Abstract
Persons with co-occurring HIV infection and cocaine use disorder tend to engage in riskier decision-making. However, the neural correlates of sensitivity to risk are not well-characterized in this population. The purpose of this study was to examine the neural interaction effects of HIV infection and cocaine use disorder to sensitivity to risk. The sample included 79 adults who differed on HIV status and cocaine use disorder. During functional magnetic resonance imaging (fMRI), participants completed a Wheel of Fortune (WoF) task that assessed neural activation in response to variations of monetary risk (i.e., lower probability of winning a larger reward). Across groups, neural activation to increasing risk was in cortical and subcortical regions similar to previous investigations using the WoF in nondrug-using populations. Our analyses showed that there was a synergistic effect between HIV infection and cocaine use in the left precuneus/posterior cingulate cortex and hippocampus, and right postcentral gyrus, lateral occipital cortex, cerebellum, and posterior parietal cortex. HIV+ individuals with cocaine use disorder displayed neural hyperactivation to increasing risk that was not observed in the other groups. These results support a synergistic effect of co-occurring HIV infection and cocaine dependence in neural processing of risk probability that may reflect compensation. Future studies can further investigate and validate how neural activation to increasing risk is associated with risk-taking behavior.
Similar content being viewed by others
Introduction
Cocaine use disorder is associated with behavioral deficits in inhibitory control, executive functioning, and decision-making, which may contribute to the risk-taking behavior commonly seen in this population (Aron & Paulus, 2007; Luijten et al., 2014; Morein-Zamir & Robbins, 2015). Cocaine disrupts the mesocorticolimbic dopaminergic system, and frequent use is postulated to result in neuroadaptations in fronto-striatal regions that are implicated in reward-based decision-making (Everitt & Robbins, 2016). These neuroadaptations likely result from the proinflammatory effects of cocaine on the central nervous system, including increased oxidative stress, induction of inflammatory cytokines, and greater permeability of the blood-brain-barrier (Buch et al., 2012). It has been proposed that dopamine dysregulation acts as a catalyst for dysfunctional immune regulation that underlies these neuroinflammatory responses (Ersche & Döffinger, 2017). Cocaine-induced neuroadaptations that affect reward processing and decision-making may lead to riskier decisions (Heil, Johnson, Higgins, & Bickel, 2006; Rosselli, Ardila, Lubomski, Murray, & King, 2001; Verdejo-Garcia, Perales, & Perez-Garcia, 2007).
Persons with cocaine use disorder have disproportionately high rates of HIV infection (Korthuis et al., 2008). This disparity is, in part, driven by engagement in high-risk sexual behaviors, such as inconsistent condom use, multiple sex partners, and trading of sex for drugs (Berg, Weatherburn, Marcus, & Schmidt, 2019; Cheng et al., 2016; Harzke, Williams, & Bowen, 2009). Cocaine users display risky behavior on varied decision-making processing, including riskier choices with more disadvantageous outcomes and a preference for immediate smaller rewards over larger delayed rewards (Spronk, van Wel, Ramaekers, & Verkes, 2013). This propensity toward risky decision making may be exacerbated by the effect of co-occurring HIV infection on the brain (Cai, Yang, Callen, & Buch, 2016).
While combination antiretroviral therapy can effectively restore immune system function and suppress many symptoms of HIV infection, neurocognitive impairment remains a prevalent comorbidity among HIV-positive individuals, with rates up to 50% (Clifford & Ances, 2013; Heaton et al., 2010). HIV-associated deficits are most prominent in the cognitive domains of attention, memory, and executive function (Brouillette et al., 2016; Eggers et al., 2017; Heaton et al., 2015). In particular, HIV infection has also been associated with increased risky decision-making (Fujiwara, Tomlinson, Purdon, Gill, & Power, 2015; Hardy, Hinkin, Levine, Castellon, & Lam, 2006; Martin et al., 2004). HIV-associated cognitive dysfunction may reflect abnormalities in underlying brain function. Functional magnetic resonance imaging (fMRI) studies of executive functioning in HIV-positive individuals have found hyperactivations in frontal brain regions both at rest and during tasks that may underlie HIV-associated neurocognitive impairment (Hakkers et al., 2017). Like cocaine-use disorder, HIV disease is associated with increased dopaminergic function that is believed to cause neuroinflammation, which increases susceptibility to viral infection and leads to dysregulation of myeloid inflammatory processes (Nolan & Gaskill, 2019). In healthy adults, decisions that involve uncertain outcomes have been shown to involve neural activation in the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (PPC) (Platt & Huettel, 2008). Risky decision making in the context of uncertain outcomes can be investigated using the Wheel of Fortune (WoF) task, which captures neural activation in response to increasingly riskier choices. Prior studies of healthy adults have shown increased activation in the dlPFC and dorsal anterior cingulate cortex (ACC) and decreased activation in the precuneus, middle temporal area, and insula (Ernst et al., 2004; Roy et al., 2011; B. W. Smith et al., 2009). Neuroeconomics provides a framework for understanding decision making based on preference formation, action selection, and evaluation of outcomes using both utility (i.e., subjective value) and probability (Sharp, Monterosso, & Montague, 2012). In making risky versus safe decisions, choice often is guided by the probability of outcomes (Sirigu & Duhamel, 2016). Individual differences in neural sensitivity to reward probabilities may help to explain risky decision making.
Relatively few studies have used neuroimaging to investigate risky decision making in persons with HIV. In an fMRI study using a task involving decisions about risky gains, HIV-positive individuals showed higher neural activation than HIV-negative individuals in ventral ACC, dlPFC, medial prefrontal cortex (mPFC), thalamus, and basal ganglia (Connolly et al., 2014). Two studies have examined the independent effects of HIV and cocaine on neural response to decision making by including four groups stratified by HIV infection and cocaine use. Using a loss aversion task, cocaine was associated with higher neural activation in regions that tracked the favorability of gambles (mPFC, dorsal ACC, precuneus, and visual cortex), whereas HIV was associated with less activation in regions that tracked the unfavorability of gambles (dlPFC, PPC, orbitofrontal cortex, insula, and striatum) (Meade et al., 2018). Using a delay discounting task, cocaine moderated the effect of HIV in the lateral and medial PFC and the cerebellum, such that the HIV-related compensatory response was diminished in participants with co-occurring cocaine dependence (Meade et al., 2017). These studies suggest that co-occurring HIV infection and cocaine dependence can have interactive effects on neural functioning associated with multiple aspects of reward-based decision making, including the valuation of potential losses and the discounting of delayed rewards.
