Abstract
Strong evidence suggests that sleep plays a role in memory consolidation, which involves both stabilizing memory into long-term storage as well as integrating new information into existing stores. The current study investigated consolidation, across a day of wakefulness or night of sleep, of emotional and neutral directly learned visual paired associates (A-B/B-C pairs) as well as formation of memory for relational pairs formed via overlapping learned components (A-C pairs). Participants learned 40 negative and 40 neutral face-object pairs followed by a baseline test in session 1 either in the morning or evening. They then spent a 12-hour retention period during which participants either went about their normal day or spent the night in the sleep lab. During session 2, participants completed a surprise test to assess their memory for relational pairs (A-C) as well as memory for direct associates (A-B/B-C). As hypothesized, the results demonstrated that a 12-hour retention period predominantly spent asleep, compared to awake, benefited memory for both relational and direct associative memory. However, contrary to the hypothesis that emotional salience would promote preferential consolidation, sleep appeared to benefit both negative and neutral information similarly for direct associative and relational memories, suggesting that sleep may interact with other factors affecting encoding (e.g., depth of encoding) to benefit direct and relational associative memory. As one of the few studies examining the role of nocturnal sleep and emotion on both direct and relational associative memory, our findings suggest key insights into how overnight sleep consolidates these different forms of memory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep plays a key role in memory consolidation. As we sleep, the experiences and information acquired during the day are stabilized and integrated into long-term memory networks (Alger, Chambers, Cunningham, & Payne, 2015; Diekelmann & Born, 2010; Payne, Ellenbogen, Walker, & Stickgold, 2008; Payne, 2011; Payne, Stickgold, Swanberg, & Kensinger, 2008; Stickgold & Walker, 2013). Importantly, new pieces of information are not always learned and stored in a distinct, isolated manner. Rather, learning often requires the direct and indirect association of different components of complex information or experiences. Associated details are interleaved with existing memories to form and modify schemata or networks linked by common features of related memories (Ellenbogen, Hu, Payne, Titone, & Walker, 2007; Preston & Eichenbaum, 2013). Schemata influence how we process the world around us, allowing us to assign order to a world filled with numerous complex stimuli. In addition, schemata are modified by our perception of the emotional tone of an experience, which includes valence (e.g., positive, negative), as well as level of arousal (e.g., calming, exciting). It is possible that emotional salience serves as an evolutionary tool to ensure memory for potentially dangerous (or beneficial) information, and sleep often has been found to preferentially preserve this material (Bennion, Payne, & Kensinger, 2015; Chambers & Payne, 2014; Payne & Kensinger, 2010, 2018; Stickgold & Walker, 2013; Walker, 2009, for review). However, to our knowledge, only one study has examined the role of sleep and emotion on both direct and indirect associative memory (Alger & Payne, 2016). Furthermore, as far as we know, none have examined the influence of a full night of sleep on both types of memories. The present study sought to replicate and extend previous research by examining the effects of emotional salience and overnight sleep, both as isolated factors and interacting variables, on the ability to both directly and indirectly associate and consolidate information.
Direct associative memory refers to an explicitly learned relationship directly relating two pieces of information. The direct associations formed can also be extended to the formation of indirect associations, often referred to as relational memories. Relational memory refers to a relationship that was not explicitly taught and has no directly learned connection but can be inferred through logical thought. For example, if you are told that Alex is older than Sarah, and Sarah is older than Michael, you have learned two explicit, direct associations. From this, you can infer that Alex is older than Michael, despite never directly learning that information, and thus creating a relational memory. Our study employed a face-object memory task, whereby a series of images consisting of pairs of faces and objects were presented in two sets. As a result, it was explicitly learned that a particular face (A) was related to an object (B), and that another face (C) was associated with the same object (B). It could then be inferred that both faces (A-C) were indirectly associated, even though that relationship was never explicitly taught. In this case, memory for A-B and B-C pairs reflects direct associative memory, whereas A-C reflects relational memory. Sleep has been shown to facilitate the formation of both direct associative and relational memories. Ellenbogen et al. (2007), for example, demonstrated a strong benefit of sleep for inferential memory consolidation. Similarly, Lau, Tucker, and Fishbein (2010) used an A-B, B-C relational memory task similar to the current task and found that a daytime nap benefited both direct and relational associative memory.
