Abstract
The purpose of this study was to investigate sex-related differences in the electrophysiological response to socioemotional stimuli (positive, negative, and ambiguous) depicting couple interactions. The associations between anxiety and avoidance attachment dimensions (measured with the Experiences in Close Relationships–Revised questionnaire) and the strength of cortico-limbic circuit intensity was explored, recorded using a 256-Hydrocel Geodesic Sensor-Net. Event-related potentials (ERPs) and standardized low-resolution electromagnetic tomography (sLORETA) data were analyzed for a total sample of 74 participants. Regression analyses showed that the women presented increased brain intensity compared with that in men, and the avoidance score was positively associated with brain intensity, particularly in response to negative socioemotional stimuli. The interaction sex per avoidance was a significant predictor of intensity in many brain areas, with women displaying significantly more pronounced positive associations between avoidance and brain intensity than men. In conclusion, the findings of the present study showed that women appeared to be more emotionally involved during the socioemotional task. Avoidance was positively associated with intensity of the cingulate and prefrontal regions, and these associations were more pronounced in women than in men. These findings suggested that avoidance seems to represent two different socioemotional strategies, in which women appear to activate an avoidant strategy to modulate increased emotional involvement in relationships, whereas men appear to adopt avoidance with a more intense emotional suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several studies have provided evidence regarding the existence of sex-related differences in socioemotional processing (Collignon et al., 2010; Kret & De Gelder, 2012). According to recent literature, women appear to be considered as more sensitive and focused on emotional aspects of social experiences compared with men (Bianchin & Angrilli, 2012; Guimond, Chatard, Martinot, Crisp, & Redersdorff, 2006). Specifically, women appear to develop a more pronounced tendency to empathize with and understand both the verbal and nonverbal information related to the behaviors of others (Proverbio, Zani, & Adorni, 2008). From an evolutionary perspective, this female propensity could be viewed as a functional adaptation associated with the care of offspring (Hampson, van Anders, & Mullin, 2006). Consistently, the many studies that have investigated sex-related differences in brain activity have indicated the activation of different neural pathways during socioemotional tasks between men and women (Althaus et al., 2014; Bianchin & Angrilli, 2012; Groen Wijers, Tucha, & Althaus, 2013). In particular, some event-related potential (ERP) studies have reported that women prioritize the processing of socially relevant and negative emotional information, showing different P100, N200, and late positive potential (LPP) amplitudes in the occipital, frontocentral, and parietal montages, respectively, compared with those in men (Groen Wijers, Tucha, & Althaus, 2013; Proverbio, Adorni, Zani, & Trestianu, 2009). Moreover, activity in the right amygdala and right prefrontal brain regions in response to pictures depicting characters in negative contexts was only observed in women, suggesting the potential role of these areas in the increased affective response to negative social information in women (Proverbio et al., 2009). Finally, women were shown to present enhanced activity in the cingulate brain areas; this neural correlate could be an expression of women’s propensity to respond empathetically to social stimuli (Proverbio et al., 2008; Sander, Frome, & Scheich, 2007).
In light of these previous studies, the investigation of how sex-related differences affect social and intimate relationships in men and women is of interest (Ratliff & Oishi, 2013). Hazan and Shaver (1987) suggested that adult romantic love represents an attachment process that is affected by infant experiences, theorizing three different forms of attachment styles: secure, anxious/ambivalent, and avoidant. Specifically, secure people feel comfortable with intimacy and maintain autonomy in their relationships, anxious people are extremely preoccupied with relationships and rely on their partners, and avoidant people withdraw from closeness and intimacy in their relationships (Hazan & Shaver, 1987; Main, Kaplan, & Cassidy, 1985).
Many studies have approached this issue from a neurobiological perspective, investigating the brain processes associated with various attachment dimensions (Cecchini, Iannoni, Pandolfo, Aceto, & Lai, 2015; Gillath, Bunge, Shaver, Wendelken, & Mikulincer, 2005; Lai, Altavilla, Ronconi, & Aceto, 2016). In particular, the anxiety dimension of attachment has been positively associated with the activation of emotion-related brain areas (the anterior temporal pole, insula, and anterior cingulate cortex) and inversely correlated with the activation of brain areas involved in emotional regulation processing (orbitofrontal cortex; Gillath et al., 2005).
Previous studies examining the association between the avoidant attachment dimension and cortico-limbic activation (amygdala, insula, anterior cingulate cortex, and prefrontal cortex) have reported contrasting findings (Vrtička, Bomdolfi, Sander, & Vuilleumier, 2012). On the one hand, some studies demonstrated decreased cortico-limbic activity as a function of increased avoidance scores in social exclusion tasks and during the presentation of positive socioemotional stimuli (Dewall et al., 2012; Vrtička, Andersson, Grandjean, Sander, & Vuilleumier, 2008). On the other hand, other studies have shown increased cortico-limbic activation in people with high scores in the avoidant attachment dimension in response to unpleasant socioemotional stimuli (Buchheim, George, Kächele, Erk, & Walter, 2006; Strathearn, Fonagy, Amico, & Montague, 2009; Vrtička & Vuilleumier, 2012). In light of these contrasting findings, whether the avoidant attachment dimension is negatively or positively associated with cortico-limbic activation during socioemotional stimuli processing remains unclear. This divergence in the findings reported by previous studies could be associated with the different socioemotional tasks that were used, which may have resulted in the activation of different neural correlates (e.g., social reward, social exclusion, emotional faces, and crying infants; Lee & Siegle, 2009).
In line with this hypothesis, a very recent metanalytic study suggested that two opposing neurobiological mechanisms may be associated with avoidance (Long, Verbeke, Ein-Dor, & Vrtička, 2020). On the one hand, deactivating strategies may be engaged, which appear to be associated with the relative insensitivity to negative social information, preventing an excessive activation of an “aversion module” (anterior cingulate cortex, insula, hippocampus, amygdala, and anterior temporal pole). On the other hand, avoidance strategies appear to result in the increased sensitivity to negative social information, accompanied by the reduced ability to regulate consequent distress, which results in the increased activation of the aversion module. Simultaneously, positive emotions associated with social contexts also appear to be suppressed. These data suggested that individuals with avoidance attachment present a blunted subjective experience and reduced brain activity in response to positive social information, whereas there is an impaired regulation of strongly negative social information (Long et al., 2020).