Building on these studies, the present analysis investigates the interactive effects of cocaine dependence and HIV infection on neural sensitivity to increasingly risky choices. Specifically, participants were asked to make choices between larger rewards with a lower probability of winning and smaller rewards with a higher probability of winning. We hypothesized that HIV and cocaine would be independently associated with decreased activation in task-related regions. Additionally, we expected that co-occurring HIV and cocaine would be associated with compensatory hyperactivations in a broader set of regions related to reward processing.
Methods
Participants
This data comes from a study on the effects of co-occurring HIV infection and cocaine use on neural activation during decision making (Meade et al., 2016). The sample included adults aged 22-55 years who were active cocaine users with HIV (COC+/HIV+, n = 19) and without HIV (COC+/HIV−, n = 18), and non-cocaine users with HIV (COC−/HIV+, n = 24) and without HIV (COC−/HIV−, n=18). The COC+ groups met the following criteria: lifetime cocaine dependence, regular cocaine use for ≥1 year, and any use in the past month. The COC− groups met the following criteria: no lifetime cocaine abuse or dependence, no history of regular cocaine use, 0 days of cocaine use in the past year, and cocaine-negative drug screen. Alcohol and marijuana use were permitted in all groups, but current alcohol or marijuana dependence were only acceptable in the COC+ group if secondary to a principal diagnosis of cocaine dependence. For other illicit drugs, individuals in all groups were excluded for lifetime regular use or dependence, use in the past year, or a positive drug screen. HIV-negative status was verified by an OraQuick© rapid test, and HIV-positive status was confirmed by medical record review. Additional exclusion criteria were: English nonfluency or illiteracy; <8th grade education; severe learning disability; serious neurological disorders not due to HIV; acute opportunistic brain infections or history of such infections without return to normal cognition; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; severe mental illness; current use of antipsychotic or mood stabilizing medications; MRI contraindications; and/or impaired mental status. Medical records were reviewed to verify the absence of exclusionary medical conditions.
Procedures
Participants were recruited via advertisements in local newspapers, websites, community-based organizations, and infectious diseases clinics. After a brief prescreener, individuals completed a 3-hour in-person screening. Eligible participants returned for the MRI scan and additional assessments. Participants were asked to abstain from alcohol, cocaine, and other illicit drugs for at least 4 hours before the visit. Research staff were trained in the detection of obvious signs of withdrawal that could interfere with the testing procedures (e.g., tremors, fatigue), but no scans were aborted for withdrawal symptoms. Participants were compensated for participation in the study. Procedures were approved by the institutional review boards at Duke University Health System and University of North Carolina at Chapel Hill.
Measures
At the screening visit, participants completed clinical interviews, computerized questionnaires, and biological tests to confirm eligibility. Module E of the Structured Clinical Interview for DSM-IV-TR identified substance use disorders (First, Spitzer, Gibbon, & Williams, 1996), and the Addiction Severity Index-Lite assessed substance use and associated impairments (McLellan et al., 1992). A nine-panel urine toxicology screen that tested for cocaine, amphetamine, barbiturates, benzodiazepine, methamphetamine, opioids, methadone, marijuana, and oxycodone was used to corroborate self-reports of recent drug use. A urine pregnancy test also was completed. The Mini International Neuropsychiatric Interview (MINI) was identified mood, anxiety, and psychotic disorders (Sheehan et al., 1998). Premorbid verbal IQ was estimated using the Wechsler Test of Adult Reading (WTAR), which asks participants to read aloud 50 words that have atypical grapheme to phoneme translations (Wechsler, 2001). Finally, computerized questionnaires assessed demographics and smoking history.
On the day of the MRI scan, timeline follow-back methodology was used to assess substance use in the past 30 days (Robinson, Sobell, Sobell, & Leo, 2014). Another urine pregnancy test was completed and a five-panel urine toxicology screen was administered that tested for cocaine, amphetamine, opioids, marijuana, and benzodiazepine. A positive screen for cocaine was not required for COC+ participants and was exclusionary for COC− participants. A positive screen for marijuana was allowed in all groups. No participants screened positive for any other illicit drug (2 participants tested positive for benzodiazepine and opioids due to a verified prescription). Nicotine use was allowed in all groups. Healthcare records were reviewed to obtain medical history and, if applicable, HIV disease indicators (e.g., CD4 cell counts).
Wheel of Fortune (WoF) task
The WoF is a computerized two-choice decision-making task that involves probabilistic monetary outcomes (Ernst et al., 2004). Participants are presented with two wheels: one with a larger reward but smaller probability of winning, and the other with a smaller reward but larger probability of winning (Fig. 1). Participants were asked which wheel they would prefer to “spin.” The 90 experimental trials varied in the probability of winning each reward, as well as the monetary amount of the rewards. The original task was modified to increase variability in the choices presented and to maximize the likelihood of participants choosing both riskier and less risky options. Of note, there was no inherent value to choosing from only one wheel. Specifically, the expected value for only choosing the riskier wheel ($227) or the less risky wheel ($290) was lower than that of a combination of risky and less risky choices based on monetary value of the choices (up to $367). Only the selection phase was presented; none of the choices were resolved during the scanning session. This simplification eliminated the possibility that outcomes on previous trials might influence choices on subsequent trials.
