Abstract
To investigate the effects of the Dejian mind-body intervention (DMBI), on depressive symptoms and electroencephalography (EEG) changes in relation to emotional processing in patients with depression. Seventy-five age-, gender-, and education-matched participants with depression were randomly assigned to receive either Cognitive Behavior Therapy (CBT) or DMBI or were placed in a control group. Overall depressive syndrome, specific mood-related symptoms (Hamilton Psychiatric Rating Scale for Depression, Beck Depression Inventory), and EEG data were collected individually during a resting state and during affective image viewing before and after 10 weeks of intervention. After intervention, both the DMBI and CBT groups showed significantly reduced levels of overall depressive syndrome and mood-related symptoms (Ps ≤ 0.002) than the control group. In addition, the DMBI group demonstrated a significantly greater extent of elevation in fronto-posterior EEG theta coherence on the right hemisphere when viewing different mood-induction (neutral, positive, and negative) stimuli than the CBT and control groups (Ps < 0.03). The elevated intra-right fronto-posterior coherence when viewing mood-induction stimuli correlated with improved mood levels after the intervention (Ps < 0.05). Our findings also showed that, only in the DMBI group, there was a significant suppression of theta source activity at the posterior and subcortical brain regions that are known to mediate negative emotional responses and the self-absorbed mode of thinking. The findings of reduced depressive symptoms and elevated frontoposterior coherence suggest that the DMBI can enhance emotional control in depression.
Similar content being viewed by others
Introduction
Excessive sensitivity and over-reactiveness to the environment in a negative manner coupled with the perseveration of negative and self-absorbed thoughts is a common cognitive-emotional pattern of patients with depression (Samson et al., 2011). Primary depression is not easily treated. A recent review of depression reported that the therapeutic effect of a combination of pharmacological and psychological treatments was significantly greater than that of pharmacological intervention alone (Cuijpers, Van Straten, Andersson, & Van Oppen, 2008). One emerging line of research has documented the positive therapeutic effects of lifestyle interventions on improving the health and well-being of patients with depression (Kushner & Mechanick, 2015). Lifestyle medicine is a branch of medicine that aims to reverse pathology and/or delay disease progression by modifying high-risk health habits, such as nutrition, physical inactivity, and chronic stress. Recently, a Chinese mindfulness-based lifestyle intervention named the Dejian mind-body intervention (DMBI) has received increasing interest in clinical settings for the treatment of patients with various psychological problems and cognitive dysfunctions, including depression (Chan et al., 2012a), autism (Chan, Sze, Siu, Lau, & Cheung, 2013b), and epilepsy (Chan, Sze, Cheung, Lam, & Shi, 2009).
The DMBI adopts a holistic approach that consists of three integrative treatment components: 1) psychological education; 2) mind-body exercises; and 3) dietary modification (Chan, Sze, Woo, & Yu, 2014). Recent empirical evidence has demonstrated that this mind-body practice has beneficial effects on mood and cognitive problems related to depression. For example, the results of a randomized, controlled study showed that DMBI could elevate mood in a group of community-dwelling adults with depressive mood (Chan, Cheung, Tsui, Sze, Shi, et al., 2011a). Furthermore, the mind-body intervention was found to be effective at reducing the overall depressive syndrome and improving depression-related symptoms, including attention deficits and sleep disturbances, of patients diagnosed with major depressive disorder (Chan, Han, Sze, Wong, & Cheung, 2013a; Chan et al., 2012b). Although the aforementioned studies on the mindfulness-based intervention are so far encouraging, it has not been conclusive. It is largely unknown how the lifestyle interventions based around mindfulness affect the neural networks in patients with major depression. Recent studies have shown that alterations in brain functions may play a role in depression (Samson et al., 2011).
Emotion involves a network of cortical-subcortical systems, including the ventral striatum/accumbens, septum, hippocampus, hypothalamus, and brainstem, which interlocks with perception, cognition, motivation, and action (Pessoa, 2017). In turn, brain areas involved in attention, working memory, and executive control, such as the lateral prefrontal cortex and supplementary motor area contribute to the regulation of emotion (Frank et al., 2014; Kohn et al., 2014; Okon-Singer, Hendler, Pessoa, & Shackman, 2015). Given that mental processes involve massive exchanges of information between pertinent brain areas, it is reasonable to posit that aberrant interactions between brain areas may be one of the primary deficits that underlies the symptom profiles of depression (Lee, Wu, Yu, Chen, & Chen, 2011; Phillips, Ladouceur, & Drevets, 2008). Indeed, increasing evidence has suggested that disordered functional connectivity between frontal and posterior brain areas of the emotional network (Isaac & Bayley, 2012) may play an important role in the development and maintenance of depressive symptomology, including impairments in emotional perception, experience, and regulation. For example, EEG studies have reported that decoupling or decreased connectivity of prefrontal-posterior brain regions is associated with individual differences in the behavioral traits of absorption and the propensity to ruminate (Reiser et al., 2012), such that individuals with higher score in these negative personality traits had lower coupling of their frontal-posterior brain regions. In addition, neuroimaging studies assessing the regulatory influence of the mood-regulating circuit in response to negative stimuli have shown a reduced level of interaction between the frontal and posterior regions in depressed patients relative to healthy controls (Anand et al., 2005; Brody et al., 2001), further suggesting that decreased frontoposterior connectivity is associated with impaired emotional regulation in response to the negative stimulus of depression.
Apart from the disordered functional connectivity between frontal and posterior cortical regions of the emotional network, a growing body of evidence has established that the posterior sensory regions, in particular the right parietal area, also play an important role in the modulation of emotional processing (Aftanas, Varlamov, Pavlov, Makhnev, & Reva, 2001; Davidson, Putnam, & Larson, 2000). For example, a boost in electromagnetic and blood flow responses of the posterior sensory brain regions have been demonstrated in response to emotional stimuli (Vuilleumier & Driver, 2007). In addition, a neurophysiological study has reported that the severity of depression was related to abnormal metabolism and blood flow in the temporo-parietal cortex (Kimbrell et al., 2002b; Sackeim et al., 1990), whereas a neuroimaging study has demonstrated heightened responsiveness of brain areas involved in emotional processing to affective material in patients with depression (Brody et al., 2001; Hamilton & Gotlib, 2008). Together, these findings suggest that both the posterior sensory region and the functional connectivity of the frontal and posterior brain regions are critically involved in the modulation of emotional arousal.