Several EEG studies have demonstrated that the N200, P200 and late components appear to be modulated by the attachment style (Krahe et al., 2015; Krahe, Drabek, Paloyelis, & Fotopoulou, 2016; Zayas, Shoda, Mischel, Osterhout, & Takahashi, 2009). Specifically, higher N200 and P200 amplitudes have been associated with increased avoidance (measured with the Experiences in Close Relationships–Revised questionnaire) in response to painful stimuli that occur in the presence of social support (Krahe et al., 2015) or to social rejection/exclusion experiences (White et al., 2012). These social neuroscience data underline how avoidance is associated with the preferential use of suppression as an emotional (self-)regulation strategy during both positive and negative social contexts (Collins & Feeney, 2004; Long et al., 2020), resulting in two possible neurobiological outcomes—either decreased activation or increased sensitivity to social information.
In light of these recent findings, a possible explanation of the contrasting results regarding the relationship between the avoidant attachment dimension and cortico-limbic activation in response to socioemotional stimuli may be ascribable to the adoption of different emotional coping strategies in men and women. In previous studies, positive associations between the avoidance attachment dimension and brain activation were only reported in women (Buchheim et al., 2006; Strathearn et al., 2009; Vrtička & Vuilleumier, 2012), whereas negative associations were either only found only in men or identified in mixed samples (Dewall et al., 2012; Vrtička et al., 2008).
In view of the divergence among these neurobiological studies, the investigation of the associations between attachment dimensions and cortico-limbic activation in men and women is of interest. To the best of our knowledge, few neurobiological studies have focused on this topic.
The primary purpose of this study was to investigate sex-related differences in the electrophysiological responses to socioemotional images of couple interactions. Moreover, the associations between attachment style dimensions and the brain responses in cortico-limbic circuits in both men and women were investigated. The hypotheses were that women would show different amplitudes and latencies for early ERP components in the occipital and temporo-parietal montages, whereas for late ERP components will be observed in the frontal montage, as well as an increased intensity in the limbic, cingulate, and prefrontal cortices in response to socioemotional images compared with the brain intensity observed in men. The association between the avoidant attachment dimension and the intensity of the limbic, cingulate, and prefrontal cortices will be significantly positive and significantly more pronounced in women than in men, particularly in response to images with negative valence.
Methods
Participants
Eighty-three right-handed, healthy volunteers participated in the study. The study was approved by the Ethics Committee of the Department of Dynamic and Clinical Psychology, Sapienza University, and all participants signed informed consent to participate in the study. The inclusion criterion was being between 18 and 35 years of age. The exclusion criteria were any history of neurological injury, psychiatric illness, drug abuse, or psychotropic medication.
Psychological assessment
The Experiences in Close Relationships–Revised scale (ECR-R; Busonera, Martini, Zavattini, & Santona, 2014; Fraley, 2012; Fraley, Waller, & Brennan, 2000; Picardi et al., 2002) was administered to assess two attachment dimensions (anxiety and avoidance). The ECR-R is a 36-item self-report questionnaire that includes 18 items for the anxiety dimension and 18 items for the avoidance dimension (with Cronbach’s α of 0.90 and 0.89, respectively; Busonera et al., 2014). The participants rated each item on a 7-point scale, ranging from 1 (totally disagree) to 7 (totally in agreement). In the sample used in the present study, the Cronbach’s α values for the anxiety and avoidance dimensions were 0.89 and 0.86, respectively.
Stimuli
A total of 120 black-and-white pictures were selected. Ninety images depicted a man and a woman engaged in couple interactions, with three different emotional valences, as follows: 30 pictures with couples in intimate interactions (happy facial expressions or direct gaze or physical contact) were chosen for the positive condition; 30 pictures with couples in conflictual interactions (sad or angry facial expressions or averted gaze or physical violence) were chosen for the negative condition; and 30 pictures with couples in neutral interaction (neutral facial expressions or neutral actions or averted gaze) were chosen for the ambiguous condition. Finally, 30 pictures depicting neutral objects were chosen to avoid habituation to the interaction stimuli.
Thirty neutral objects were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008). Ninety pictures of couple interactions were chosen as follows: from the IAPS were selected all the available pictures depicting couple interactions (n = 15) and from the internet were selected the remaining pictures (n = 75) according to the criteria described above (positive, negative, and ambiguous conditions). All 120 pictures were adjusted for luminance and contrast using the Gnu Image Manipulation Program (GIMP; Version 2.8, Free Software Foundation, Inc.) and Microsoft Office Picture Manager software, setting an identical resolution (640 × 480 pixels) for each image.
A preliminary assessment was performed by 19 volunteers to evaluate the emotional valence (on a Likert scale from 1 = very negative to 7 = very positive) and the emotional arousal (on a Likert scale from 1 = no intense to 7 = extreme intense) of each of the 90 couple interaction images.
A repeated-measures analysis of variance (ANOVA), with condition (positive vs. negative vs. ambiguous) as the within-subjects factor, performed on the emotional valence score showed a main effect of the condition, F(2, 36) = 283.2, p < .001, in which the positive condition reported a higher positive score than the negative (p < .001) and ambiguous (p < .001) conditions, and the negative condition reported a higher negative score compared with the ambiguous condition (p < .001). A repeated-measures ANOVA, with condition (positive vs. negative vs. ambiguous) as the within-subjects factor, performed on the emotional arousal showed a main effect of the condition, F(2, 36) = 6.7, p = .003, in which the ambiguous condition reported a lower arousal rating than the positive (p < .001) and negative (p = .039) conditions.
Experimental procedure
Participants were seated at a viewing distance of 80 cm from a PC monitor (27 cm, 75 Hz, 1,024 × 768). The stimuli were presented using E-Prime (Version 2.0.8.90; Psychology Software Tools, Inc., Pittsburgh, PA, USA). The participants were instructed to pay attention to the images and evaluate their emotional valence. Each trial began with a fixation cross displayed for 1,000 ms, followed by a picture (positive vs. negative vs. ambiguous vs. neutral), which was presented for 2,000 ms. Participants then rated the emotional valence of the visual stimuli on a 7-point scale, ranging from 1 (very negative) to 7 (very positive). Each trial ended with an interstimulus interval (ISI) with a random duration ranging from 300 to 500 ms. A total of 120 trials (30 trials per condition) were presented in a random order (see Fig. 1). After the visual task, the ECR-R was administered.