Illustration of the adapted Wheel of Fortune task. For each pair of wheels, the green slice corresponds to the probability of winning the monetary amount listed above. The participant was asked which wheel they prefer to spin. The left/right location of the riskier option was randomized across trials within individuals to minimize lateralized motion preparation to the choice period. Each trial was 6 seconds in length and included the choice presentation (2 s) and the response period (4 s), followed by an exponentially distributed variable intertrial interval of 2-8 s. After the 2 s choice presentation, the words “choose now” appeared, indicating that the participant could make their selection by pressing a button on the response pad that corresponded to the left or right choice. Once the participant made a choice, that wheel was underlined for the remainder of the response period
The probability of the larger reward ranged from 5% to 45% in 5% intervals, while the probability of the smaller reward ranged from 95% to 55%, respectively. For example, the riskiest choice with the greatest discrepancy had probabilities of 5% for the larger reward and 95% for the smaller reward, while the least risky choice had probabilities of 45% for the larger reward and 55% for the smaller reward. The associated monetary rewards varied from $5.50 to $10.00 for the larger rewards and $0.50 to $5.00 for the smaller rewards, with the monetary amounts orthogonalized across the different probability pairs. An additional 30 trials served as controls, in which the size of the rewards differed but both options had a 50% probability of winning. Because the larger monetary reward was clearly advantageous, this was considered the correct choice. The control trials were included to assess task comprehension and attention. Participants completed four runs of 30 trials each. The order of runs was randomized across participants, but the sequence of trials within each run was the same.
Participants were trained on the WoF task on the day of the scan session. After receiving instructions, they completed a shortened version of the WoF on a computer out of the MRI scanner. Participants received clarifications about the task as needed. To increase motivation, participants were informed that one trial would be selected after the scan. Based on their choice during the scan, they spun a wooden wheel with the corresponding probability. If the arrow landed on the green section, they earned the associated reward; otherwise, they did not earn the reward.
MRI data acquisition
MRI data were acquired during a 90-minute session with a 3.0T GE Discovery MR750 whole-body scanner at the Brain Imaging and Analysis Center at Duke University Hospital. T2-weighted axial echo-planar imaging (EPI) was used to collect whole-brain BOLD signal with the following parameters: TR = 2,000 ms, TE = 27 ms, flip angle = 77°, FOV = 25.6 cm, in-plane matrix size = 64*64, slice thickness = 4 mm, number of slices = 39. High-resolution T1-weighted structural images were acquired with the following parameters: TR = 8.10 ms, TE = 3.18 ms, FOV = 25.6 cm, flip angle = 12°, in-plane matrix = 256*256; slice thickness = 1 mm, number of slices = 172. The images were acquired in the anterior to posterior direction (axially) for the EPI, and left to right (sagitally) for the T1-weighted images.
Quality control
Of the 90 study completers, 79 passed all quality control checks and were included in the final sample. Of the 11 participants who were excluded, 2 had relative mean displacement >0.3 mm, 3 skipped >15% of trials, and 6 demonstrated irrational choice on the WoF task. Task performance was evaluated for each individual using a multivariate logistic regression model predicting riskier choice, with probability and monetary amount entered as regressors. The unstandardized regression coefficients (β values) are expected to be positive for both probability (more likely to choose the riskier wheel as probability of winning increases) and reward (more likely to choose the riskier wheel as reward amount increases). Irrational choice was defined as negative β-values for probability and/or amount, coupled with visual inspection of heat maps. Furthermore, specific runs were excluded due to excessive motion, skipped trials, or image acquisition problems (six had one run excluded, one had two runs excluded, and two had three runs excluded).
fMRI data analysis
Functional and anatomical data were pre-processed and analyzed using FSL’s FEAT (Smith et al., 2004). Preprocessing steps included: motion correction with MCFLIRT; slice-timing correction using Fourier-space time-series phase-shifting; spatial smoothing with a Gaussian kernel of 5-mm full-width at half-maximum; high-pass temporal filtering (Gaussian-weighted least squares straight line fitting with σ = 60 s); grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; and skull stripping of structural images with BET. Registration of functional data to the T1-weighted anatomical slices and registration of structural images to the 2-mm Montreal Neurological Institute (MNI) standard-space template were done using FLIRT utilizing a 12-parameter affine transformation. Individual time-series statistical analyses were performed using FILM with local autocorrelation correction for two separate contrasts examining sensitivity to risk.
The primary contrast modeled neural activation in response to increasing risk (i.e., decreasing probability of winning a larger reward). For this first-level general linear model (GLM), two regressors were defined. The first included all WoF trials. The second modeled the paired probabilities associated with the smaller and larger rewards, respectively, on a 1-10 scale (i.e., 1 = 50% or 50%, 10 = 95% or 5%). The probability regressor was separately orthogonalized from the regressor containing all trials. A binary variable of whether they chose the risky or safe option was included as a covariate to control for choice. This regressor structure modeled increasing risk on a continuous scale while controlling for choice. Standard motion parameters also were added to each GLM, and temporal filtering was applied to each regressor before convolution with a double-gamma hemodynamic response function. A second-level fixed-effects analysis was then used to average activation across the separate runs of each participant. For both the first and second level individual analyses, results were corrected for multiple comparisons using a cluster thresholding approach of Z > 2.3 and p < 0.05.
For the third-level group analyses, we utilized a more stringent cluster threshold of Z > 2.6 and p < 0.05 to protect against spurious group-level effects using FMRIB’s Local Analysis of Mixed Effects (FLAME 1+2). To examine if there were neural differences related to increasing risk between groups, a whole-brain voxel-wise one-way ANCOVA was performed on the contrast of interest. Root mean square of the intensity difference of volume N to volume N+1 (DVARS) (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012) was calculated to assess motion. Motion (DVARS) and age (in years) were included as covariates of no interest. Both sets of analyses were restricted to a gray matter mask.
For each significant cluster identified in the ANCOVA, percent signal change was extracted using Featquery. Further analyses were conducted in SPSS 24.0. Group averages were examined to determine the direction of effects. Finally, a 2 (HIV+ vs. HIV−) x 2 (COC+ vs. COC−) ANOVA was conducted to examine the main effects of cocaine and HIV and their interaction.
Results
Participant characteristics
Participant characteristics by group are described in Table 1. The sample included 50 men and 29 women, primarily African-American (83.54%), with a mean age of 42.13 years (standard deviation [SD] = 8.95). Years of education ranged from 8 to 22 (mean [M] = 13.53, SD = 2.57), and participants had an average premorbid IQ of 91.57 (SD = 16.67). There were no group differences on these demographic characteristics.