However, learning in the real world is frequently accompanied by an emotional response to stimuli, which has implications for associative and relational memory formation, especially over a period of sleep. Not all memories are remembered equally, and negative information in particular tends to be preferentially remembered when compared to neutral stimuli (see Kensinger, 2009 for review; see also Payne, Chambers, & Kensinger, 2012; Payne et al., 2015; Payne, Stickgold, et al., 2008; Payne & Kensinger, 2010). This preferential consolidation of emotional information may extend to associative and relational memory. For instance, the prioritized binding theory (MacKay & Ahmetzanov, 2005) posits that negative stimuli are remembered better than neutral ones as a result of binding processes triggered by emotion. These processes link the emotional event to contextual details, such as location, or in the present study’s case, a neutral paired image of a face.
Although there is a wealth of evidence supporting a role for sleep in the preferential consolidation of emotional information, there are several studies that conclude otherwise. Sleep, even a brief nap, has been often shown to benefit memory for negative information (Groch, Wilhelm, Diekelmann, & Born, 2013; Hu, Stylos-Allan, & Walker, 2006; Nishida, Pearsall, Buckner, & Walker, 2009; Tempesta, Socci, De Gennaro, & Ferrara, 2018). Moreover, several studies demonstrate a selective benefit for emotional information after sleep, such that that the preference for processing negative stimuli comes at the expense of memory for neutral stimuli (Alger, Chen, & Payne, 2018; Payne et al., 2008, Payne et al., 2012; Payne, 2015). However, there are studies, including nap protocols, that show no effect of valence on memory performance after sleep, suggesting that the role of sleep on emotional memory consolidation is still being defined (Bennion, Payne, & Kensinger, 2016; Cairney, Durrant, Hulleman, & Lewis, 2014; Cellini, Torre, Stegagno, & Sarlo, 2016; Lehmann, Seifritz, & Rasch, 2016).
Furthermore, the effects of sleep on emotional associative memory, both direct and relational, are understudied, leaving a gap in knowledge that needs to be addressed. A study by Lehmann et al. (2016) found that a full night of sleep benefited both negative and neutral direct associative memories similarly, with neither valence showing preferential consolidation. Because this study tested only direct associates, the relationship between overnight sleep and indirectly associated, relational emotional memory pairs remains unclear. To our knowledge, only one study has examined the role of sleep with regard to both emotional direct associative memory consolidation and the formation of emotional relational memories. Using a daytime nap protocol, Alger and Payne (2016) found, as hypothesized, that those who napped had better retention of the direct associative pairs than those who remained awake. However, even though the negative direct associative pairs were remembered better overall compared with the neutral pairs, their results showed a selective preservation of neutral associations following the nap. Whereas memory for negative pairs deteriorated at similar rates between conditions, neutral pairs deteriorated more across a period of wakefulness than across a period of sleep. Relational memory, on the other hand, showed the nap group outperforming the wake group on both negative and neutral relational pairs. The authors suggested that because neutral pairs were perhaps more difficult to encode, learning them required a deeper level of encoding, which in turn led to sleep-based reactivation and preferential consolidation of neutral pairs across the nap (Talamini, Nieuwenhuis, Takashima, & Jensen, 2008). Given the lack of research investigating the development of memory on this task across a night of sleep, and the unexpected behavioral findings of Alger and Payne’s nap study (2016), the current study sought to determine if these results would be replicated when extended to an overnight design.