Task procedure. The task started with instructions for the participants. They were instructed to pay attention to the images and evaluate their emotional values. Each trial started with a fixation cross, displayed for 1,000 ms, followed by the picture (positive vs. negative vs. ambiguous vs. neutral), presented for 2,000 ms. Then the participants rated the image on a 7-point scale ranging from 1 (very negative) to 7 (very positive); the trial ended with an interstimulus interval (ISI) ranging from 300 to 500 ms. A total of 120 trials (30 trials for each condition) were presented in random order
Electroencephalographic (EEG) registration and analysis
Electroencephalographic (EEG) signal was recorded continuously at 250 Hz using Net Station 4.4.2 and a 256-Hydrocel Geodesic Sensor Net, with an impedance below 50 kΩ and referenced to the vertex (Cz).
The acquired data were digitally filtered (30 Hz low-pass) off-line. The EEG data for each subject was segmented into epochs starting 100 ms before the presentation of the stimulus until 700 ms after stimulus onset. The artefact detention was set to 200 μV for bad channels (noisy electrodes), to 150 μV for eye blinks (detected on specific electrodes pairs: left: 37-241/32-241 and right: 18-238/25-238), and to 100 μV for the electrodes detecting eye movements (left: 252 and right: 226; Electrical Geodesic, Inc., Eugene, OR, USA; Bourisly & Shuaib, 2018; Lai et al., 2018; Luan, Yao, & Bai, 2017; Picton et al., 2000; Schreiter, Chmielewski, & Beste, 2018). The segments containing eye blinks, eye movements, or more than 15 bad channels were excluded. Baseline correction −100 ms before stimulus onset was applied.
Through the visual inspection of ERPs components, the following intervals were set: from 80 to 160 ms for the P100, and from 160 to 220 ms for the N200 (early components); from 220 to 300 ms for the P250, from 300 to 500 for the LC1, and from 500 to 700 for the LC2 (late components).
After the EEG signal cleaning from artefacts, as reported by previous studies (Lai et al., 2020; Lai, Pellicano, et al., 2018; Picton et al., 2000; Tanner, Morgan-Short, & Luck, 2015), the following electrode locations were chosen for each montage: occipital (O1, O2), temporo-parietal (left: 65, 75, 84, 86, 97, 107, 108; right: 150, 160, 161, 162, 173, 179, 180), and frontal (left: 22, 23, 24, 28, 30, 47, 49; right: 6, 7, 13, 207, 214, 215, 221).
Data were analyzed on peak amplitudes and latencies for the following: at P100 on the occipital and temporo-parietal montages; at N200 on the temporo-parietal montage; and at P250 on the temporo-parietal and frontal montages. Moreover, at LC1 and LC2, the data were analyzed just on peak amplitudes of the temporo-parietal and frontal montages.
Source analysis (sLORETA)
The EEG signal was processed through GeoSource 2.0 software (Electrical Geodesic, Inc., Eugene, OR, USA) to identify the locations of the neural generators of the ERP components.
Standardized low-resolution electromagnetic tomography (sLORETA; Pascual-Marqui, 2002), was used to identify source locations based on a probabilistic map of the MNI305 average (Montreal Neurological Institute 305 subjects). Gray matter volume was parcellated into 7-mm voxels, with each voxel serving as a source location with three orthogonal orientation vectors (Cecchini, Aceto, Altavilla, Palumbo, & Lai, 2013; Lai et al., 2016; Lai et al., 2017; Lai et al., 2018; Lai et al., 2020; Lai, Pellicano, et al., 2018; Lancaster et al., 2000; Luciani et al., 2014; Massaro et al., 2018). This parcellation resulted in a total of 2,447 source triplets whose anatomical labels were estimated through the use of a Talairach daemon (Cecchini et al., 2013; Lancaster et al., 2000; Luciani et al., 2014).
In accordance with the hypotheses and based on previous literature (Long et al., 2020; Vrtička & Vuilleumier, 2012) regarding associations between the avoidance dimension and neurobiological responses to socioemotional stimuli, the following regions of interest (ROIs) were chosen for sLORETA analyses: limbic, anterior cingulate cortex, posterior cingulate cortex, and prefrontal cortex. For each ROI, the following Brodmann areas (BAs) in both hemispheres were selected: amygdala, insula, amygdala-hippocampus junction, and hippocampus for the limbic ROI; BA24, BA32, and BA33 for the anterior cingulate cortex ROI; BA23, BA30, and BA31 for the posterior cingulate cortex ROI; and BA09, BA10, BA11, BA46, and BA47 for the prefrontal cortex ROI.
Statistical analysis
Differences between men and women were examined using t-tests on the number of trials (positive, negative, and ambiguous) without artefacts that were inserted in the final analyses, and on ECR-R scores (anxiety and avoidance).
For behavioral data analyses, the 2 × 3 repeated-measures analyses of covariance (ANCOVAs), with sex (men vs. women) as the between-subjects factor, condition (positive vs. negative vs. ambiguous) as the within-subjects factor, and age and the ECR-R scores (anxiety and avoidance) as covariates, were conducted on the reaction times and the emotional valence assignment of the visual stimuli. Correlation analyses (Pearson’s r) were performed between ECR-R scores (anxiety and avoidance) and reaction times, and emotional valence assignment of the visual stimuli. The reaction times and emotional valence assignment scores were subjected to multiple factorial regression analyses with age, sex, ECR-R-anxiety score, ECR-R-avoidance score, and the Sex × ECR-R Score interaction as predictors.
To analyze ERPs data, the 2 × 3 × 2 repeated-measures ANCOVAs, with sex (men vs. women) as the between-subjects factor, condition (positive vs. negative vs. ambiguous) and hemisphere (left vs. right) as the within-subjects factors, and with age and the ECR-R scores (anxiety and avoidance) as covariates were conducted on the amplitude and latency of early and late components on the occipital, temporo-parietal and frontal montages.