Given that the reported abnormality of functional brain connectivity and amplified activation of the posterior sensory regions are implicated in the dysfunctional modulation of the impact of social-emotional information on depressed patients, it is conceivable that the documented positive effects of DMBI are associated with alterations in these neurophysiological mechanisms that underlie the impairments in emotional processing in patients with major depressive disorder. The present study thus aimed to evaluate the effect of the DMBI on mood and its modulating effect on the neural networks, as measured by EEG, in patients with major depressive disorder. We hypothesized that compared with baseline, DMBI would result in 1) reduced depressive symptoms; 2) increased neural connectivity between the frontal and posterior brain regions over the right hemisphere; and 3) an overall reduction in the arousal level of the posterior sensory regions when confronted with social-emotional information during an affective image viewing task. Specifically, we aimed to examine whether the intervention effects on neural activity was specific to negative picture viewing, or if the effects would also exist when viewing neutral and positive affective images.
Methods
Participants
Seventy-five adults from the outpatient clinic at the West Kowloon Psychiatric Centre voluntarily participated in the study. All participants had a primary diagnosis of major depressive disorder made by a clinical psychologist based on the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders-IV-TR (Segal, 2010) and the Chinese-bilingual version of the Structural Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV (Chinese-bilingual SCID-I/P version) (So et al., 2003). None of the included participants had a positive history of head injury, seizure, stroke, other central nervous system diseases, other comorbid psychiatric illnesses, or reports of strong suicidal ideation. Only participants who had a diagnosis of major depressive disorder were included in the study. Throughout the study, all participants continued their regular follow-up care, including psychiatric consultation, and schedule of antidepressant medications. All the participants were taking antidepressants, and none of their medications were modified during the study period.
An independent medical professional who was blinded to the experimental design randomly assigned the participants to the cognitive behavioural therapy (CBT), DMBI, or control groups using a computer-generated random number list. Patients who were eligible to participate in the study and who had signed informed consent were informed about their group allocation via a phone call from the medical professional. However, they were blinded to the potential benefits of the two intervention techniques. The group allocation information was given to the clinical psychologists to administer the appropriate intervention. To be included in the final analyses, participants were required to attend at least 70% of the intervention sessions and complete all assessments.
Sample size determination
The required sample size for the present study was based on our previous study of the effects of the DMBI in community-dwelling adults with depressive mood (Chan, Cheung, Tsui, Sze, & Shi, 2011b), which indicated a mean effect size (Cohen’s f) of 0.425 when the DMBI group was compared with the CBT group to reduce depressive mood. Power calculations indicated that a sample of 57 subjects (19 subjects per group) would provide an 80% (β = 0.20) chance of detecting a significant group difference (α = 0.05) given that a real group difference existed. To allow for a 30% attrition rate, 75 subjects (25 subjects per group) were recruited in the present study.
Interventions
Both treatment groups underwent 10 weekly 90-minute group training sessions. The control group continued their follow-up care but they did not receive any psychological intervention during the study period. The two intervention groups were designed to have similar structures and formats with respect to group size, the venue of intervention, the duration and frequency of the sessions, didactic teaching on the fundamental principles and corresponding techniques, in-session sharing, and home allocation. The CBT group was ran by a clinical psychologist with more than ten years of clinical experience in regularly conducting this type of group training in a hospital. Another clinical psychologist who also had more than ten years of clinical experience and who developed the DMBI ran the group.
Dejian mind-body intervention
The DMBI is an integrative lifestyle intervention for the mind and body. The goal of the intervention is to alleviate psychological distress and physical health problems by understanding the root of the problems in accordance with Buddhist philosophy, practising Nei Gong (a form of mind-body exercise) and modifying dietary habits. The details of the intervention regime have been described in our previous studies (Chan et al., 2012a), and a demonstration of the mind-body exercises can be found at www.neuropsywellbeing.com. Briefly, a key aspect of the DMBI is that individuals are encouraged to execute their treatment regime naturally in adherence with their own lifestyle and to feel the change that occurs in their minds and bodies by modifying their thoughts, diet, and exercise habits.
Cognitive behavioural therapy
The protocol included the typical components of CBT and began with psychoeducation on the biopsychosocial model of depression and the triadic relationship between mood state, cognition, and behaviour (Bieling, McCabe, & Antony, 2009). Through the introduction of the triadic relationship, the participants were guided to positively modify their cognitive processes and behaviour to trigger a positive change in mood. Participants were guided to challenge their maladaptive thought patterns and beliefs (e.g., overgeneralizing, magnifying negatives and minimizing positives, catastrophizing) and attempt to think realistically and effectively to reduce emotional distress and self-defeating behaviours. Furthermore, the participants were taught to practice progressive muscle relaxation as a means of inducing a relaxed state of mind (Gaylord, Orme-Johnson, & Travis, 1989).
Data collection
Each participant was individually assessed by a psychiatrist and a trained research assistant with regard to his/her medication record, depression-related symptoms, and EEG activity before and at the end of the 10-week intervention programme. The psychiatrist and the research assistant who conducted the assessment procedures were blinded to the group allocation of each participant. The primary outcome measures were the participants’ moods between the pre- and post-assessments. The participants’ overall depressive syndrome was evaluated according to the 17-item Hamilton Psychiatric Rating Scale for Depression (Hamilton, 1967). The maximum possible score of the Hamilton Psychiatric Rating Scale for Depression is 52, with higher scores indicating a greater degree of depression. In addition to the total sum of the scores, the global depression subscale score was computed as a measure of mood-related symptoms. The global depression subscale consists of eight mood-related items and was adopted based on the five-factor model (Gibbons, Clark, & Kupfer, 1993). The participants’ severity of depression was also measured using the 21-item Chinese version of the Beck Depression Inventory-II (Chang, 2007), with higher scores indicating a more severe degree of depression. The possible maximum score was 63. The cognitive-affective subscale score was computed based on the two-factor model (Storch, Roberti, Roth, & anxiety, 2004) as a measure of mood-related symptoms. While the Hamilton Psychiatric Rating Scale for Depression was rated by the psychiatrists who were blinded to the group allocation, the Beck Depression Inventory-II was rated by the participants themselves. Moreover, the scores obtained from the Hamilton Psychiatric Rating Scale for Depression and the Beck Depression Inventory-II were used only as supporting information and not as part of the screening criteria in the recruitment process.