We explored the possibility that emotional associative and relational memory formation are sensitive to the inherent differences in the sleep physiology of overnight sleep compared with nap physiology of previous research. With regard to the sleep physiology underlying consolidation and integration, research suggests rapid eye movement (REM) and nonrapid eye movement (NREM) sleep may play distinct and possibly complementary roles. REM sleep appears to be important for consolidating emotional memories, sometimes at the expense of neutral memories (see Genzel, Spoormaker, Konrad, & Dresler, 2015 for review; see also Goldstein & Walker, 2014; Nishida et al., 2009; Payne et al., 2012, 2008; Plihal & Born, 1997). REM sleep also is known to aid in the process of memory integration, including creative problem solving (Cai, Mednick, Harrison, Kanady, & Mednick, 2009), making this stage of sleep important for associative memory. Strong evidence also suggests a role for NREM sleep in declarative memory consolidation (Diekelmann & Born, 2010, for review), with several more recent studies also suggesting a role for SWS in emotional memory consolidation and memory integration (Alger et al., 2018; Gais & Born, 2004; Lewis & Durrant, 2011; Payne & Kensinger, 2018; Payne et al., 2015). Furthermore, there is some evidence that it is not purely the presence of REM or SWS, but rather the interactions between the two that lead to memory consolidation (Batterink, Westerberg, & Paller, 2017; Giuditta, 2014; Giuditta et al., 1995). One proposed theory, known as the sequential hypothesis, suggests that memory consolidation is the result of selective strengthening or elimination during SWS followed by a reactivation and then reintegration of the strengthened memories during REM sleep.
Research examining relational memory has predominantly been conducted using daytime naps as the sleep opportunity. In these studies, the findings regarding sleep physiology contributions to relational memory formation are somewhat conflicting. Lau et al. (2010) demonstrated that SWS facilitated the formation of relational memories, using a face-object task with only neutral information. Alger and Payne (2016) found that REM sleep had an inverse relationship with direct associative and relational memories. Not only was more REM sleep in the nap unexpectedly related to greater forgetting of memory for the direct associates, but it also predicted greater formation of the relational memories of neutral, not emotional, content. The authors did not find a relationship between SWS and memory performance. Other nap studies have found no effect of either REM sleep (Cellini et al., 2016) or SWS (Groch et al., 2013) on emotional declarative memory. This is in contrast to a substantial literature linking REM sleep with emotional memory consolidation (see Hutchison & Rathore, 2015; Payne & Kensinger, 2010; van der Helm & Walker, 2009 for review). Notably, shorter sleep opportunities (i.e., naps) rarely contain the SWS-REM cycles that are present in overnight sleep and may be a requisite to complete the consolidation and formation of the direct associative and relational memories, both emotional and neutral. Furthermore, daytime naps and nocturnal sleep are associated with different phases of the circadian rhythm, including differences in cortisol phase (Weitzman et al., 1971), which may impact emotional memory consolidation (Payne & Kensinger, 2018; Payne, in press). Taking these equivocal findings into account, and in light of the unexpected findings of Alger and Payne (2016), the current study aimed determine whether the unexpected relationships between REM sleep and direct and relational associative memory would extend to overnight sleep physiology.
To our knowledge, the current study is the first to look at the combined effects of sleep and valence on both indirect and direct associative memory using an overnight sleep design. First, given the evidence that sleep supports both emotional and nonemotional declarative memory consolidation, we predicted that memory retention for both direct associative and relational memory would be greater following a night of sleep when compared to an equivalent period of daytime wake. In addition, given the wealth of evidence demonstrating the benefit of emotional salience to memory, we expected that negative pairs would be better remembered than the neutral pairs overall. Furthermore, we anticipated that a full night of sleep, with multiple sleep cycles, would lead to preferential memory for both negative direct and relational associations over the neutral information due to selective processing of emotional information. This finding would be contrary to Alger and Payne (2016), who found preferential preservation of memory for neutral direct associates and equal facilitation of neutral and negative relational associations over a nap. Finally, in light of previous studies correlating REM sleep in different forms of memory consolidation, including emotional and associative memory (Alger & Payne, 2016; Goldstein & Walker, 2014; Groch et al., 2013; Nishida et al., 2009; Ravassard et al., 2016), we predicted a preservation of memory associated with high percentages of REM sleep.