For the sLORETA data analyses, a correlation analysis (Pearson’s r) was performed between the ECR-R scores (anxiety and avoidance) and the mean intensity of each BA in each ROI in response to each condition for the early and late ERP components (Bonferroni correction was applied, and the p value was set at ≤ .0003; 15 BAs × 2 hemispheres × 5 components: 0.05/150 = 0.0003). For each BA in each ROI that showed a significant correlation between the ECR-R-anxiety score, the ECR-R-avoidance score, and the BA mean intensity, a multiple factorial regression analysis was performed, including age, sex, ECR-R-anxiety score, ECR-R-avoidance score, and the Sex × ECR-R Score interaction as predictors.
All statistical analyses were performed with Statistica 10.0 (StatSoft Inc.).
Results
From among the initial 83 participants, nine were excluded due to the presence of artefacts in the EEG data. The total sample consisted of 74 participants (37 men and 37 women; mean age for men = 24.8 ± 3.9 years; women = 23.8 ± 3.1), t(72) = 1.3, p = .195.
The t tests performed between men and women on the number of positive, negative, and ambiguous trials without artefacts that were included in the final analyses did not show any significant differences (see Table 1).
The mean ECR-R score for the anxiety dimension for men was 3.6 ± 0.8, whereas the mean score for women was 3.5 ± 1.2; the mean ECR-R score for the avoidance dimension for men was 3.0 ± 0.9, whereas the mean score for women was 2.7 ± 0.8. No significant differences were found between men and women for either the anxiety, t(72) = 0.5, p = .635, or avoidance, t(72) = 1.4, p = .156, dimensions.
Behavioral data
A 2 (sex: men vs. women) × 3 (condition: positive vs. negative vs. ambiguous) repeated-measures ANCOVA, with age and ECR-R scores (anxiety and avoidance) as covariates, performed on the reaction times and emotional valence assignment of the visual stimuli showed the following results. For the reaction times, a main effect of the condition was identified, F(2, 138) = 3.9, p = .023; ƞp2 = 0.05, in which the ambiguous condition elicited longer reaction times compared with the reaction times for the positive (p < .001) and negative (p < .001) conditions, and the negative condition elicited longer reaction times than the positive condition (p < .001). For the emotional valence assignment in response to visual stimuli, the ANCOVA showed a main effect of the condition, F(2, 138) = 34.7, p < .001, ƞp2 = 0.33, in which the positive condition presented a greater positive valence than either the negative (p < .001) or ambiguous (p < .001) conditions, and the ambiguous condition presented a greater positive valence than the negative (p < .001) condition. A Sex × Condition interaction, F(2, 138) = 3.3, p = .039, ƞp2 = 0.05, was also identified, in which men showed a lower positive score in response to the positive condition than did women (p = .006). Finally, an Avoidance × Condition interaction, F(2, 138) = 10.8, p < .001, ƞp2 = 0.13, was found.
The correlation analyses (Pearson’s r) performed between ECR-R scores (anxiety and avoidance) and the reaction times and emotional valence assignment of the visual stimuli revealed the following results. The ECR-R anxiety score did not show any significant correlations with either the reaction times or the emotional valence assignment of the visual stimuli. The ECR-R avoidance score was negatively correlated with the positive valence assignment of the visual stimuli (r = −0.49, p < .001). Coherently, the multiple factorial regression on the emotional valence assignment in response to the positive condition, with age, sex, ECR-R-anxiety score, ECR-R-avoidance score, and the Sex × ECR-R Score interaction as predictors showed a significant model. The sex and avoidance resulted as significant predictors (see Table 2).
ERPs
To analyze ERP data, the 2 (sex: men vs. women) × 3 (condition: positive vs. negative vs. ambiguous) × 2 (hemisphere: left vs. right) repeated-measures ANCOVAs, with age and ECR-R scores (anxiety and avoidance) as covariates, were performed on ERP amplitude (see Table 3 and Fig. 2). A main effect of sex was found on the occipital montage at P100 and on the temporo-parietal montage at P100 and P250, in which men showed a greater amplitude compared with those in women, and on the frontal montage at LC2, where men showed a lower amplitude than women. A Sex × Condition interaction was identified for the temporo-parietal montage at P100, in which men showed a greater amplitude in response to negative and ambiguous stimuli compared with women. In addition, ambiguous stimuli elicited a greater amplitude in men compared with positive stimuli, whereas in women this effect was inverted. A Sex × Condition × Hemisphere interaction was observed for the temporo-parietal montage at LC2. An Anxiety × Condition interaction was observed for the occipital montage at P100 and for the temporo-parietal montage at N200, P250, and LC1. An Avoidance × Condition interaction was identified for the occipital montage at P100. An Avoidance × Hemisphere interaction was observed for the temporo-parietal montage at P250. An Avoidance × Condition × Hemisphere interaction was found on the temporo-parietal montage at P250 and LC1 and on the frontal montage at P250. All the effects, also the not significant ones, were reported in Table 1 of the supplementary material (see Table 1s).
ANCOVAs (sex: men vs. women) × (condition: positive vs. negative vs. ambiguous) × hemisphere (left vs. right), with age and the ECR-R scores (anxiety and avoidance) as covariates performed on the latency, showed the following results (see Table 3 and Fig. 2). A main effect of sex was observed for the occipital and temporo-parietal montages at P100, in which men showed a longer latency compared than women. A main effect of age was identified for the frontal montage at P250, in which age was negatively associated with the latency. An Anxiety × Condition × Hemisphere interaction was found on the temporo-parietal montage at P100.
sLORETA
The correlation analyses performed between the ECR-R scores (anxiety and avoidance) and the mean intensity of each BA in each ROI in response to each condition, for each ERP component, is discussed below.
The ECR-R anxiety score did not show significant correlations with the brain intensity of any ROI, for any component (see Table 4). The ECR-R avoidance score was significantly positively correlated with the brain intensity of all ROIs in all components (see Table 4): for the early components (P100 and N200), the ECR-R avoidance scores correlated with brain intensity observed in the limbic, cingulate, and prefrontal cortices, mainly in response to negative and ambiguous conditions (see Table 4); for the late components (P250, LC1, and LC2), ECR-R avoidance scores correlated with the intensity of the limbic ROI in response to all conditions, with the intensity of the cingulate cortices in response to negative and ambiguous conditions, and with the intensity of the prefrontal cortex exclusively in response to negative condition (see Table 4).