Lifetime years of cocaine use ranged from 2-33 years (M = 16.75, SD = 8.19) with a mean of 10.00 (SD = 7.49) days of use in the past 30 days. Participants had last used cocaine an average of 2.71 (SD = 4.59) days before the MRI scan, and 83.78% had a positive urine screen for cocaine on the day of the MRI scan. Cocaine use characteristics did not differ by HIV status.
All HIV+ participants were in HIV care and prescribed antiretroviral medications. They had been diagnosed with HIV for 1-26 years (M = 9.37, SD = 7.04), 69.77% had a suppressed viral load at <50 copies/mL, and 41.86% had an AIDS diagnosis. Most recent CD4 count ranged from 79 to 2,377 (median = 598.00, interquartile range [IQR] = 553.00), and nadir CD4 count ranged from 0 to 718 (median = 202.00, IQR = 327.00). HIV characteristics did not differ by COC status.
Task performance
Behavioral performance indicated that participants understood and were engaged with the task (Table 1). Participants responded to an average of 117.78 of the 120 trials (SD = 4.10). Reaction time was significantly faster for control trials (M = 0.56 s, SD = 0.25) than experimental trials (M = 0.86 s, SD = 0.32; t(78) = 9.68; p < 0.001). On control trials, participants chose the larger wheel 97.72% of the time (SD = 3.97%). On experimental trials, they chose the larger reward wheel 41.70% of the time (SD = 25.82%). As shown in Fig. 2, participants were less likely to choose the riskier wheel as the probability of winning on that wheel decreased. A repeated measures ANOVA showed a strong main effect for probability [F(8, 600) = 118.10, p < 0.001], but there was no group-by-probability interaction effect [F(24, 600) = 0.72, p = 0.832]. There were no differences between groups on additional metrics of task performance (Table 1).
Whole-sample task activation
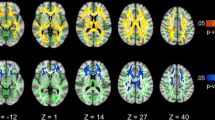
Increasing risk was positively associated with BOLD signal in bilateral regions of the lateral occipital, inferior temporal, and dorsomedial prefrontal cortices and the left dorsal ACC, inferior frontal gyrus, and lateral orbitofrontal cortex (Table 2; Fig. 3). In addition, increasing risk was negatively associated with BOLD signal in bilateral regions of the medial occipital, precuneus/posterior cingulate cortex (PCC), PPC, insular, opercular, motor, mid-cingulate, ventral ACC, orbitofrontal cortices and the striatum, hippocampus, amygdala, and cerebellum.
Mean activation and deactivation maps for the Wheel of Fortunate task. Overall task activation across all participants for (A) increasing risk and (B) decreasing risk. Cluster information is described in Table 3. Activation maps were thresholded using clusters determined by Z > 2.6 and a corrected cluster significance threshold of p = 0.05. Images are in radiological orientation (L = left)
Group differences in task activation
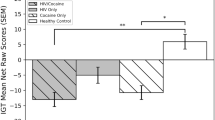
There were significant differences in neural activation across the groups in six clusters (Table 3). These clusters included bilateral precuneus/PCC, superior parietal lobule of the PPC, and postcentral gyrus. In the left hemisphere, there were differences in the hippocampus, parahippocampus, inferior and middle temporal gyri, and fusiform cortex. In the right hemisphere, there were differences in the inferior parietal lobule of the PPC, postcentral gyrus, motor areas (precentral gyrus, juxtapositional lobule, and superior frontal gyrus), parietal and central opercula, and cerebellum. In each of these clusters, there was a significant HIV*COC interaction effect (Table 4). As shown in Fig. 4, while the other three groups generally deactivated in each of the clusters, the HIV+/COC+ group had higher neural activations.
Neural activation in group-differentiating clusters on the Wheel of Fortune task. On the left, images show the clusters that exhibited significant F-values between groups. The z-statistic images were thresholded at 2.6 with a cluster threshold of p = 0.05. Images are in radiological orientation (L = left). On the right, bar graphs show the average percent BOLD signal change by group (error bars represent standard error from the mean. *p < 0.05
Discussion
The results of this study support synergistic effects of HIV infection and cocaine dependence on the neural substrates underlying risky decision making. Specifically, in response to monetary choices that were increasingly risky, the COC+/HIV+ group exhibited higher neural activation across multiple regions, including the left precuneus/PCC and hippocampus, and right postcentral gyrus, lateral occipital cortex, cerebellum, and PPC. Overall, our findings suggest that persons with co-occurring HIV infection and cocaine dependence may have less efficiency when processing risk probabilities across two options.
The WoF task elicited neural activations across all groups in regions consistent with previous studies (Ernst et al., 2004; Roy et al., 2011; Smith et al., 2009). Specifically, we observed activations in response to increasing risk in a set of regions involved in decision-making and probability assessment, including the PPC, dorsal ACC, inferior frontal gyrus, and dorsomedial prefrontal cortices. The WoF task also produced neural hypoactivation in bilateral regions of the medial occipital, precuneus, PPC, insular, opercular, motor, mid-cingulate, ventral ACC, orbitofrontal cortices and the left striatum, and right hippocampus, amygdala, and cerebellum in response to increasing risk. Although we hypothesized that HIV infection and cocaine use disorder would be independently associated with decreased neural activation in reward-related regions, our results did not support this. This may be due to risk sensitivity not relying on primary reward processing areas, but instead relying on decision making and probability assessment regions.