The secondary outcomes were the treatment effects on the participants’ neurophysiological responses underlying emotional processing. EEG coherence is commonly used to assess the cortical connectivity between brain regions in response to cognitive and affective processes (Rippon, Brock, Brown, & Boucher, 2007). EEG coherence measures the neural synchrony between two brain areas in terms of the EEG signals that are recorded at different sites of the scalp (Nunez & Srinivasan, 2006). High EEG coherence is indicative of a high level of coupling between two brain areas, and low EEG coherence is suggestive of a low level of coupling or decoupling of the brain regions (Murias, Webb, Greenson, & Dawson, 2007). In addition to the level of synchronicity, different EEG frequencies have been shown to correlate with different cognitive processes (Rippon et al., 2007). EEG studies have widely reported that the theta band (4-7.5 Hz) is involved with the modulation of emotional arousal (Aftanas et al., 2001; Knyazev & Slobodskoy-Plusnin, 2009). Hence, EEG data were collected from each participant during an eyes-closed resting condition, followed by three affective image viewing conditions in a sound- and light-attenuated room. EEG activity was recorded using the Deymed TruScan measuring set with 19 electrodes positioned according to the International 10-20 System (Jasper, 1958). Because decreased frontoposterior connectivity and hypofunction of the right cortex have been reported to be associated with impaired emotional regulation in depression (Moratti, Rubio, Campo, Keil, & Ortiz, 2008; Reiser et al., 2012), the intra-right frontoposterior coherence will be our region of interest in the present study. Coherence values on the left and the right hemispheres in the theta band (4-7.5 Hz) were computed to reflect the level of functional connectivity between the frontal and posterior brain regions during the affective image viewing tasks. EEG theta coherence connecting the frontal (F3, F4, F7, F8) and posterior (P3, P4, O1, O2) scalp regions was measured and clustered into left and the right frontoposterior coherence pairs (i.e., left-frontal-to-left-posterior regions, and right-frontal-to-right-posterior regions).
For the different affective image viewing conditions, the participants were required to focus their attention on three sets of coloured pictures that varied in emotional arousal and valence and were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2008). The three sets of pictures consisted of exemplars from three emotion categories, namely, neutral, positive, and negative. Three blocks of 20 pictures from each category were displayed, in the sequence of neutral, positive, and negative, on a monitor screen with a 10-second interstimulus interval. Neutral pictures depicted household objects, transportation, people, and animals in mundane contexts. Positive pictures depicted scenes of happiness, achievement, interpersonal bonding, and charming animals and children. Negative pictures depicted scenes of disgusting pests, interpersonal attacks, mutilation, sickness, and death. EEG data were captured during each block of image viewing and then visually scanned for artefacts due to eye blinks and body movements, which were edited using Neuroguide v.2.9.1. Artefact-free EEG signals were fast Fourier transformed, and the power spectrum at the theta frequency band (4-7.5 Hz) was extracted for subsequent coherence analyses and source localization.
To localize the sources of the EEG theta activity in response to the treatment conditions, standardized low-resolution brain electromagnetic tomography (sLORETA) was employed to analyse the current density (the source activity of the scalp EEG signals) in the present study. sLORETA is a standardized inverse solution that computes the three-dimensional cortical distribution of the electric neuronal source activity from scalp EEG measurements to yield images of standardized current density with exact and zero-error localization (Pascual-Marqui, 2002). The computations of sLORETA were based on a realistic head model (Fuchs et al., 2002) using the Montreal Neurological Institute (MNI) 152 template (Mazziotta et al., 2001), with the three-dimensional solution space restricted to cortical gray matter, as determined by the probabilistic Talairach atlas (Lancaster et al., 2000). The intracerebral volume was partitioned in 6239 voxels (voxel size: 5 mm x 5 mm x 5 mm). sLORETA images represented the standardized electric activity at each voxel in neuroanatomic MNI space as the exact magnitude of the estimated current density. Anatomical labels as Brodmann areas were reported using MNI space, with correction to Talairach space (Brett et al., 2002). sLORETA has been validated against fMRI and well documented to provide accurate estimations of the potential sources of the scalp EEG (Dümpelmann, Ball, & Schulze-Bonhage, 2012). The neural networks involved in emotional processing, based on findings of past neuroimaging studies, have been shown to link to the prefrontal cortex, the parietal cortex, and the well-documented emotional centre of the limbic lobe and insular cortex (Isaac & Bayley, 2012; Sliz & Hayley, 2012; Zhang, Peng, Sweeney, Jia, & Gong, 2018). Therefore, in the present study, these brain structures were selected as the regions of interest (ROIs) for sLORETA statistical comparison.
Data analysis
Baseline group differences in demographic and clinical characteristics were explored with ANOVAs and chi-squared tests. Yates’s continuity correction was applied if more than 20% of the cells had expected counts less than 5. The Shapiro-Wilks test was not significant (Ps < 0.05), indicating normal distribution for most of the dependent variables of the mood and EEG data. Therefore, ANOVA and t-tests were performed to compare the changes in depressive symptoms and EEG coherence between the CBT, DMBI, and control groups. The main analyses of the effects of the interventions on mood measures (i.e., the total and the two subscale scores of the Hamilton Psychiatric Rating Scale for Depression and the Beck Depression Inventory-II) were performed using a mixed ANOVA with time (pre-, post-assessments) as the within-subjects factor and group (controls, CBT, DMBI) as the between-subjects factor. This was followed by post hoc paired t tests in each condition. In addition, a four-way (time x coherence pair x group x condition) mixed-design ANOVA was performed to analyse the effects of the interventions on theta coherence. Also, a repeated ANOVA was performed on theta coherence to examine the pattern of changes before and after intervention within the three groups of participants. Because specific hypotheses were tested and the number of comparisons was large within a relatively small group of participants, the t test was adopted without adjustments to the alpha level to avoid lowering the power of the tests. Effect sizes (Cohen’s d statistic) were calculated to evaluate the degree of change from baseline to post-intervention. Any between-group differences were analysed using ANOVAs or independent-sample t tests.