For each BA that showed a significant correlation with the ECR-R scores, a multiple factorial regression analysis was performed using age, sex, anxiety, avoidance, Sex × Anxiety, And Sex × Avoidance as predictors. As reported in Table 5, the regression models were all significant. The avoidance predictor was always positively and statistically significant coherently with the inclusion criteria applied starting from the significant correlations. The sex predictor was significant (with women showing increased brain intensity compared with men) for the early and late prefrontal cortex ROI intensity in response to positive condition; for the early and late cingulate and prefrontal cortices ROIs intensity in response to negative condition; and for the early posterior cingulate cortex ROI intensity in response to ambiguous condition. Sex × Avoidance was a significant predictor for posterior cingulate and prefrontal cortices ROIs intensity in response to positive condition, and for early and late cingulate and prefrontal cortices ROIs intensity in response to the ambiguous and negative conditions.
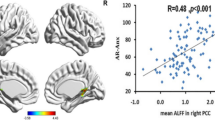
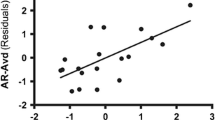
As shown by the inclination of the regression lines in Fig. 3, the different avoidance/BA intensity associations in men versus women (represented by the significant Sex × Avoidance interaction in the regression models) showed a stronger positive association in women compared with men mostly for the late components of the cingulate and prefrontal cortices ROIs in response to negative condition.
The associations in men and women between avoidance (x-axis) and the Brodmann areas (BA) intensity (y-axis) where the Sex × Avoidance interaction resulted as a significant predictor (.002 < p > .047) in the regression model (at P100, N200, P250, LC1, and LC2 components). In all the significant Sex × Avoidance interactions, the women showed more pronounced positive association compared with men. (n = 74)
Discussion
The main finding of this study was that avoidance was positively associated with cortico-limbic brain intensity. Consistent with the hypotheses, this association presented a clear sex-related difference, for which the regression slopes in women were significantly more pronounced than those in men. As hypothesized, the findings of this study suggested that the avoidant attachment might be a sex-related adaptive strategy that involves different affective and cognitive processes in response to socioemotional distress. According to the attachment theory, the internal working models seem to be behavioral strategies that allow the regulation and maintenance of a minimum level of internal security, through the deactivation or hyperactivation of the attachment system (Mikulincer & Shaver, 2003).
The present findings suggested that in women, the avoidance strategy might be associated with an effort to regulate the hyperactivation of the attachment system induced by socioemotional cues, whereas the avoidance strategy in men appears to be associated with a more intense emotional suppression. One interpretation of this divergence between avoidant strategies in men and women is the general female tendency to be more receptive to emotional aspects in relational contexts (Proverbio et al., 2009). Several behavioral (Bachrach, Croon, & Bekker, 2015; Li & Fung, 2014) and neurobiological (George, Ketter, Parekh, Herscovitch, & Post, 1996; Piefke, Weiss, Markowitsch, & Fink, 2005; Proverbio et al., 2008) studies have supported the increased tendency among women to empathize and understand others and revealed the different cortico-limbic correlates in response to socioemotional stimuli between men and women.
In this study, the Sex × Avoidance interaction effect in regression analyses showed sex-related differences in the association between avoidance scores and brain intensity, particularly evident in response to the negative socioemotional stimuli, involving primarily the left and right anterior (BA32 and BA33) and posterior cingulate (BA23 and BA31) cortices in the late components and the left prefrontal cortex (BA11) in both early and late components. In response to the positive and ambiguous conditions, significantly different associations between men and women were less frequently observed, and primarily involved the right posterior cingulate cortex (BA23), the left anterior cingulate (BA32), and the left prefrontal cortices (BA11). These findings were also supported by the Sex × Condition interaction effect that as observed for the amplitudes of early ERP components, which confirmed the pivotal role of negative socioemotional cues for the activation of the attachment system, mostly in women. Consistently, recent studies (Proverbio et al., 2009; Stevens & Hamann, 2012) have demonstrated that women responded more strongly to negative emotional stimuli, particularly in the prefrontal brain areas, compared with men.
Moreover, the behavioral results of the present study revealed that men attributed fewer positive values to positive socioemotional stimuli than women did, which appears to suggest that men are less likely to express positive values on relational contexts (Tamres, Janicki, & Helgeson, 2002). As suggested by Buck and others (Buck, 1977, 1991; Chaplin, 2015; Levenson, Carstensen, & Gottman, 1994), this could indicate that men become internally aroused, but “keep in” their emotions through unknown regulatory mechanisms, whereas women express these emotions more freely. Consistent with this interpretation, in the present study, the ERP data showed a main effect of sex, in which women were observed to have shorter latencies and lower amplitudes within the early occipital and temporo-parietal components, and larger amplitudes in the late frontal component compared with those in men.
Coherently, in the regression analyses, sex was a significant predictor of the brain intensity, showing increased intensity in the cingulate and prefrontal cortices in women compared with that in men. Specifically, differences between men and women were found in the intensity levels of the left anterior cingulate (BA32), the right posterior cingulate (BA23 and BA31), and the left prefrontal cortex (BA11), in response to negative socioemotional stimuli, whereas differences were found only on the left prefrontal (BA11) in response to the positive condition and only on the right posterior cingulate cortex (BA23) in response to the ambiguous condition. These findings suggested that the women appeared to be more responsive to the socioemotional cues, primarily to the negative cues, compared with the responsiveness of men (Proverbio et al., 2009).
In contrast to our findings for avoidance, the anxiety dimension did not show any significant associations with any brain intensity, suggesting that anxiety may be less associated with the neurobiological pathways during socioemotional tasks (Ran & Zhang, 2018; Vrtička et al., 2008).