Materials and methods
Participants
Sixty-six healthy volunteers were recruited to participate from the undergraduate population at the University of Notre Dame. All participants were recruited via the SONA online participant management system, through which all eligible undergraduates registered to volunteer for research in exchange for course credit or monetary payment, as well as through flyers posted around campus. Before arriving at the lab, participants were assigned to either wake or sleep conditions based on their schedule availability. Of the original 66 participants, 2 met exclusion criteria, 4 withdrew before completing the study, and 5 were excluded due to data collection errors. Therefore, 55 participants completed the study: (sleep condition: n = 27, 13 females, age: mean ± SEM, 19.36 ± 0.29, range: 18-26; wake condition: n = 28, 17 females, age: 19.79 ± 0.67, range: 18-36). Based on effect sizes reported by (Lipinska, Stuart, Thomas, Baldwin, & Bolinger, 2019) and an online power analysis tool (WebPower; Zhang & Yuan, 2018), we determined that a sample size of at least n = 22 within the sleep condition would be needed to detect the preferential consolidation of negative relative to neutral information after sleep.
All participants were in good health and free of any sleep disturbances or mental illness, as assessed by a standard screening form and a 3-day sleep log completed prior to arriving in lab. Participants were excluded for any of the following reasons: prior participation in related studies in the lab, history of psychiatric, neurological, endocrine, metabolic, or sleep disorders, and current use of any drug that effects the central nervous system. Participants also had normal or corrected-to-normal vision and identified English as their first language. Participants completed a series of questionnaires throughout the experiment to gather further demographic information and as part of a standard battery to assess psychological conditions, sleep habits, alertness, etc. Questionnaires included the Beck Depression Index (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), Beck Anxiety Index (BAI; Beck, Epstein, Brown, & Steer, 1988), Acute Life Events Questionnaire (ALEQ; Haeffel et al., 2007), State Trait Anxiety Index (STAI-X1 and X2; Spielberger, 2010), Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), Morningness-Eveningness Questionnaire (MEQ; Horne & Ostberg, 1976), and Stanford Sleepiness Scale (SSS; Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973). This study was conducted according to the principles expressed in the University of Notre Dame Human Subjects Institutional Review Board, with all subjects providing written informed consent.
Memory task
The present study used an adapted associative inference task (Bunsey & Eichenbaum, 1996; Lau et al., 2010; Preston, Shrager, Dudukovic, & Gabrieli, 2004) previously used by Alger and Payne (2016). The stimuli consisted of two sets of photograph pairs, with 40 pairs per set. Each pair consisted of a face and an object, with the same 40 objects used in both sets. The faces in each pair were forward facing and looking at the camera in front of a gray background with a neutral expression. An equal number of male and female faces were used, representing a variety of ethnic backgrounds. The objects were easily identifiable and presented on a white background. Half of the objects were of a negative valence (e.g., a bloody knife) while the other half were of neutral valence (e.g., an apple). Images were previously rated and normed for valence and arousal by Alger and Payne (2016) to ensure correct categorization as negative and neutral images. Participants encoded two sets of 40 face-object pairs (an A-B set and a B-C set) on a computer, with a presentation time of 3.0 seconds per pair. Four lists, each containing the same 80 pairs randomly divided into A-B and B-C sets, were created to balance presentation and avoid order and list effects. Additionally, in each list, half of the objects were paired with male faces and half were paired with female faces to eliminate sex bias. All faces across the four counterbalanced lists appeared with both a negative and a neutral object. Each object appeared with both a male and female face at some point across the lists. Participants viewed the complete set of all 40 A-B twice through, with a 1-minute break in between exposures of each set, followed by baseline testing of memory for A-B pairs. They then viewed the B-C set of 40 pairs twice, followed by baseline testing of memory for B-C pairs. This encoding design was determined following a pilot study (n = 12) that resulted in a level of face-object pair learning high enough to ensure adequate baseline memory (approximately 75% retention when tested immediately following encoding).
Procedure
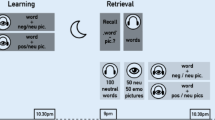
Participants in both conditions completed a two-part study with a 12-hour break between sessions (see Fig. 1 for experimental protocol). At either 8:30 PM (sleep condition) or 9:00 AM (wake condition), participants arrived at the lab and provided informed consent. They then filled out a series of questionnaires on the computer.
