduction to the event-related potential technique. Cambridge: The MIT Press.
Luck, S. J., & Gaspelin, N. (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology, 54(1), 146–157.
Luu, P., Collins, P., & Tucker, D. M. (2000). Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General, 129(1), 43.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24(1), 167–202.
Mukamel, R., Ekstrom, A. D., Kaplan, J., Iacoboni, M., & Fried, I. (2010). Single-neuron responses in humans during execution and observation of actions. Current Biology, 20(8), 750–756.
Murray, M. M., Brunet, D., & Michel, C. M. (2008). Topographic ERP Analyses: A Step-by-Step Tutorial Review. Brain Topography, 20(4), 249–264. https://doi.org/10.1007/s10548-008-0054-5
Nieuwenhuis, S., Yeung, N., & Cohen, J. D. (2004). Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology, 41(1), 157–160.
Nunez, P. L., & Srinivasan, R. (2006). Electric fields of the brain: the neurophysics of EEG. Oxford University Press, USA.
Oldfield, R. C. (1971). Assesment and analysis of handedness - Edinburgh Inventory. Neuropsychologia, 9(1), 97-.
Olvet, D. M., & Hajcak, G. (2009). The stability of error-related brain activity with increasing trials. Psychophysiology, 46(5), 957–961. https://doi.org/10.1111/j.1469-8986.2009.00848.x
Pailing, P. E., & Segalowitz, S. J. (2004). The effects of uncertainty in error monitoring on associated ERPs. Brain and Cognition, 56(2), 215–233.
Pearce, E., Launay, J., & Dunbar, R. I. M. (2015). The ice-breaker effect: singing mediates fast social bonding. Royal Society Open Science, 2(10), 150221. http://rsos.royalsocietypublishing.org/content/2/10/150221.abstract
Pfabigan, D. M., Holzner, M.-T., & Lamm, C. (2016). Performance monitoring during a minimal group manipulation. Social Cognitive and Affective Neuroscience, 11(10), 1560–1568.
Picton, T. W., & Hillyard, S. A. (1972). Cephalic skin potentials in electroencephalography. Electroencephalography and Clinical Neurophysiology, 33(4), 419–424.
Prinz, W. (1990). A common coding approach to perception and action. In Relationships between perception and action (pp. 167–201). Springer.
Prinz, W. (1997). Perception and action planning. European Journal of Cognitive Psychology, 9(2), 129–154.
Prinz, W. (2005). 5 an ideomotor approach to imitation. Perspectives on Imitation: Mechanisms of Imitation and Imitation in Animals, 1, 141.
Proverbio, A. M., & Riva, F. (2009). RP and N400 ERP components reflect semantic violations in visual processing of human actions. Neuroscience Letters, 459(3), 142–146.
Ratner, K. G., & Amodio, D. M. (2013). Seeing “us vs. them”: Minimal group effects on the neural encoding of faces. Journal of Experimental Social Psychology, 49(2), 298–301.
Rauchbauer, B., Majdandžić, J., Hummer, A., Windischberger, C., & Lamm, C. (2015). Distinct neural processes are engaged in the modulation of mimicry by social group-membership and emotional expressions. Cortex, 70, 49–67.
Rauchbauer, B., Majdandžić, J., Stieger, S., & Lamm, C. (2016). The Modulation of Mimicry by Ethnic Group-Membership and Emotional Expressions. PLoS ONE, 11(8), e0161064. Retrieved from 10.1371%2Fjournal.pone.0161064
Rauchbauer, B., Pfabigan, D. M., & Lamm, C. (2018). Event-related potentials of automatic imitation are modulated by ethnicity during stimulus processing, but not during motor execution. Scientific Reports, 8(1), 1–15.
Rizzolatti, G., & Craighero, L. (2004). The mirror neuron system. Annual Review of Neuroscience, 27(1), 169–192. https://doi.org/10.1146/annurev.neuro.27.070203.144230
Rizzolatti, G., Fogassi, L., & Gallese, V. (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience, 2(9), 661–670.
RStudio Team. (2020). RStudio: Integrated development for R. RStudio, PBC: Boston, MA, USA; 2020. Retrieved from https://rstudio.com/. Accessed 2020-12-04.
Searle, S. R., Speed, F. M., & Milliken, G. A. (1980). Population marginal means in the linear model: an alternative to least squares means. The American Statistician, 34(4), 216–221.
Shibasaki, H., & Kato, M. (1975). Movement-associated cortical potentials with unilateral and bilateral simultaneous hand movement. Journal of Neurology, 208(3), 191–199.
Simons, R. F. (2010). The way of our errors: Theme and variations. Psychophysiology, 47(1), 1–14.
Skrandies, W. (1990). Global field power and topographic similarity. Brain Topography, 3(1), 137–141.
Sowden, S., Koehne, S., Catmur, C., Dziobek, I., & Bird, G. (2016). Intact automatic imitation and typical spatial compatibility in autism spectrum disorder: Challenging the broken mirror theory. Autism Research, 9(2), 292–300.