The ERP data showed that age was negatively associated with the latency of the P250 component in the frontal montage; however, this finding should be considered in light of the specific age range recruited for this study and which characterized the final sample (18–35-year-olds). This result could reflect the greater emotional involvement and the lowered inhibition in intimacy during relationships that occur with increasing of age, as reported in previous studies (Levenson et al., 1994; Radmacher & Azmitia, 2006).
Our study has some limitations. The scores of the attachment dimensions were assessed by a self-administered questionnaire, which could potentially favor social desirability bias and the acquiescent response bias. Due to the small age range of the participants (18–35 years), these findings cannot be generalized to populations with different ages.
In conclusion, the findings of the present study showed that women appear to be more emotionally involved during a socioemotional task. Avoidance was positively associated with the cingulate and prefrontal intensity levels, and these associations were more pronounced in women. These findings suggested that avoidance appears to represent two different socioemotional strategies, in which women appear to activate the avoidant strategy to modulate higher emotional involvement in relationships, whereas men appeared to adopt it with a more intense emotional suppression.
Whether these differences are inherited or whether they are linked to more complex sociocultural factors associated with the social learning of stereotypical sex roles remains unclear (Lungu, Potvin, Tikàsz, & Mendrek, 2015). How sociocultural factors modulate the associations between avoidance attachment and brain activation should be explored in the future.
From a clinical perspective, the findings of this study suggested that the avoidant attachment could require different levels of attention when treating men and women, with the treatment of avoidant women focused on the regulation of emotional hyperactivation.
In conclusion, based on the present results, future research that examines the neural correlates of avoidant attachment styles should consider sex-related differences.
References
Althaus, M., Groen, Y., van der Schaft, L., Minderaa, R. B., Tucha, O., Mulder, L. J., & Wijers, A. A. (2014). Sex differences in orienting to pictures with and without humans: Evidence from the cardiac evoked response (ECR) and the cortical long latency parietal positivity (LPP) PLOS ONE, 9(10), Article e108224. https://doi.org/10.1371/journal.pone.0108224
Bachrach, N., Croon, M. A., & Bekker, M. H. (2015). The role of sex, attachment and autonomy-connectedness in personality functioning. Personality and Mental Health, 9(4), 330–344. https://doi.org/10.1002/pmh.1309
Bianchin, M., & Angrilli, A. (2012). Gender differences in emotional responses: A psychophysiological study. Physiology & Behavior, 105(4), 925–932. https://doi.org/10.1016/j.physbeh.2011.10.031
Bourisly, A. K., & Shuaib, A. (2018). Sex differences in electrophysiology: P200 event-related potential evidence. Translational Neuroscience, 9, 72–77. https://doi.org/10.1515/tnsci-2018-0013
Buchheim, A., George, C., Kächele, H., Erk, S., & Walter, H. (2006). Measuring adult attachment representation in an fMRI environment: Concepts and assessment. Psychopathology, 39(3), 136–143. https://doi.org/10.1159/000091799
Buck, R. (1977). Nonverbal communication of affect in preschool children: Relationships with personality and skin conductance. Journal of Personality and Social Psychology, 35(4), 225. https://doi.org/10.1037/0022-3514.35.4.225
Buck, R. (1991). Temperament, social skills, and the communication of emotion. In D. G. Gilbert & J. J. Connolly (Eds.), Personality, social skills, and psychopathology (pp. 85–105). Springer. https://doi.org/10.1007/978-1-4899-0635-9_4
Busonera, A., Martini, P. S., Zavattini, G. C., & Santona, A. (2014). Psychometric properties of an Italian version of the Experiences in Close Relationships–Revised (ECR-R) scale. Psychological Reports, 114(3), 785–801. https://doi.org/10.2466/03.21.PR0.114k23w9
Cecchini, M., Aceto, P., Altavilla, D., Palumbo, L., & Lai, C. (2013). The role of the eyes in processing an intact face and its scrambled image: A dense array ERP and low-resolution electromagnetic tomography (sLORETA) study. Social Neuroscience, 8(4), 314–325. https://doi.org/10.1080/17470919.2013.797020
Cecchini, M., Iannoni, M. E., Pandolfo, A. L., Aceto, P., & Lai, C. (2015). Attachment style dimensions are associated with brain activity in response to gaze interaction. Social Neuroscience, 10(3), 282–93. https://doi.org/10.1080/17470919.2014.998344
Chaplin, T. M. (2015). Gender and emotion expression: A developmental contextual perspective. Emotion Review, 7(1), 14–21. https://doi.org/10.1177/1754073914544408
Collignon, O., Girard, S., Gosselin, F., Saint-Amour, D., Lepore, F., & Lassonde, M. (2010). Women process multisensory emotion expressions more efficiently than men. Neuropsychologia, 48(1), 220–225. https://doi.org/10.1016/j.neuropsychologia.2009.09.007
Collins, N. L., & Feeney, B. C. (2004). Working models of attachment shape perceptions of social support: Evidence from experimental and observational studies. Journal of Personality and Social Psychology, 87(3), Article 363e383. https://doi.org/10.1037/0022-3514.87.3.363
Dewall, C. N., Masten, C. L., Powell, C., Combs, D., Schurtz, D. R., & Eisenberger, N. I. (2012). Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience, 7(2), 184–192. https://doi.org/10.1093/scan/nsq107
Fraley, R. C. (2012). Information on the Experiences in Close Relationships–Revised (ECR-R) Adult Attachment Questionnaire. Retrieved from http://labs.psychology.illinois.edu/~rcfraley/measures/ecrr.htm
Fraley, R. C., Waller, N. G., & Brennan, K. A. (2000). An item response theory analysis of self-report measures of adult attachment. Journal of personality and social psychology, 78(2), 350. https://doi.org/10.1037/0022-3514.78.2.350
George, M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P., & Post, R. M. (1996). Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biological Psychiatry, 40(9), 859–8871. https://doi.org/10.1016/0006-3223(95)00572-2
Gillath, O., Bunge, S. A., Shaver, P. R., Wendelken, C., & Mikulincer, M. (2005). Attachment-style differences in the ability to suppress negative thoughts: Exploring the neural correlates. NeuroImage, 28(4), 835–847. https://doi.org/10.1016/j.neuroimage.2005.06.048
Guimond, S., Chatard, A., Martinot, D., Crisp, R. J., & Redersdorff, S. (2006). Social comparison, self-stereotyping, and gender differences in self-construals. Journal of Personality and Social Psychology, 90(2), 221. https://doi.org/10.1037/0022-3514.90.2.221
Groen, Y., Wijers, A. A., Tucha, O., & Althaus, M. (2013). Are there sex differences in ERPs related to processing empathy-evoking pictures?. Neuropsychologia, 51(1), 142–155. https://doi.org/10.1016/j.neuropsychologia.2012.11.012
Hampson, E., van Anders, S. M., & Mullin, L. I. (2006). A female advantage in the recognition of emotional facial expressions: Test of an evolutionary hypothesis. Evolution and Human Behavior, 27(6), 401–416. https://doi.org/10.1016/j.evolhumbehav.2006.05.002
Hazan, C., & Shaver, P. (1987). Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology, 52(3), 511–524. https://doi.org/10.1037/0022-3514.52.3.511
Krahe, C., Drabek, M. M., Paloyelis, Y., & Fotopoulou, A. (2016). Affective touch and attachment style modulate pain: A laser-evoked potentials study. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708). https://doi.org/10.1098/rstb.2016.0009.