Experimental protocol. Participants arrived to lab at either 9:00 AM (wake condition) or 8:30 PM (sleep condition) for session 1, which included a series of background questionnaires, and the encoding portion of the face-object memory task. Both conditions then had a 12-hour break during which the wake condition experienced normal daytime wake and the sleep condition spent the night in our lab with PSG monitoring. During session 2, participants completed a second set of questionnaires as well as a surprise relational memory test followed by a general associative memory retest
Participants then began the encoding session of the Face-Object memory task. Participants were instructed to attend to the face-object pairs and try to associate them in their mind. Each face-object pair in both the A-B and B-C sets was visible for 3.0 seconds with an interstimulus interval of 0.5 seconds. A baseline recognition test of these direct associative pairs was given immediately following encoding of each individual set, rather than at the end of all the encoding (i.e., encode A-B, encode A-B, baseline test A-B set, and repeat for B-C; Fig. 2). Participants viewed a face on the screen presented with four objects (two neutral, two negative) and were told to choose the object that had been paired with the cued face during encoding. The three “incorrect” options were all objects that had been previously viewed in the set to ensure familiarity, controlling for the possibility that choices could be eliminated, because they were recognized as novel. The three incorrect answers had all been paired with a face of the same gender as the cued face. Baseline testing was conducted for all 80 pairs encoded in session 1.
Procedure for encoding and baseline testing in session 1. During the encoding session, participants viewed two sets of 40 face-object pairs (A-B list and B-C list), with the same 40 objects kept the same in both sets of pairs. Half of the objects were negative salience (spider) and half were neutral (tea cup). Immediately following encoding, participants completed a recognition task to assess baseline memory. (Encode A-B; Test A-B; Encode B-C; Test B-C). In the recognition task, participants were given a face and asked to choose among the four given objects the one that had been previously paired with the given face. Objects were identical between sets and therefore had been paired with two different faces by the end of the session. (Reprinted with author permission from Alger & Payne, 2016)
The encoding session was followed by a 12-hour retention period. Participants in the wake condition left the lab and went about their daily life but were instructed to avoid napping, rigorous exercise, caffeine, alcohol, or unnecessary drugs before returning for session 2. Participants in the sleep condition remained in the lab overnight and were prepared for overnight sleep with standard polysomnographic procedures. Participants watched a nonarousing movie while electrodes were applied, which occurred between 10:30-11:30 PM and took approximately 35 minutes. Subjects were monitored online using digital EEG acquisition software (Comet System-Grass/Twin PSG Clinical Software) using a standard polysomnography montage that included electrooculography (EOG), electromyography (EMG), and electroencephalography (EEG) from F3, F4, C3, C4, and Cz channels with each electrode referenced to the contralateral mastoid. The participants went to bed in individual sound- and light-attenuated bedrooms between 11:00-11:30 PM and were awakened between 7:30-8:00 AM to allow for an 8-hour sleep opportunity. Participants were restricted from access to mobile devices that may have disturbed sleep. Once awake, the electrodes were removed and participants were given 30 minutes to move about the lab to ensure they were fully alert before starting session 2. Sleep recordings were scored for sleep stages following the standards of Rechtschaffen and Kales (1968) to be used for correlational analysis.
During session 2, participants first completed a second, shorter set of questionnaires to account for any state differences between the sessions. Participants then were given a surprise memory test for the 40 indirect relational pairs created by two faces sharing a relationship with the same object. The participants were given a cued face on the screen and asked to match it to one of the four possible faces shown (Fig. 3). This relationship had not been directly learned and thus was a test of indirect association. This was followed by a final retest on all previously learned 80 face-object pairs (i.e., direct associative memory) together. The unexpected relational memory test always came before the expected associative memory retest to avoid a priming effect. Participants also were asked during the debriefing session whether they had expected the relational memory test or rehearsed the face-object pairs to confirm that the test had indeed been unexpected and that any memory effects were not due to rehearsal during the 12-hour break between sessions.