In addition, Pearson’s correlations (two-tailed) were calculated to explore possible associations between the pre-post changes in EEG coherence during picture viewing and mood-related measures post-assessment. Specifically, Pearson’s correlations between the total scores of the Hamilton Psychiatric Rating Scale for Depression and the Beck Depression Inventory-II at post-assessment and the frontoposterior theta coherence pair on the right hemisphere that demonstrated significant increases during picture viewing (see Results) were calculated. Furthermore, sLORETA using voxel-by-voxel paired-sample t tests, which adopts a nonparametric permutation approach to estimate, via randomization, the empirical probability distribution for the max-statistics, were performed to compare the pre-post changes in the theta current density level for each group.
Results
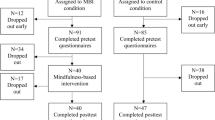
Study enrolment is shown in the flow chart (Fig. 1). The demographic and clinical characteristics of the participants before intervention are presented in Table 1. Three participants scored within the normal range in either or both the Hamilton Psychiatric Rating Scale for Depression and the Beck Depression Inventory-II. ANOVA and chi-squared tests showed no significant group differences in age, years of education, or gender (Ps > 0.19). In addition, there were no significant group differences in the aspects of depression severity assessed by the Structural Clinical Interview-based diagnoses, onset age, illness duration, course of illness, total score on the Hamilton Psychiatric Rating Scale for Depression, and the Beck Depression Inventory-II at baseline (Ps > 0.42).
Treatment effects on overall depressive syndrome and specific mood-related symptoms
The results of mixed ANOVA showed a significant group × time interaction in the cognitive-affective subscale score of the Beck Depression Inventory (F(2, 43) = 3.32, P = 0.046). There also was a trend toward significance for the group × time interaction in the total scores of the Beck Depression Inventory-II (F(2, 43) = 2.69, P = 0.079) and the Hamilton Psychiatric Rating Scale for Depression (F(2, 45) = 2.44, P = 0.099). There was no significant group x time interaction effect in the subscale score of the Hamilton Psychiatric Rating Scale for Depression. In addition, there was a significant main effect of time for all measures for overall depressive syndrome (Ps < 0.001). The post hoc paired t test results showed that participants from both the CBT and the DMBI groups exhibited a significant reduction in all scores on the Hamilton Psychiatric Rating Scale for Depression and the Beck Depression Inventory-II (Table 2) (Ps ≤ 0.008). These results suggest reduced levels of overall depressive syndrome and mood-related symptoms after the CBT or DMBI. Regarding the percentage change, the CBT group showed a 40.5% to 50.3% reduction in overall depressive syndrome and a 50.4% to 53.2% reduction in mood-related symptoms, whereas the DMBI group showed a 36.6% to 49.3% reduction in overall depressive syndrome and a 37.9% to 51.5% reduction in mood-related symptoms.
While patients in the control group showed a similar yet smaller reduction in the total score of the Beck Depression Inventory-II (P = 0.045), they exhibited no significant reduction in the total score of the Hamilton Psychiatric Rating Scale for Depression (P = 0.20). Similarly, while patients in the control group showed a similar reduction in the global depression subscale score of the Hamilton Psychiatric Rating Scale for Depression (P = 0.002), they exhibited no significant reduction in the cognitive-affective subscale score of the Beck Depression Inventory (P = 0.094). With respect to the percentage change, the control group showed an 8.1% to 17.5% reduction in overall depressive syndrome and a 7.3% to 36.5% reduction in mood-related symptoms. The percentage changes of both overall depressive syndrome and specific mood-related symptoms in the control group were smaller than those in the CBT and the DMBI groups.
The ANOVA results showed a significant difference in the cognitive-affective subscale score of the Beck Depression Inventory (P = 0.046). There was also a trend towards significance in the total scores of the Beck Depression Inventory-II (P = 0.079) and the Hamilton Psychiatric Rating Scale for Depression (P = 0.099). Planned independent-sample t tests revealed that there were larger pre-post changes in the total and global depression subscale scores of the Beck Depression Inventory-II in the DMBI group than in the control group (Ps ≤ 0.066). There also were larger pre-post changes in the total scores of the Beck Depression Inventory and the Hamilton Psychiatric Rating Scale for Depression and in the global depression subscale score of the Beck Depression Inventory in the CBT group than in the control group (Ps < 0.046). No significant difference was found between CBT and DMBI in the total scores and the subscale scores of the Beck Depression Inventory-II and Hamilton Psychiatric Rating Scale for Depression (Ps > 0.45). Taken together, the present findings suggest that the CBT and the DMBI groups showed more marked improvements in overall depressive syndrome and specific mood-related symptoms than the control group.
Treatment effects on state-specific frontoposterior coherence during affective picture viewing
At baseline, the three groups showed comparable levels of intra-hemispheric theta coherence, p > 0.05. Three-way (Time x Hemisphere x Condition) repeated measures ANOVAs were performed to explore the pattern of changes before and after intervention across the left and right hemispheres of the three affective viewing conditions for the CBT, DMBI, and control groups. The repeated ANOVA showed no significant time × hemisphere × condition in affective picture viewing in any of the three groups (Ps > 0.094). The lack of significant interaction effects could possibly due to small sample size that might have suppressed the power of statistics. During picture viewing, there was a significant main effect of the Hemisphere across the three groups, F values range from 56.99 to 80.46, p < 0.001. There was also a significant main effect of Time in the DMBI group (F = 5.46, p = 0.035), and a significant interaction effect between Time and Condition in the CBT group (F = 5.84, p = 0.007). The results of subsequent post hoc paired t tests showed that the DMBI group demonstrated a postintervention elevation in right frontoposterior coherence regardless of the type of picture during picture viewing (Ps ≤ 0.031). The CBT group showed a significant increase over the right frontoposterior coherence pairs only when viewing negative pictures (P = 0.009), and the control group did not show any significant change during the different picture viewing conditions (Ps ≥ 0.43). There was no significant change in left frontoposterior coherence in any group across the different picture viewing conditions (Ps ≥ 0.095) (Table 3). These results suggest that only the DMBI intervention induced extensive alterations in frontoposterior functional connectivity over the right hemisphere.
To examine whether the pattern of frontoposterior coherence change observed in the DMBI group was a state-specific change during picture viewing or a general change after the treatment, the same statistical analyses were performed on the coherence pairs acquired during the eyes-closed resting state. The results showed that there were no significant changes in frontoposterior coherence in any of the three groups (Ps > 0.070), suggesting that the elevated pattern of frontoposterior coherence in the DMBI and the CBT groups was a unique change in response to affective picture viewing rather than a general change after the treatment.