Krahe, C., Paloyelis, Y., Condon, H., Jenkinson, P. M., Williams, S. C. R., & Fotopoulou, A. (2015). Attachment style moderates partner presence effects on pain: A laser-evoked potentials study. Social Cognitive and Affective Neuroscience, 10(8), Article 1030e1037. https://doi.org/10.1093/scan/nsu156
Kret, M. E., & De Gelder, B. (2012). A review on sex differences in processing emotional signals. Neuropsychologia, 50(7), 1211–1221. https://doi.org/10.1016/j.neuropsychologia.2011.12.022
Lai, C., Altavilla, D., Mazza, M., Scappaticci, S., Tambelli, R., Aceto, P., Luciani, M., Corvino, S., Martinelli, D., Alimonti, F., & Tonioni, F. (2017). Neural correlate of Internet use in patients undergoing psychological treatment for Internet addiction. Journal of Mental Health, 26(3), 276–282. https://doi.org/10.1080/09638237.2017.1294745
Lai, C., Altavilla, D., Ronconi, A., & Aceto, P. (2016). Fear of missing out (FOMO) is associated with activation of the right middle temporal gyrus during inclusion social cue. Computers in Human Behavior, 61, 516–521. https://doi.org/10.1016/j.chb.2016.03.072
Lai, C., Luciani, M., Di Giorgio, C., Fiorini, R., Yaya, G., Pellicano, G. R., Mazza, M., Altavilla, D., & Aceto, P. (2018). Brain functional connectivity of meaning attribution in patients with psychosis: Preliminary electroencephalographic observations. Schizophrenia Research, 199, 449–451. https://doi.org/10.1016/j.schres.2018.04.005
Lai, C., Pellicano, G. R., Altavilla, D., Proietti, A., Lucarelli, G., Massaro, G., Luciani, M., & Aceto, P. (2018). Violence in video game produces a lower activation of limbic and temporal areas in response to social inclusion images. Cognitive, Affective, & Behavioral Neuroscience, 1–12. Advance online publication. https://doi.org/10.3758/s13415-018-00683-y
Lai, C., Pellicano, G. R., Ciacchella, C., Guidobaldi, L., Altavilla, D., Cecchini, M., Begotaraj, E., Aceto, P., & Luciani, M. (2020). Neurophysiological correlates of emotional face perception consciousness. Neuropsychologia, 146, Article 107554. https://doi.org/10.1016/j.neuropsychologia.2020.107554
Lancaster, J. L., Woldorff, M. G., Parsons, L. M., Liotti, M., Freitas, C. S., Rainey, L., Kochunov, P. V., Nickerson, D., Mikiten, S. A., & Fox, P. T. (2000). Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping, 10(3), 120–131. https://doi.org/10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual (Technical Report A-8). Gainesville.
Lee, K. H., & Siegle, G. J. (2009). Common and distinct brain networks underlying explicit emotional evaluation: A meta-analytic study. Social Cognitive and Affective Neuroscience, 7(5), 521–534. https://doi.org/10.1093/scan/nsp001
Levenson, R. W., Carstensen, L. L., & Gottman, J. M. (1994). Influence of age and gender on affect, physiology, and their interrelations: A study of long-term marriages. Journal of Personality and Social Psychology, 67(1), 56. https://doi.org/10.1037/0022-3514.67.1.56
Li, T., & Fung, H. H. (2014). How avoidant attachment influences subjective well-being: An investigation about the age and gender differences. Aging & Mental Health, 18(1), 4–10. https://doi.org/10.1080/13607863.2013.775639
Long, M., Verbeke, W., Ein-Dor, T., & Vrtička, P. (2020). A functional neuro-anatomical model of human attachment (NAMA): Insights from first-and second-person social neuroscience. Cortex, 126, 281–321. https://doi.org/10.1016/j.cortex.2020.01.010
Luan, J., Yao, Z., & Bai, Y. (2017). How social ties influence consumer: Evidence from event-related potentials. PLOS ONE, 12(1), Article e0169508. https://doi.org/10.1371/journal.pone.0169508
Luciani, M., Cecchini, M., Altavilla, D., Palumbo, L., Aceto, P., Ruggeri, G., Vecchio, F., & Lai, C. (2014). Neural correlate of the projection of mental states on the not-structured visual stimuli. Neuroscience Letters, 573, 24–29. https://doi.org/10.1016/j.neulet.2014.05.008
Lungu, O., Potvin, S., Tikàsz, A., & Mendrek, A. (2015). Sex differences in effective fronto-limbic connectivity during negative emotion processing. Psychoneuroendocrinology, 62, 180–188. https://doi.org/10.1016/j.psyneuen.2015.08.012
Main, M., Kaplan, N., & Cassidy, J. (1985). Security in infancy, childhood, and adulthood: A move to the level of representation. In I. Bretherton & E. Waters (Eds.), Growing points in attachment theory and research: Monographs of the Society for Research in Child Development, 50 (1/2, Serial No. 209), 66–106. https://doi.org/10.2307/3333824
Massaro, G., Altavilla, D., Aceto, P., Pellicano, G. R., Lucarelli, G., Luciani, M., & Lai, C. (2018). Neurophysiological correlates of collective trauma recall in 2009 L'Aquila earthquake survivors. Journal of Traumatic Stress, 31(5), 687–697. https://doi.org/10.1002/jts.22334
Mikulincer, M., & Shaver, P. R. (2003). The attachment behavioral system in adulthood: Activation, psychodynamics, and interpersonal processes. In M. P. Zanna (Ed.), Advances in experimental social psychology (Vol. 35, pp. 53–152). Elsevier. https://doi.org/10.1016/S0065-2601(03)01002-5
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl. D), 5–12.