Procedure for relational memory retest. Indirect memory for the faces related by a shared object was tested during session 2, 12 hours after encoding. In this test, participants were given a face and asked to choose, from the four given faces, the face that had been previously paired with the same object as the given face. In the above example, this requires the participant to remember that the given face had been previously paired with the spider and then recall which of the four options also had been paired with the spider during encoding. This task was a “surprise” test as participant had not been made aware that the indirect (A-C) pairs existed. (Reprinted with author permission from Alger & Payne, 2016)
Data analysis
Face-Object Memory task responses were recorded as a proportion of correct hits (0.00 to 1.00, correct responses/total number of possible correct responses). All statistical tests were performed using SPSS Statistics software, version 24. As the primary concern of this experiment was the effect of sleep on associative memory, the results of both the direct and indirect associative memory task were compared between the sleep and wake conditions. For direct associative memory, a 2 x 2 x 2 mixed ANOVA was performed to assess the retention of memory for direct face-object pairs over the 12-hour break, with condition (sleep, wake) as the between-subjects factor and session (baseline, retest) and valence (negative, neutral) as the within-subjects factors. For relational memory, a 2 x 2 mixed ANCOVA was conducted with condition as the between-subjects factor and valence as the within-subjects factor, with negative and neutral baseline associative memory performance included as covariates to match the two levels of relational memory valence. Post hoc independent t-tests were also performed between each group for negative and neutral retest and relational memory tests to assess between-groups differences. Furthermore, paired t-tests were also run to look at the effect of valence within groups. The relationships between time spent in slow wave sleep (SWS) and rapid eye movement (REM) sleep and both relational and associative memory were assessed using Pearson’s correlations.
Results
Questionnaires
All questionnaire data was analyzed using two-tailed independent t-tests to check for any potential group differences, and no significance impact with regards to memory performance was found. Participants in both the wake and sleep condition had slept similar amounts both in the previous night (t(53) = 0.38, p = 0.70) and over the past 3 days (t(53) = −1.38, p = 0.17), as indicated in a self-report sleep log (Table 1). As participants self-selected their condition, the groups were also analyzed for differences in morningness-eveningness type, but no difference was found (t(53) = 0.32, p = 0.75).
Baseline memory performance
Baseline performance was defined as the number of correctly remembered face-object associative memory pairs divided by the total possible pairs both for A-B and B-C sets for each valence, yielding a proportion of correct hits. A one-way ANOVA was conducted to compare A-B and B-C performance at baseline to ensure that there were no differences in how the sets were encoded across conditions. There were no significant differences for any measure, overall, F(1,108) = 0.74, p = 0.39 (M ± SEM, 0.91 ± 0.02 for A-B, 0.89 ± 0.02 for B-C), or within negative and neutral sets, F(1, 108) = 0.57, p = 0.45; F(1,108) = 0.78, p = 0.38 (M ± SEM for negative: A-B 0.93 ± 0.02, B-C 0.91 ± 0.02; for neutral: A-B 0.90 ± 0.02, B-C 0.87 ± 0.03). Therefore, in all further analyses, performance on A-B and B-C pairs of each valence was collapsed to comprise an overall “baseline” and “retest” performance.
Independent t-tests were performed to verify that direct associative memory performance was similar between conditions at baseline. There were no significant differences between sleep and wake groups for baseline measures of either negative pairs, t(53) = −0.90, p = 0.37 (M ± SEM for wake: 0.90 ± 0.02, for sleep: 0.93 ± 0.02) or neutral pairs, t(53) = −0.86, p = 0.39 (wake: 0.87 ± 0.03; sleep: 0.91 ± 0.03) (Table 2). In addition, a paired t-test was performed on both the wake and sleep groups to compare encoding at baseline due to valence. Both groups correctly paired significantly more negative pairs at baseline than neutral pairs (wake: t(27) = 2.41, p = 0.02; sleep: t(26) = 4.22, p < 0.001). This confirms that both groups encoded the information similarly.