Association between pre-post changes in frontoposterior theta coherence during picture viewing and depression measures after intervention
Results on the combined-group analysis showed that the total score of the Beck Depression Inventory-II at post-assessment was negatively correlated with the pre-post changes in frontoposterior coherence on the right hemisphere when viewing the three different types of mood-induction (neutral, positive, and negative) pictures (Ps ≤ 0.019). In addition, the total score of the Hamilton Psychiatric Rating Scale for Depression at post-assessment was found to correlate with the pre-post changes in frontoposterior coherence on the right hemisphere during neutral picture viewing (P ≤ 0.020) (Table 4; Fig. 2). Although the significant association between changes in the mood and EEG measures disappeared when the subgroup analyses were performed separately or combined for the DMBI and CBT groups. This lack of correlational relationship could be due to the lower power as a result of the smaller number of participants or a more restricted range of scores in the treatment groups. Furthermore, no significant correlations were found between the pre-post changes in the frontoposterior coherences and the pre-post changes of the total scores in Beck Depression Inventory-II nor Hamilton Psychiatric Rating Scale for Depression (Ps > 0.20). To further explore whether there was a relationship between the severity of depression symptoms at baseline and the change in level of depression symptoms after the intervention, the Pearson’s correlation analyses were performed for the two treatment groups. Results showed that for the participants in the DMBI and the CBT groups, there were significant correlations between the baseline total scores and the pre-post changes in Hamilton Psychiatric Rating Scale for Depression (r = 0.52, P = 0.002), as well as between the baseline total scores and the pre-post changes of the total scores in the Beck Depression Inventory-II (r = 0.60, P < 0.001). These results suggest that there was a relationship between the severity of depressive symptoms at baseline and the change in level of depressive symptoms after the intervention, and that participants with higher levels of depressive symptoms would show greater pre-post changes in Beck Depression Inventory-II and Hamilton Psychiatric Rating Scale for Depression scores than those with mild symptoms of depression. Taken together, our findings suggest that participants with a lower level of depressive symptoms after intervention had greater increases in frontoposterior theta coherence during the viewing of affective pictures, but the treatment effect may be related to the baseline levels of severity in these individuals.
Scatter diagrams of the relationships between the mood measures (Beck Depression Inventory-II: BDI-II, Hamilton Psychiatric Rating Scale for Depression: HRSD) and pre-post changes in EEG theta coherence during (a) positive, (b) negative, and (c) neutral affective picture viewing of the cognitive behaviour therapy (CBT) and Dejian mind-body intervention (DMBI) groups
Suppressed source activity over posterior and subcortical brain regions
The results of the sLORETA voxel-by-voxel paired t tests showed that after intervention, the DMBI group demonstrated a substantial reduction in the theta source activity level during picture viewing (Fig. 3). Specifically, for the DMBI group, the degree of activity suppression was higher and spread across the four regions of interest (i.e., prefrontal cortex, parietal cortex, limbic lobe, insular cortex) bilaterally when neutral pictures were viewed after intervention. During positive and negative picture viewing, theta activity subsided with a relatively greater involvement of the parietal and insular lobes of the left hemisphere. There was greater bilateral limbic activity suppression during positive picture viewing than during negative picture viewing. The regions that yielded the greatest pre-post difference were the precuneus (BA19 & 31) in the parietal lobe and the posterior cingulate cortex (BA31) in the limbic lobe. For the CBT group, there was no change in theta source activity during neutral and positive picture viewing, but there was a significant increase in theta activity in the insular cortex (BA 13) during negative picture viewing. In contrast, the control group showed significantly increased theta activity in the limbic lobe during neutral picture viewing and increased theta activity in all regions of interest, except the parietal lobe, during positive and negative picture viewing. Furthermore, there was a significant increase in theta activity in the prefrontal cortex, with relatively larger involvement of the right hemisphere when viewing affective but not neutral pictures.
Graphical representation of the LORETA t-statistics comparing the pre-post changes in theta source activity during affective picture viewing of the control, cognitive behaviour therapy (CBT), and Dejian mind-body intervention (DMBI) groups. The DMBI group demonstrated maximally suppressed theta source activity (shown in blue color) in the parietal and insula cortices, whereas the control group showed no change in the theta activity, and the CBT group showed significantly increased activity (shown in yellow) over the frontal, insula, and anterior cingulate cortices
Discussion
The main purposes of the present study were to examine the effects of a mindfulness-based mind-body intervention and to explore whether changes in the EEG pattern generated after the practice of this intervention were associated with successful treatment. Compared with the controls, participants in both the CBT and the DMBI groups showed significant improvements in their depressive symptoms, measured with the Hamilton Psychiatric Rating Scale for Depression and Beck Depression Inventory-II. Of which, both the CBT and the DMBI demonstrated similar effects on reducing clinical symptoms in medicated patients with major depression, which suggest that both psychological intervention methods can be considered as an alternative or adjunctive treatment for depression than pharmacological treatment alone.
The findings of the present study also have provided support for the two psychological interventions for modulating neural activity patterns in response to emotionally laden stimuli among patients with depression. The change in the functional connectivity of the neural system associated with the two intervention methods is in agreement with neuroimaging and neuropsychological studies, indicating that affective perception involves the coordinated activation of widely distributed cortical networks (Miskovic & Schmidt, 2010), and that depressive symptoms, such as heightened sensitivity to negative affective stimuli and self-absorption, are attributed to disordered connectivity between frontal and posterior cortices (Keil et al., 2009). Nevertheless, although both DMBI and CBT were associated with a similar pattern of enhanced frontal-posterior coherence when viewing negative pictures, only the DMBI group showed a significantly elevated state-dependent EEG coherence between the frontal and posterior cortical regions, particularly over the right hemisphere, across different mood-induction (neural, positive, and negative) conditions during the affective image viewing task. The absence of change in left frontoposterior coherence and the presence of a salient increase in right frontoposterior coherence during affective picture viewing in the two treatment groups, are in line with existing scientific evidence of the emotion-modulating role played by the functional coupling of the right frontal-posterior brain circuit in the activation, arousal and expression of emotion in depression (Aftanas et a., 2001; Lee et al., 2011; Moratti et al., 2008). Additionally, our findings also suggest that the changes in neural activity patterns generated by the CBT might be different from the neural activity changes generated by the mindfulness-based lifestyle intervention. This may be explained by the fact that the focus of CBT is on the analytical evaluation of a scenario, especially a negative one (Bieling et al., 2009), whereas the DMBI was developed based on the Chinese mindfulness philosophy, which emphasizes modest reactions to the ever-changing world based on the understanding of cause-and-effect relationships and the laws of nature (Chan et al., 2012b). One can reasonably assume that individuals who receive the DMBI would be less prone to being emotionally aroused by affective pictures of different valence.