Picardi, A., Vermigli, P., Toni, A., D’amico, R., Bitetti, D., & Pasquini, P. (2002). Il questionario “Experiences in Close Relationships” (ECR) per la valutazione dell’attaccamento negli adulti: ampliamento delle evidenze di validità per la versione italiana [The “Experiences in Close Relationships” (ECR) questionnaire for the assessment of attachment in adults: Expansion of evidence of validity for the Italian version]. Giornale Italiano di Psicopatologia, 8(3), 282–294. http://www.cnr.it/prodotto/i/69186
Picton, T. W., Bentin, S., Berg, P., Donchin, E., Hillyard, S. A., Johnson, R., Miller, G. A., Ritter, W., Ruchkin, D. S., Rugg, M. D., & Taylor, M. J. (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(2), 127–152. https://doi.org/10.1111/1469-8986.3720127
Piefke, M., Weiss, P. H., Markowitsch, H. J., & Fink, G. R. (2005). Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Human Brain Mapping, 24(4), 313–324. https://doi.org/10.1002/hbm.20092
Proverbio, A. M., Adorni, R., Zani, A., & Trestianu, L. (2009). Sex differences in the brain response to affective scenes with or without humans. Neuropsychologia, 47(12), 2374–2388. https://doi.org/10.1016/j.neuropsychologia.2008.10.030
Proverbio, A. M., Zani, A., & Adorni, R. (2008). Neural markers of a greater female responsiveness to social stimuli. BMC Neuroscience, 9(56). https://doi.org/10.1186/1471-2202-9-56
Radmacher, K., & Azmitia, M. (2006). Are there gendered pathways to intimacy in early adolescents’ and emerging adults’ friendships?. Journal of Adolescent Research, 21(4), 415–448. https://doi.org/10.1177/0743558406287402
Ran, G., & Zhang, Q. (2018). The neural correlates of attachment style during emotional processing: An activation likelihood estimation meta-analysis. Attachment & Human Development, 20(6), 626–633. https://doi.org/10.1080/14616734.2018.1465105
Ratliff, K. A., & Oishi, S. (2013). Gender differences in implicit self-esteem following a romantic partner’s success or failure. Journal of Personality and Social Psychology, 105(4), 688. https://doi.org/10.1037/a0033769
Sander, K., Frome, Y., & Scheich, H. (2007). FMRI activations of amygdala, cingulate cortex, and auditory cortex by infant laughing and crying. Human Brain Mapping, 28(10), 1007–1022. https://doi.org/10.1002/hbm.20333
Schreiter, M. L., Chmielewski, W. X., & Beste, C. (2018). How socioemotional setting modulates late-stage conflict resolution processes in the lateral prefrontal cortex. Cognitive, Affective, & Behavioral Neuroscience, 18(3), 521–535. https://doi.org/10.3758/s13415-018-0585-5
Stevens, J. S., & Hamann, S. (2012). Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia, 50(7), 1578–1593. https://doi.org/10.1016/j.neuropsychologia.2012.03.011
Strathearn, L., Fonagy, P., Amico, J., & Montague, P. R. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34(13), 2655–2666. https://doi.org/10.1038/npp.2009.103
Tamres, L. K., Janicki, D., & Helgeson, V. S. (2002). Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personality and Social Psychology Review, 6(1), 2–30. https://doi.org/10.1207/S15327957PSPR0601_1
Tanner, D., Morgan-Short, K., & Luck, S. J. (2015). How inappropriate high-pass filters can produce artifactual effects and incorrect conclusions in ERP studies of language and cognition. Psychophysiology, 52, 997–1009. https://doi.org/10.1111/psyp.12437
Vrtička, P., Andersson, F., Grandjean, D., Sander, D., & Vuilleumier, P. (2008). Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLOS ONE, 3(8), Article e2868. https://doi.org/10.1371/journal.pone.0002868
Vrtička, P., Bomdolfi, G., Sander, D., & Vuilleumier, P. (2012). The neuronal substrates of social emotion perception and regulation are modulated by adult attachment style. Social Neuroscience, 7(5), 473–493. https://doi.org/10.1080/17470919.2011.647410
Vrtička, P., & Vuilleumier, P. (2012). Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience, 6(212). https://doi.org/10.3389/fnhum.2012.00212
White, L. O., Wu, J., Borelli, J. L., Rutherford, H. J., David, D. H., Kim-Cohen, J., Mayes, L. C., & Crowley, M. J. (2012). Attachment dismissal predicts frontal slow-wave ERPs during rejection by unfamiliar peers. Emotion, 12(4), 690–700. https://doi.org/10.1037/a0026750
Zayas, V., Shoda, Y., Mischel, W., Osterhout, L., & Takahashi, M. (2009). Neural responses to partner rejection cues. Psychological Science, 20(7), 813–821. https://doi.org/10.1111/j.1467-9280.2009.02373.x
Acknowledgements
We thank Lisa Giles, from Blue Pencil Science (http://www.bluepencilscience.com/) for editing an English draft of this manuscript. The project was funded by Ateneo Sapienza 2013 and 2016.
Funding
Open Access funding provided by Università degli Studi di Roma La Sapienza.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of interest statement
The authors have no conflicts of interest to declare.
Additional information
Open practices statements
The data and materials for all experiments are available and may be requested by mail.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 65 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altavilla, D., Ciacchella, C., Pellicano, G.R. et al. Neural correlates of sex-related differences in attachment dimensions. Cogn Affect Behav Neurosci 21, 191–211 (2021). https://doi.org/10.3758/s13415-020-00859-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-020-00859-5