Neural compensation theories posit that, in response to damage in regions involved with specific cognitive functions, additional regions that are not typically activated for a specific task must be engaged to maintain performance (Barulli & Stern, 2013; Fornito, Zalesky, & Breakspear, 2015). We found that the COC+/HIV+ group exhibited greater neural activation in regions whose primary function is not reward processing, but rather, to facilitate communication across the brain to enable complex cognition (van den Heuvel & Sporns, 2013). Neural hyperactivation may serve as a mechanism to compensate for decreased efficiency and neural capacity in persons with co-occurring HIV infection and cocaine use disorder. We speculate that this group may require more cognitive resources to assess the relative risk of two uncertain choices. Our team also has observed increased neural activation in COC+/HIV+ individuals in the PPC, precuneus, and cerebellum when performing decision-making tasks that involve gambles with potential losses and with delays to reward receipt (Meade et al., 2018; Meade et al., 2017). A possible mechanistic explanation for the unique neural sensitivity to risk in the COC+/HIV+ group is that chronic cocaine use and HIV infection are both independently associated with increased neuroinflammation that is then associated with altered cognitive functioning (Ersche & Döffinger, 2017; Rubin et al., 2018). Neuroinflammation in both cocaine use disorder and HIV disease are hypothesized to result from altered dopaminergic function. Specifically, both cocaine and the HIV-tat protein bind to the dopamine transporter and block the reuptake of dopamine, resulting in increased extracellular levels of dopamine (Dahal, Chitti, Nair, & Saxena, 2015). Increased dopamine levels are postulated to then increase neuroinflammation and the permeability of the blood-brain-barrier resulting in cellular pathology (Dahal et al., 2015). This cellular pathology could explain the altered neural function observed in the COC+/HIV+ group.
The PCC has been implicated in risky decision making and the subjective valuation of choice in multiple nonhuman primate and human studies (Platt & Huettel, 2008). This region is activated in response to greater uncertainty of rewards (Huettel, Song, & McCarthy, 2005; K. Smith, Dickhaut, McCabe, & Pardo, 2002) and higher probability of winning during a betting task (Studer, Apergis-Schoute, Robbins, & Clark, 2012). While increased neural activation of this region may not be aberrant, hyperactivation may signal neural inefficiency reflective of difficulty in assessing risk probability. In a functional network connectivity analysis of persons with HIV, decreased PCC efficiency was identified in persons with cognitive dysfunction compared to those without cognitive dysfunction (Ventura et al., 2018). The authors theorized that this “hypermodulation” of the PCC was due to a need to compensate for HIV-associated neural injury to avoid or postpone cognitive deterioration. The present study suggests that persons with HIV who actively use cocaine may be at greater risk for dysfunctional activation in the PPC in response to risky decisions.
Research has shown that the hippocampus is negatively affected by both cocaine use disorder (Yamaguchi et al., 2005) and HIV infection (Torres-Munoz et al., 2001). The hippocampus has been identified as critical neural circuitry associated with multiple facets of cocaine use disorder, including viewing cocaine-related stimuli, cocaine craving, and cocaine relapse (Castilla-Ortega et al., 2016). Cocaine administration has been shown to potentiate hippocampus fMRI signal in humans (Breiter et al., 1997), and greater intrinsic connectivity in the hippocampus has been negatively associated with cocaine abstinence during a Stroop task (Mitchell et al., 2013). Lower hippocampal volume is associated with HIV infection (Fleischman et al., 2018; Kallianpur et al., 2013; Thames et al., 2017), which may be attributable to neuroinflammation (Rubin et al., 2018) and immune activation (Fleischman et al., 2018). Increased hippocampus activation during risky decision making may reflect previous damage to this region as a result of the combined effects of HIV infection and cocaine use.
In the sample overall, participants on average chose the riskier wheel 42% of the time, with no difference between groups, suggesting that the task was optimally designed to elicit within-subject variability in choice. The lack of behavioral differences was unexpected, given that prior studies have found that persons with HIV make more disadvantageous decisions on gambling tasks (Fujiwara et al., 2015; Hardy et al., 2006; Iudicello et al., 2013; Thames et al., 2012). We speculate that neural hyperactivation may have compensated for potential loss in function in core reward processing regions, allowing participants to perform the task effectively. The observed neural hyperactivations may also relate to greater probability discounting, which is the devaluation of uncertain outcomes (Bickel, Johnson, Koffarnus, MacKillop, & Murphy, 2014). A prior study found that cocaine administration is dose-dependently associated with risky sexual health probability discounting (Johnson, Herrmann, Sweeney, LeComte, & Johnson, 2017). Future work could explore how this construct relates to risk-taking in HIV+ individuals. Our finding of altered neural activation despite no group differences has been reported in previous studies on HIV infection (Connolly et al., 2014) and cocaine use (Gowin, May, Wittmann, Tapert, & Paulus, 2017; Worhunsky, Potenza, & Rogers, 2017; Zhang et al., 2018). Differences in task difficulty could produce variations in performance between groups. While the absence of group differences was unexpected, it eliminates the possibility that group differences on neural activation patterns were confounded by behavioral performance. A primary motivation for utilizing fMRI is that it can be more sensitive to group differences relative to behavioral task performance.
This study is the first to examine the effects of co-occurring HIV infection and cocaine use disorder on neural sensitivity to risk. Strengths of the study include the factorial design, use of an established paradigm to investigate risk in the context of monetary choices, and a matched sample with well-characterized substance use histories. Nevertheless, as with all studies, there are limitations that must be acknowledged. First, the relatively small sample size for each group may have limited power to detect small effects, although we still observed robust interactions. To minimize spurious results, we used a conservative cluster threshold for the group comparisons. Second, the prevalence of alcohol and nicotine was higher in COC+ compared with COC−. This was expected, as co-occurrence of these substances is very common in persons who use cocaine (Pennings, Leccese, & Wolff, 2002), and we allowed this to ensure ecological validity and generalizability of results. We did not control for other substances in our analyses due to collinearity problems. Studies with larger samples are needed to tease apart the potential effects of polysubstance use on neural activation. There are other potential confounding factors that we were unable to control for due to low frequency (e.g., mood disorder). While monetary rewards are a “real-world” secondary reinforcer that is fairly generalizable across many populations, it is possible that the salience of monetary rewards differed across groups and that domain-specific rewards would more effectively assess decision making among cocaine users. However, with the inclusion of non-cocaine users, we selected a reinforcer that would be relevant to all groups. Finally, because this study was cross-sectional, we are unable to make causal inferences. Longitudinal studies are needed to determine the direction of effects and if neural sensitivity to risk is predictive of real-world behaviors.
In conclusion, COC+/HIV+ participants demonstrated neural hyperactivation in response to increasing risk in multiple regions associated with compensatory activation, suggesting a synergistic effect of HIV and cocaine on brain function that may be mediated by altered dopaminergic function. Utilizing behavioral economic theory, we speculate that this neural activation profile is related to a dysfunctional weighting of risk probability from both cocaine and HIV-related pathology. The neural representation of this deficit may be less efficient neural processing of risk leading to neural overcompensation. When confronted with real-life decisions, these neural mechanisms may not be able to adequately inhibit behavior. Identifying neural correlates of risk sensitivity has the potential to identify treatment targets for clinical intervention to help reduce risk-taking behaviors.