Spengler, S., Bird, G., & Brass, M. (2010). Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biological Psychiatry, 68(12), 1148–1155.
Tanaka, J. W., Kiefer, M., & Bukach, C. M. (2004). A holistic account of the own-race effect in face recognition: Evidence from a cross-cultural study. Cognition, 93(1), B1–B9.
Tarr, B., Launay, J., & Dunbar, R. I. M. (2016). Silent disco: dancing in synchrony leads to elevated pain thresholds and social closeness. Evolution and Human Behavior, 37(5), 343–349. https://doi.org/10.1016/j.evolhumbehav.2016.02.004
The Jamovi Project. (2020). jamovi (Version 1.2) [Computer sotware].
Tillman, C. M., & Wiens, S. (2011). Behavioral and ERP indices of response conflict in Stroop and flanker tasks. Psychophysiology, 48(10), 1405–1411.
Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., … Nelson, C. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–249.
Vidal, F, Hasbroucq, T., Grapperon, J., & Bonnet, M. (2000). Is the ‘error negativity’specific to errors? Biological Psychology, 51(2–3), 109–128.
Vidal, Franck, Burle, B., Bonnet, M., Grapperon, J., & Hasbroucq, T. (2003). Error negativity on correct trials: a reexamination of available data. Biological Psychology, 64(3), 265–282.
West, R., & Alain, C. (1999). Event-related neural activity associated with the Stroop task. Cognitive Brain Research, 8(2), 157–164.
West, R., Bowry, R., & McConville, C. (2004). Sensitivity of medial frontal cortex to response and nonresponse conflict. Psychophysiology, 41(5), 739–748.
West, R., Jakubek, K., Wymbs, N., Perry, M., & Moore, K. (2005). Neural correlates of conflict processing. Experimental Brain Research, 167(1), 38–48.
Wickham, H. (2016). ggplot2: elegant graphics for data analysis. springer.
Wilcox, R. (2011). Modern statistics for the social and behavioral sciences: A practical introduction. CRC Press.
Willadsen-Jensen, E. C., & Ito, T. A. (2008). A foot in both worlds: Asian Americans’ perceptions of Asian, White, and racially ambiguous faces. Group Processes & Intergroup Relations, 11(2), 182–200.
Willenbockel, V., Sadr, J., Fiset, D., Horne, G. O., Gosselin, F., & Tanaka, J. W. (2010). Controlling low-level image properties: the SHINE toolbox. Behavior Research Methods, 42(3), 671–684.
Wu, Y. C., & Coulson, S. (2005). Meaningful gestures: Electrophysiological indices of iconic gesture comprehension. Psychophysiology, 42(6), 654–667. https://doi.org/10.1111/j.1469-8986.2005.00356.x
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931.
Acknowledgments
The study was partly supported by the Vienna Science and Technology Fund (WWTF, CS11-005, to CL). Birgit Rauchbauer was supported by the Fondation pour la Recherche Médicale (FRM, SPF20171039127) and benefited from the grants ANR-16-CONV-0002 (ILCB), ANR-11-LABX-0036 (BLRI), and the Excellence Initiative of Aix-Marseille University (A*MIDEX). The authors thank Andreas Gartus for highly appreciated technical support; Ludwig Huber (Veterinary University of Vienna, Messerli Research Institute) for valuable discussions of the research design; Amie Fairs for fabulous support regarding the statistical analyses; and three anonymous reviewers for valuable and constructive feedback during the review process. Participants’ financial remuneration was funded by a scholarship of the University of Vienna awarded to Corinna Lorenz. Parts of the present data were presented at the Annual Meeting of the Society for Psychophysiological Research 2017, in Vienna, Austria.
Author information
Authors and Affiliations
Contributions
All authors contributed sufficiently to qualify for authorship. Each author declares that they have no potential conflict of interest. We warrant that the material contained in the manuscript represents original work, has not been published anywhere (also not on a website), and is not under consideration for publication elsewhere.
Conceptualization: BR CLA DMP.
Data curation: BR CLA DMP.
Formal analysis: BR CLO DMP.
Funding acquisition: CLO CLA.
Investigation: BR CLO DMP.
Methodology: BR CLO DMP.
Project administration: BR CLA DMP.
Resources: CLA.
Software: BR CLO DMP.
Supervision: CLA DMP.
Validation: BR CLA DMP.
Visualization: BR DMP.
Writing – original draft: BR DMP.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Open Practice Statement
Single subject and summary level data, as well as the R script for LMM are available on the Open Science Framework (OSF; https://osf.io/): https://osf.io/ymeqs/?view_only=718ae4058be443ae8333c771631d4bd4; the experiment was not preregistered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1382 kb)
Rights and permissions
About this article
Cite this article
Rauchbauer, B., Lorenz, C., Lamm, C. et al. Interplay of self-other distinction and cognitive control mechanisms in a social automatic imitation task: An ERP study. Cogn Affect Behav Neurosci 21, 639–655 (2021). https://doi.org/10.3758/s13415-021-00878-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00878-w