In addition to showing that DMBI was associated with changes in functional coupling between frontal and posterior cortical regions in patients with depression, results from this study also bear on the electrophysiological changes with respect to the ROIs associated with affective perception. Our results from the EEG-based sLORETA analysis have shown that the DMBI group displayed a substantial decrease in state-dependent EEG source activity in the social-emotional network involving the prefrontal and parietal cortices, limbic lobe, and insula across the three mood-induction conditions (neutral, positive, negative) during affective picture viewing tasks. Again, such changes in the neural system were not observed in the CBT and waitlist control groups. These results support the idea that general reductions in emotional arousal also may be one of the principal psychological responses generated by DMBI. Our findings are in agreement with recent studies that reported the salient role of posterior brain areas, in particular the parietal cortex, in mediating sensory information (Miskovic & Schmidt, 2010) and may play a critical role in neuropsychological dysfunctions, including emotional arousal and emotion processing, that contribute to the maintenance of a depressive state in patients with depression (Lee et al., 2011). Indeed, neurophysiological results have shown abnormalities in glucose metabolism rate and regional cerebral blood flow to the temporo-parietal cortex in patients with major depressive disorder, and the extent of these abnormalities were associated with the severity of the depressive symptoms (Kimbrell et al., 2002a). However, this contrasts with previous studies on depression characterized by low emotional arousal (Heller and Nitschke, 1997; Moratti et al., 2015). This inconsistent finding might be attributed to cultural difference in the neural-bases underlying depression in Chinese patients with major depressive disorder. Our findings are consistent with a previous study of Chinese patients with depression that documented low emotional arousal to positive stimuli but enhanced arousal to negative stimuli in the patients with depression relative to the healthy individuals (Liu, Wang, Zhao, Ning, & Chan, 2012). Alternatively, our results also may reflect the concurrent heightened responsivity to negative stimuli associated with generalized anxiety that does not meet the diagnostic threshold but may occur as a symptom of clinical depression (Gelenberg, et al., 2010; Kemp & Felmingham, 2008; Larson, Nitschke, & Davidson, 2007). Further studies are needed to verify this speculation and to better understand the neural substrates underlying comorbidity of depression and anxiety. This limitation notwithstanding, our results support the idea that the therapeutic depression-reducing effects of DMBI are associated with a robust enhancement in frontoposterior connectivity of the emotion network as well as changes in arousal of the posterior sensory regions, which in turn allows mood improvement to occur through better coherent, reciprocal activity between posterior sensory regions and higher-order cortical structures, such as the prefrontal cortex. On the other hand, because participants in the CBT were guided to think critically about their maladaptive thoughts and beliefs, this may in turn affect the functioning of the prefrontal cortex, and an increase in source activity may result (Clark & Beck, 2010).
In summary, this study showed that the regular practice of psychological interventions, including CBT and the DMBI, could improve core depressive symptoms in patients with major depressive disorder. The beneficial effects of these psychological interventions were mediated by the enhancement of neural connectivity, involving changes in EEG theta coherence. However, decreased activity in the posterior sensory regions that are known to mediate negative emotional responses and the self-absorbed mode of thinking (Heller and Nitschke, 1997) was observed only with the practice of the DMBI. Hence, our results suggest that the different EEG characteristics were generated by the two different psychological treatment methods. While CBT showed an enhanced higher-order frontal network associated with the cognitive control of negative emotion, the DMBI not only altered the frontoposterior functional connectivity of the emotion network but also resulted in changes in the arousal of the posterior sensory regions.
Collectively, our findings are in line with the general understanding of the electrophysiological nature of psychological treatment methods in which theta EEG coherence has been associated with changes in affective processes (Aftanas et al., 2001; Reiser et al., 2012). However, some limitations should be noted. First, this study only examined the effects of 10-week DMBI and CBT programs, and their long-term effects remain uncertain. Second, the generalization of the findings may be limited due to the relatively small sample size, overrepresentation of clinically depressed patients, and loss of patients during the course of the study. Our sample is therefore subject to confounding and biases that might not reflect the general patient population with depression. Future studies that recruit a larger sample size with longitudinal follow-up and that include patients with varied severities of depressive symptoms and different clinical populations will extend the current knowledge to a wider population. Furthermore, the duration of medication taken by the patients is another limitation as the longer-term medicated patients might show a different pattern of functional connectivity from those that were taking the medications for a short period. In addition, the DMBI might be more culturally relevant and familiar among Asian ethnic groups. The applicability and effectiveness of its therapeutic effects on non-Chinese or non-Asian patients with clinical depression in the West and the effects of the duration of medication require further investigation.
Despite these limitations, the potentials of the DMBI to enhance emotional control suggest that this mind-body lifestyle intervention is clinically applicable. It also shows great potential as an adjunct therapy for treating clinical populations with depression given its cost-effectiveness and ease of learning and practice. In addition, because all participants in the current study were taking antidepressants to treat depression, this suggests that the mindfulness-based lifestyle intervention and the CBT have equivalent benefits and can be as effective, if not more so, than drug therapy alone for treating major depressive disorder.
References
Aftanas, L., Varlamov, A., Pavlov, S., Makhnev, V., & Reva, N. (2001). Affective picture processing: event-related synchronization within individually defined human theta band is modulated by valence dimension. Neuroscience letters, 303(2), 115-118.
Anand, A., Li, Y., Wang, Y., Wu, J., Gao, S., Bukhari, L., … Lowe, M. J. J. B. P. (2005). Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. 57(10), 1079-1088. Retrieved from https://www.sciencedirect.com/science/article/pii/S0006322305001861?via%3Dihub
Bieling, P. J., McCabe, R. E., & Antony, M. M. (2009). Cognitive-behavioral therapy in groups: Guilford Press.
Brett, M., Johnsrude, I. S., and Owen, A. M. (2002). The problem of functional localization in the human brain. Nat. Rev. Neurosci. 3, 243-249.