References
Aron, J. L., & Paulus, M. P. (2007). Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction, 102(Suppl 1), 33-43. https://doi.org/10.1111/j.1360-0443.2006.01778.x
Barulli, D., & Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502-509. doi:https://doi.org/10.1016/j.tics.2013.08.012
Berg, R. C., Weatherburn, P., Marcus, U., & Schmidt, A. J. (2019). Links between transactional sex and HIV/STI-risk and substance use among a large sample of European men who have sex with men. BMC Infectious Diseases, 19(1), 686. doi:https://doi.org/10.1186/s12879-019-4326-3
Bickel, W. K., Johnson, M. W., Koffarnus, M. N., MacKillop, J., & Murphy, J. G. (2014). The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annual Review of Clinical Psychology, 10, 641-677. doi:https://doi.org/10.1146/annurev-clinpsy-032813-153724
Breiter, H. C., Gollub, R. L., Weisskoff, R. M., Kennedy, D. N., Makris, N., Berke, J. D., … Hyman, S. E. (1997). Acute effects of cocaine on human brain activity and emotion. Neuron, 19(3), 591-611.
Brouillette, M. J., Yuen, T., Fellows, L. K., Cysique, L. A., Heaton, R. K., & Mayo, N. E. (2016). Identifying neurocognitive decline at 36 months among HIV-positive participants in the CHARTER cohort using group-based trajectory analysis. PLoS ONE, 11(5), e0155766. doi:https://doi.org/10.1371/journal.pone.0155766
Buch, S., Yao, H., Guo, M., Mori, T., Seth, P., Wang, J., & Su, T. P. (2012). Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Current HIV Research, 10(5), 425–428.
Cai, Y., Yang, L., Callen, S., & Buch, S. (2016). Multiple faceted roles of cocaine in potentiation of HAND. Current HIV Research, 14(5), 412-416.
Castilla-Ortega, E., Serrano, A., Blanco, E., Araos, P., Suarez, J., Pavon, F. J., … Santin, L. J. (2016). A place for the hippocampus in the cocaine addiction circuit: Potential roles for adult hippocampal neurogenesis. Neuroscience and Biobehavioral Reviews, 66, 15-32. doi:https://doi.org/10.1016/j.neubiorev.2016.03.030
Cheng, T., Johnston, C., Kerr, T., Nguyen, P., Wood, E., & DeBeck, K. (2016). Substance use patterns and unprotected sex among street-involved youth in a Canadian setting: a prospective cohort study. BMC Public Health, 16, 4. doi:https://doi.org/10.1186/s12889-015-2627-z
Clifford, D. B., & Ances, B. M. (2013). HIV-associated neurocognitive disorder. The Lancet Infectious Diseases, 13(11), 976-986. doi:https://doi.org/10.1016/S1473-3099(13)70269-X
Connolly, C. G., Bischoff-Grethe, A., Jordan, S. J., Woods, S. P., Ellis, R. J., Paulus, M. P., … Translational Methamphetamine Aids Research Center (TMARC) Group (2014). Altered functional response to risky choice in HIV infection. PLoS ONE, 9(10), e111583. doi:https://doi.org/10.1371/journal.pone.0111583
Dahal, S., Chitti, S. V., Nair, M. P., & Saxena, S. K. (2015). Interactive effects of cocaine on HIV infection: implication in HIV-associated neurocognitive disorder and neuroAIDS. Frontiers in Microbiology, 6, 931. doi:https://doi.org/10.3389/fmicb.2015.00931
Eggers, C., Arendt, G., Hahn, K., Husstedt, I. W., Maschke, M., Neuen-Jacob, E., … Straube, E. (2017). HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. Journal of Neurology, 264(8), 1715-1727.
Ernst, M., Nelson, E. E., McClure, E. B., Monk, C. S., Munson, S., Eshel, N., … Pine, D. S. (2004). Choice selection and reward anticipation: an fMRI study. Neuropsychologia, 42(12), 1585-1597. https://doi.org/10.1016/j.neuropsychologia.2004.05.011
Ersche, K. D., & Döffinger, R. (2017). Inflammation and infection in human cocaine addiction. Current opinion in behavioral sciences, 13, 203-209.
Everitt, B. J., & Robbins, T. W. (2016). Drug addiction: updating actions to habits to compulsions ten years on. Annual Review of Psychology, 67, 23-50. doi:https://doi.org/10.1146/annurev-psych-122414-033457
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (1996). Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute.
Fleischman, D. A., Arfanakis, K., Leurgans, S., Keating, S. M., Lamar, M., Bennett, D. A., … Barnes, L. L. (2018). Neopterin is associated with hippocampal subfield volumes and cognition in HIV. Neurology Neuroimmunology Neuroinflammation, 5(4), e467. doi:https://doi.org/10.1212/NXI.0000000000000467
Fornito, A., Zalesky, A., & Breakspear, M. (2015). The connectomics of brain disorders. Natural Reviews Neuroscience, 16(3), 159-172. doi:https://doi.org/10.1038/nrn3901
Fujiwara, E., Tomlinson, S. E., Purdon, S. E., Gill, M. J., & Power, C. (2015). Decision making under explicit risk is impaired in individuals with human immunodeficiency virus (HIV). Journal of Clinical and Experimental Neuropsychology, 37(7), 733-750. doi:https://doi.org/10.1080/13803395.2015.1057481
Gowin, J. L., May, A. C., Wittmann, M., Tapert, S. F., & Paulus, M. P. (2017). Doubling down: increased risk-taking behavior following a loss by individuals with cocaine use disorder is associated with striatal and anterior cingulate dysfunction. Biol Psychiatry Cogn Neurosci Neuroimaging, 2(1), 94-103. doi:https://doi.org/10.1016/j.bpsc.2016.02.002
Hakkers, C. S., Arends, J. E., Barth, R. E., Du Plessis, S., Hoepelman, A. I. M., & Vink, M. (2017). Review of functional MRI in HIV: Effects of aging and medication. Journal of Neurovirology, 23(1), 20-32. doi:https://doi.org/10.1007/s13365-016-0483-y
Hardy, D. J., Hinkin, C. H., Levine, A. J., Castellon, S. A., & Lam, M. N. (2006). Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology, 20(3), 355-360. doi:https://doi.org/10.1037/0894-4105.20.3.355
Harzke, A. J., Williams, M. L., & Bowen, A. M. (2009). Binge use of crack cocaine and sexual risk behaviors among African-American, HIV-positive users. AIDS Behav, 13(6), 1106-1118. doi:https://doi.org/10.1007/s10461-008-9450-9
Heaton, R. K., Clifford, D. B., Franklin, D. R., Jr., Woods, S. P., Ake, C., Vaida, F., … Grant, I. (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75(23), 2087-2096. doi:https://doi.org/10.1212/WNL.0b013e318200d727
Heaton, R. K., Franklin, D. R., Jr., Deutsch, R., Letendre, S., Ellis, R. J., Casaletto, K., … Grant, I. (2015). Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clinical Infectious Diseases, 60(3), 473-480. doi:https://doi.org/10.1093/cid/ciu862
Heil, S. H., Johnson, M. W., Higgins, S. T., & Bickel, W. K. (2006). Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors, 31(7), 1290-1294. doi:https://doi.org/10.1016/j.addbeh.2005.09.005
Huettel, S. A., Song, A. W., & McCarthy, G. (2005). Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience, 25(13), 3304-3311. doi:https://doi.org/10.1523/JNEUROSCI.5070-04.2005
Iudicello, J. E., Woods, S. P., Cattie, J. E., Doyle, K., Grant, I., & The H. I. V. Neurobehavioral Research Program Group. (2013). Risky decision-making in HIV-associated neurocognitive disorders (HAND). The Clinical Neuropsychologist, 2(256-275), 256. doi:https://doi.org/10.1080/13854046.2012.740077
Johnson, M. W., Herrmann, E. S., Sweeney, M. M., LeComte, R. S., & Johnson, P. S. (2017). Cocaine administration dose-dependently increases sexual desire and decreases condom use likelihood: The role of delay and probability discounting in connecting cocaine with HIV. Psychopharmacology (Berl), 234(4), 599-612. doi:https://doi.org/10.1007/s00213-016-4493-5
Kallianpur, K. J., Shikuma, C., Kirk, G. R., Shiramizu, B., Valcour, V., Chow, D., … Sailasuta, N. (2013). Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology, 80(19), 1792-1799. doi:https://doi.org/10.1212/WNL.0b013e318291903f
Korthuis, P. T., Zephyrin, L. C., Fleishman, J. A., Saha, S., Josephs, J. S., McGrath, M. M., … Gebo, K. A. (2008). Health-related quality of life in HIV-infected patients: the role of substance use. AIDS Patient Care & STDs, 22(11), 859-867.
Luijten, M., Machielsen, M. W., Veltman, D. J., Hester, R., de Haan, L., & Franken, I. H. (2014). Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of Psychiatry and Neuroscience, 39(3), 149-169.
Martin, E. M., Pitrak, D. L., Weddington, W., Rains, N. A., Nunnally, G., Nixon, H., … Bechara, A. (2004). Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society, 10(7), 931-938. doi:https://doi.org/10.10170/S1355617704107054
McLellan, A. T., Kushner, H., Metzger, D., Peters, R., Smith, I., Grissom, G., … Argeriou, M. (1992). The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment, 9(3), 199-213. doi:https://doi.org/10.1016/0740-5472(92)90062-S
Meade, C. S., Cordero, D. M., Hobkirk, A. L., Metra, B. M., Chen, N.-K., & Huettel, S. A. (2016). Compensatory activation in fronto-parietal cortices among HIV-infected persons during a monetary decision-making task. Human Brain Mapping, 37(7), 2455-2467. doi:https://doi.org/10.1002/hbm.23185
Meade, C. S., Hobkirk, A. L., Towe, S. L., Chen, N., Bell, R. P., & Huettel, S. A. (2017). Cocaine dependence modulates the effect of HIV infection on brain activation during intertemporal decision making. Drug and Alcohol Dependence, 178, 443-451. doi:https://doi.org/10.1016/j.drugalcdep
Meade, C. S., Addicott, M., Hobkirk, A. L., Towe, S. L., Chen, N.-K., Sridharan, S., & Huettel, S. A. (2018). Cocaine and HIV are independently associated with neural activation in response to gain and loss valuation during economic risky choice. Addiction Biology, 23(2), 796-809. doi:https://doi.org/10.1111/adb.12529
Mitchell, M. R., Balodis, I. M., Devito, E. E., Lacadie, C. M., Yeston, J., Scheinost, D., … Potenza, M. N. (2013). A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. The American Journal of Drug and Alcohol Abuse, 39(6), 392-402. doi:https://doi.org/10.3109/00952990.2013.841711
Morein-Zamir, S., & Robbins, T. W. (2015). Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Research, 1628(Pt A), 117-129. doi:https://doi.org/10.1016/j.brainres.2014.09.012
Nolan, R., & Gaskill, P. J. (2019). The role of catecholamines in HIV neuropathogenesis. Brain Research, 1702, 54-73. doi:https://doi.org/10.1016/j.brainres.2018.04.030
Pennings, E. J., Leccese, A. P., & Wolff, F. A. (2002). Effects of concurrent use of alcohol and cocaine. Addiction, 97(7), 773-783. doi:https://doi.org/10.1046/j.1360-0443.2002.00158.x
Platt, M. L., & Huettel, S. A. (2008). Risky business: the neuroeconomics of decision making under uncertainty. Nature Neuroscience, 11(4), 398-403. doi:https://doi.org/10.1038/nn2062
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142-2154. doi:https://doi.org/10.1016/j.neuroimage.2011.10.018
Robinson, S. M., Sobell, L. C., Sobell, M. B., & Leo, G. I. (2014). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors, 28(1), 154-162. doi:https://doi.org/10.1037/a0030992
Rosselli, M., Ardila, A., Lubomski, M., Murray, S., & King, K. (2001). Personality profile and neuropsychological test performance in chronic cocaine-abusers. International Journal of Neuroscience, 110(1-2), 55-72. doi:https://doi.org/10.3109/00207450108994221
Roy, A. K., Gotimer, K., Kelly, A. C., Castellanos, F. X., Milham, M. P., & Ernst, M. (2011). Uncovering putative neural markers of risk avoidance. Neuropsychologia, 49(5), 937-944.