Brody, A. L., Saxena, S., Mandelkern, M. A., Fairbanks, L. A., Ho, M. L., & Baxter Jr, L. R. (2001). Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biological Psychiatry, 50(3), 171-178.
Chan, A. S., Cheung, M.-C., Tsui, W. J., Sze, S. L., & Shi, D. (2011a). Dejian mind-body intervention on depressive mood of community-dwelling adults: a randomized controlled trial. Evidence-Based Complementary and Alternative Medicine, 2011.
Chan, A. S., Cheung, M.-C., Tsui, W. J., Sze, S. L., Shi, D. J. E.-B. C., & Medicine, A. (2011b). Dejian mind-body intervention on depressive mood of community-dwelling adults: a randomized controlled trial. 2011.
Chan, A. S., Han, Y. M., Sze, S. L., Wong, Q. Y., & Cheung, M.-C. (2013a). A randomized controlled neurophysiological study of a Chinese Chan-based mind-body intervention in patients with major depressive disorder. Evidence-Based Complementary and Alternative Medicine, 2013.
Chan, A. S., Sze, S. L., Cheung, M.-C., Lam, J. M., & Shi, D. J. C. J. (2009). Dejian mind-body intervention improves the functioning of a patient with chronic epilepsy: a case report. 2(1), 9080.
Chan, A. S., Sze, S. L., Siu, N. Y., Lau, E. M., & Cheung, M.-C. J. P. O. (2013b). A Chinese mind-body exercise improves self-control of children with autism: a randomized controlled trial. 8(7), e68184. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3707921/pdf/pone.0068184.pdf
Chan, A. S., Sze, S. L., Woo, J., & Yu, R. H. J. F. I. A. N. (2014). A Chinese Chan-based lifestyle intervention improves memory of older adults. 6, 50. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972479/pdf/fnagi-06-00050.pdf
Chan, A. S., Wong, Q. Y., Sze, S. L., Kwong, P. P., Han, Y. M., & Cheung, M.-C. J. J. o. A. D. (2012a). A Chinese Chan-based mind–body intervention for patients with depression. 142(1-3), 283-289. Retrieved from https://www.sciencedirect.com/science/article/pii/S0165032712003205?via%3Dihub
Chan, A. S., Wong, Q. Y., Sze, S. L., Kwong, P. P., Han, Y. M., & Cheung, M.-C. J. T. S. W. J. (2012b). A Chinese Chan-based mind-body intervention improves sleep on patients with depression: a randomized controlled trial. 2012.
Chang, H. (2007). Depressive symptom manifestation and help-seeking among Chinese college students in Taiwan. International Journal of Psychology, 42(3), 200-206.
Clark, D. A., & Beck, A. T. J. T. i. c. s. (2010). Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. 14(9), 418-424. Retrieved from https://www.sciencedirect.com/science/article/pii/S1364661310001440?via%3Dihub
Cuijpers, P., Van Straten, A., Andersson, G., & Van Oppen, P. (2008). Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. Journal of consulting and clinical psychology, 76(6), 909.
Dümpelmann, M., Ball, T., & Schulze-Bonhage, A. J. H. b. m. (2012). sLORETA allows reliable distributed source reconstruction based on subdural strip and grid recordings. 33(5), 1172-1188. Retrieved from https://onlinelibrary.wiley.com/doi/pdf/10.1002/hbm.21276
Davidson, R. J., Putnam, K. M., & Larson, C. L. (2000). Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science, 289(5479), 591-594.
Frank, D. W., Dewitt, M., Hudgens-Haney, M., Schaeffer, D. J., Ball, B. H., Schwarz, N. F., ... Sabatinelli, D. (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews, 45, 202-211.
Fuchs, M., Kastner, J., Wagner, M., Hawes, S., and Ebersole, J.S. (2002). A standardized boundary element method volume conductor model. Clin. Neurophysiol. 113, 702-712.
Gaylord, C., Orme-Johnson, D., & Travis, F. J. I. J. o. N. (1989). The effects of the transcendental mediation technique and progressive muscle relaxation on EEG coherence, stress reactivity, and mental health in black adults. 46(1-2), 77-86.
Gelenberg, A. J., Freeman, M. P., Markowitz, J. C., Rosenbaum, J. F., Thase, M. E., Trivedi, M. H., & Silbersweig, D. A. (2010). American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry, 167(suppl).
Gibbons, R. D., Clark, D. C., & Kupfer, D. J. J. J. o. p. r. (1993). Exactly what does the Hamilton depression rating scale measure? , 27(3), 259-273. Retrieved from https://www.sciencedirect.com/science/article/pii/0022395693900373?via%3Dihub
Heller, W., & Nitscke, J. B. (1997). Regional brain activity in emotion: A framework for understanding cognition in depresion. Cognition & Emotion, 11(5-6), 637-661.
Hamilton, J. P., & Gotlib, I. H. J. B. p. (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. 63(12), 1155-1162. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2474758/pdf/nihms53679.pdf
Hamilton, M. (1967). Development of a rating scale for primary depressive illness. British journal of social and clinical psychology, 6(4), 278-296.
Isaac, L., & Bayley, P. J. (2012). EEG coherence between prefrontal and posterior cortical regions is related to negative personality traits. Frontiers in human neuroscience, 6, 269.
Jasper, H. H. J. E. C. N. (1958). The 10-20 electrode system of the International Federation. 10, 371-375.
Keil, A., Sabatinelli, D., Ding, M., Lang, P. J., Ihssen, N., & Heim, S. (2009). Re-entrant projections modulate visual cortex in affective perception: Evidence from Granger causality analysis. Human brain mapping, 30(2), 532-540.
Kemp, A. H., & Felmingham, K. L. (2008). The psychology and neuroscience of depression and anxiety: Towards an integrative model of emotion disorders. Psychology & Neuroscience, 1(2), 177.
Kimbrell, T. A., Ketter, T. A., George, M. S., Little, J. T., Benson, B. E., Willis, M. W., … Post, R. M. (2002a). Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biological Psychiatry, 51(3), 237-252.
Kimbrell, T. A., Ketter, T. A., George, M. S., Little, J. T., Benson, B. E., Willis, M. W., … Post, R. M. J. B. P. (2002b). Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. 51(3), 237-252. Retrieved from https://www.sciencedirect.com/science/article/pii/S0006322301012161?via%3Dihub
Knyazev, G., & Slobodskoy-Plusnin, J. (2009). Substance use underlying behavior: investigation of theta and high frequency oscillations in emotionally relevant situations. Clinical EEG and neuroscience, 40(1), 1-4.