Rubin, L. H., Sacktor, N., Creighton, J., Du, Y., Endres, C. J., Pomper, M. G., & Coughlin, J. M. (2018). Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS, 32(12), 1661-1667. doi:https://doi.org/10.1097/QAD.0000000000001858
Sharp, C., Monterosso, J., & Montague, P. R. (2012). Neuroeconomics: a bridge for translational research. Biological Psychiatry, 72(2), 87-92. doi:https://doi.org/10.1016/j.biopsych.2012.02.029
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., … Dunbar, G. C. (1998). The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 30), 22-33.
Sirigu, A., & Duhamel, J. R. (2016). Reward and decision processes in the brains of humans and nonhuman primates. Dialogues Clinical Neuroscience, 18(1), 45-53.
Smith, B. W., Mitchell, D. G., Hardin, M. G., Jazbec, S., Fridberg, D., Blair, R. J. R., & Ernst, M. (2009). Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. NeuroImage, 44(2), 600-609.
Smith, K., Dickhaut, J., McCabe, K., & Pardo, J. V. (2002). Neuronal substrates for choice under ambiguity, risk, gains, and losses. Management Science, 48(6), 711-718. https://doi.org/10.1287/mnsc.48.6.711.194
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, Supplement 1, S208-S219. doi:https://doi.org/10.1016/j.neuroimage.2004.07.051
Spronk, D. B., van Wel, J. H. P., Ramaekers, J. G., & Verkes, R. J. (2013). Characterizing the cognitive effects of cocaine: A comprehensive review. Neuroscience & Biobehavioral Reviews, 37(8), 1838-1859. doi:https://doi.org/10.1016/j.neubiorev.2013.07.003
Studer, B., Apergis-Schoute, A. M., Robbins, T. W., & Clark, L. (2012). What are the Odds? The Neural Correlates of Active Choice during Gambling. Frontiers Neuroscience, 6, 46. doi:https://doi.org/10.3389/fnins.2012.00046
Thames, A. D., Streiff, V., Patel, S. M., Panos, S. E., Castellon, S. A., & Hinkin, C. H. (2012). The role of HIV infection, cognition, and depression in risky decision-making. The Journal of Neuropsychiatry and Clinical Neurosciences, 24(3), 340-348. doi:https://doi.org/10.1176/appi.neuropsych.11110340
Thames, A. D., Kuhn, T. P., Williamson, T. J., Jones, J. D., Mahmood, Z., & Hammond, A. (2017). Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV- adults. Drug and Alcohol Dependence, 170, 120-127. doi:https://doi.org/10.1016/j.drugalcdep.2016.11.007
Torres-Munoz, J., Stockton, P., Tacoronte, N., Roberts, B., Maronpot, R. R., & Petito, C. K. (2001). Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. Journal of Neuropathology and Experimental Neurology, 60(9), 885-892. doi:https://doi.org/10.1093/jnen/60.9.885
van den Heuvel, M. P., & Sporns, O. (2013). Network hubs in the human brain. Trends in Cognitive Sciences, 17(12), 683-696. doi:https://doi.org/10.1016/j.tics.2013.09.012
Ventura, N., Douw, L., Correa, D. G., Netto, T. M., Cabral, R. F., Lopes, F. C. R., & Gasparetto, E. L. (2018). Increased posterior cingulate cortex efficiency may predict cognitive impairment in asymptomatic HIV patients. Neuroradiology Journal, 31(4), 372-378. doi:https://doi.org/10.1177/1971400918782327
Verdejo-Garcia, A. J., Perales, J. C., & Perez-Garcia, M. (2007). Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behaviors, 32(5), 950-966. doi:https://doi.org/10.1016/j.addbeh.2006.06.032
Wechsler, D. (2001). Wechsler Test of Adult Reading (WTAR) Manual. San Antonio, TX: Harcourt Assessment.
Worhunsky, P. D., Potenza, M. N., & Rogers, R. D. (2017). Alterations in functional brain networks associated with loss-chasing in gambling disorder and cocaine-use disorder. Drug and Alcohol Dependence, 178, 363-371. doi:https://doi.org/10.1016/j.drugalcdep.2017.05.025
Yamaguchi, M., Suzuki, T., Seki, T., Namba, T., Liu, J., Arai, H., … Shiga, T. (2005). Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse, 58(2), 63-71. doi:https://doi.org/10.1002/syn.20182
Zhang, Y., Zhang, S., Ide, J. S., Hu, S., Zhornitsky, S., Wang, W., … Li, C. R. (2018). Dynamic network dysfunction in cocaine dependence: Graph theoretical metrics and stop signal reaction time. Neuroimage Clinical, 18, 793-801. doi:https://doi.org/10.1016/j.nicl.2018.03.016
Acknowledgements
This study was funded by grant K23-DA028660 from the United States National Institutes of Health. We are grateful to the UNC Center for AIDS Research (P30-AI50410) for its assistance with patient recruitment. The NIH had no further role in study design, data collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
Open practice statement
This study was not formally preregistered. Study data have not yet been provided to open source databases.
Author information
Authors and Affiliations
Contributions
CSM, SLT, SH, and RPB were responsible for study concept and design. RPB and ZL performed the functional magnetic resonance imaging analyses. CSM, SLT, SH, ZL, and RPB assisted with data analysis and interpretation. RPB drafted the manuscript. All authors critically reviewed content and approved the final version for publication.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bell, R.P., Towe, S.L., Lalee, Z. et al. Neural sensitivity to risk in adults with co-occurring HIV infection and cocaine use disorder. Cogn Affect Behav Neurosci 20, 859–872 (2020). https://doi.org/10.3758/s13415-020-00806-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-020-00806-4