Kohn, N., Eickhoff, S. B., Scheller, M., Laird, A. R., Fox, P. T., & Habel, U. (2014). Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. Neuroimage, 87, 345-355.
Kushner, R. F., & Mechanick, J. I. J. U. E. (2015). Lifestyle medicine-An emerging new discipline. 11(1), 36-40.
Lancaster, J. L., Woldorff, M. G., Parsons, L. M., Liotti, M., Freitas, C. S., Rainey, L., et al. (2000). Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 10, 120-131.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2008). International affective picture system (IAPS): affective ratings of pictures and instruction manual. University of Florida, Gainesville.
Larson, C. L., Nitschke, J. B., & Davidson, R. J. (2007). Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion, 7(1), 182.
Lee, T. W., Wu, Y. T., Yu, Y. W. Y., Chen, M. C., & Chen, T. J. (2011). The implication of functional connectivity strength in predicting treatment response of major depressive disorder: a resting EEG study. Psychiatry Research: Neuroimaging, 194(3), 372-377.
Liu, W. H., Wang, L. Z., Zhao, S. H., Ning, Y. P., & Chan, R. C. (2012). Anhedonia and emotional word memory in patients with depression. Psychiatry Research, 200(2-3), 361-367.
Mazziotta, J., Toga, A., Evans, A., Fox, P., Lancaster, J., Zilles, K., et al. (2001). A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1293-1322.
Miskovic, V., & Schmidt, L. A. (2010). Cross-regional cortical synchronization during affective image viewing. Brain research, 1362, 102-111.
Moratti, S., Rubio, G., Campo, P., Keil, A., & Ortiz, T. J. A. o. g. p. (2008). Hypofunction of right temporoparietal cortex during emotional arousal in depression. 65(5), 532-541.
Moratti, S., Strange, B., & Rubio, G. (2015). Emotional arousal modulation of right temporoparietal cortex in depression depends on parental depression status in women: First evidence. Journal of affective disorders, 178, 79-87.
Murias, M., Webb, S. J., Greenson, J., & Dawson, G. (2007). Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biological Psychiatry, 62(3), 270-273.
Nunez, P. L., & Srinivasan, R. (2006). Electric fields of the brain: the neurophysics of EEG: Oxford University Press, USA.
Okon-Singer, H., Hendler, T., Pessoa, L., & Shackman, A. J. (2015). The neurobiology of emotion–cognition interactions: fundamental questions and strategies for future research. Frontiers in human neuroscience, 9, 58.
Pascual-Marqui, R.D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24, 5-12.
Pessoa, L. (2017). A network model of the emotional brain. Trends in Cognitive Sciences, 21(5), 357-371.
Phillips, M. L., Ladouceur, C. D., & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry, 13(9), 833.
Reiser, E. M., Schulter, G., Weiss, E. M., Fink, A., Rominger, C., Papousek, I. J. B., & Cognition. (2012). Decrease of prefrontal–posterior EEG coherence: Loose control during social–emotional stimulation. 80(1), 144-154. Retrieved from https://www.sciencedirect.com/science/article/pii/S0278262612000863?via%3Dihub
Rippon, G., Brock, J., Brown, C., & Boucher, J. (2007). Disordered connectivity in the autistic brain: challenges for the ‘new psychophysiology’. International journal of psychophysiology, 63(2), 164-172.
Sackeim, H. A., Prohovnik, I., Moeller, J. R., Brown, R. P., Apter, S., Prudic, J., … Mukherjee, S. (1990). Regional cerebral blood flow in mood disorders: I. Comparison of major depressives and normal controls at rest. Archives of general psychiatry, 47(1), 60-70.
Samson, A. C., Meisenzahl, E., Scheuerecker, J., Rose, E., Schoepf, V., Wiesmann, M., & Frodl, T. J. J. o. p. r. (2011). Brain activation predicts treatment improvement in patients with major depressive disorder. 45(9), 1214-1222. Retrieved from https://www.sciencedirect.com/science/article/pii/S0022395611000586?via%3Dihub
Segal, D. L. (2010). Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). The Corsini Encyclopedia of Psychology, 1-3.
Sliz, D., & Hayley, S. (2012). Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Frontiers in Human Neuroscience, 6, 323.
So, E., Kam, I., Leung, C., Chung, D., Liu, Z., & Fong, S. J. H. K. J. o. P. (2003). The Chinese-bilingual SCID-I/P Project: Stage 1--Reliability for mood disorders and schizophrenia. 13(1), 7-19.
Storch, E. A., Roberti, J. W., Roth, D. A. J. D., & anxiety. (2004). Factor structure, concurrent validity, and internal consistency of the beck depression inventory—second edition in a sample of college students. 19(3), 187-189. Retrieved from https://onlinelibrary.wiley.com/doi/pdf/10.1002/da.20002
Vuilleumier, P., & Driver, J. J. P. T. o. t. R. S. B. B. S. (2007). Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. 362(1481), 837-855. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2430001/pdf/rstb20072092.pdf
Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., & Gong, Q. Y. (2018). Brain structure alterations in depression: psychoradiological evidence. CNS Neuroscience & Therapeutics, 24(11), 994-1003.
Acknowledgments
The authors would like to especially thank Venerbale Master Dejian of the Songshan monastery for his teaching and support. They are also grateful to Ms. Lan He, Ms. Man-ying Mo, and Ms. Maggie Ng for their effort in data collection and/or data management. This study was supported by donations from Mr. Sau-wang Law and Mr. Sau-hung Li to The Chinese University of Hong Kong.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants. This study was conducted in accordance with the Helsinki Declaration of the World Medical Association Assembly. The research protocol was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee and the Kowloon West Cluster Clinical Research Ethics Committee of the Hospital Authority in Hong Kong. The study was registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-11001740). All participants signed written informed consent forms before the study.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, Y.M.Y., Sze, S.L., Wong, Q.Y. et al. A mind-body lifestyle intervention enhances emotional control in patients with major depressive disorder: a randomized, controlled study. Cogn Affect Behav Neurosci 20, 1056–1069 (2020). https://doi.org/10.3758/s13415-020-00819-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-020-00819-z