Direct associative (face-object) memory
A 2 x 2 x 2 mixed ANOVA found significant main effects of session F(1,53) = 92.53, p < 0.001, ηp2 = 0.64, and valence F(1,53) = 16.07, p < 0.001, ηp2 = 0. 23, indicating that, in both conditions, memory decreased from baseline to retest, and negative face-object pairs were remembered better overall than neutral pairs. The main effect of condition approached significance, F(1,53) = 3.74, p = 0.06, ηp2 = 0.07. This marginal main effect was further qualified by a significant two-way interaction between session and condition F(1,53) = 4.86, p = 0.03, ηp2 = 0.08, demonstrating, as hypothesized, superior preservation of memory for direct associates in those who slept overnight compared with those in the wake condition. There were no significant two-way interactions between valence and condition F(1,53) = 1.73, p = 0.19, or valence and session F(1,53) = 0.39, p = 0.54. There also was no significant three-way interaction between group, session and valence, F(1,53) = 1.15, p = 0.29, indicating that sleep similarly benefitted memory for both negative and neutral information. In order to further explore these effects and compare our results with those of the nap study by Alger and Payne (2016), post hoc analyses were conducted based on a-priori predictions. Two-tailed independent t-tests compared the change in memory performance from baseline to retest. There was a significant group difference for neutral face-object pairs, t(53) = −2.34, p = 0.02, d = 0.69, and a trending difference for negative face-object pairs t(53) = −1.83, p = 0.07, d = 0.40, indicating that overnight sleep played a role in the consolidation of both neutral and negative face-object pairs (Fig. 4).
Relational (face-face) memory
Relational memory was calculated as the percentage of correctly matched, indirectly associated face-face pairs divided by the total number of correct pairs possible, yielding a proportion correct. Performance on the relational memory task was dependent on how well the initial face-object direct associates were learned with a significant positively correlation between baseline performance on the face-object pairs and relational face-face memory for both negative, (r(53) = 0.62, p < 0.001) and neutral (r(53) = 0.65, p < 0.001) pairs. Thus, the corresponding baseline performance was included as a covariate when analyzing the relational memory data (Table 2).
A 2 x 2 mixed ANCOVA revealed a significant main effect of condition, F(1,51) = 18.22, p < 0.001, ηp2 = 0.26, suggesting that the sleep condition participants remembered significantly more face-face pairs than the wake group participants. Follow-up independent t-tests were conducted to confirm that the sleep condition had better relational memory than the wake condition for both negative, t(53) = -4.12, p < 0.001, d = 1.13, and neutral relational memory, t(53) = −3.04, p = 0.004, d = 0.83 (Fig. 5). There were no significant effects of valence, F(1,51) = 0.10, p = 0.76, suggesting that negative and neutral relational pairs were remembered roughly the same overall. There also was no significant interaction between valence and condition, F(1,51) = 0.92, p = 0.34, indicating that differences in memory for the negative and neutral pairs did not differ between sleep and wake conditions.
Relational memory performance between groups. A highly significant group difference was found between the sleep (n = 27) and wake (n = 28) groups both for negative (p = 0.001) and neutral (p = 0.004) relational memory. There was no significant effect of valence in the sleep (p = 0.12) or the wake (p = 0.24) conditions. Performance at baseline was used as a covariate when comparing relational memory between and within conditions. Error bars represent SEM. *p < 0.05; **p < 0.01
Sleep stages and memory
All polysomnography (PSG) data from the sleep condition were scored for sleep stages according to the standard of (Rechtschaffen & Kales, 1968). Three participants’ PSG data sets were excluded due to equipment malfunction during the night, resulting in 24 scored PSG recordings (see Table 3 for all sleep statistics and correlations). Pearson’s correlations were conducted between sleep stages of interest, reflected as percentages of total sleep time, and both associative and relational memory. No significant correlations were found between any sleep stages and memory performance of either valence, contrary to the findings of Alger and Payne (2016).
Discussion
As hypothesized, we found that individuals who slept overnight had better memory retention for both associative memory (directly learned face-object pairs) and relational memory (indirectly inferred face-face pairs) compared with those who remained awake during the day. We also predicted that the sleep group would preferentially remember the negative associations over the neutral pairs for both memory tasks, due to the emotional salience of the negative pairs. However, our study revealed a complex set of findings with regards to valence. Those who slept between sessions forgot significantly less neutral and nearly significantly less negative associative (face-object) pairs compared with those who spent the day awake, suggesting that overnight sleep benefitted memory relative to wakefulness, regardless of valence. With regard to relational memory, there again was no difference due to valence between conditions, which was contrary to our hypothesis that negative would be preferentially remembered over neutral pairs. In addition, there were no significant relationships between any sleep stage and associative or relational memory.
To our knowledge, the current study is the first to extend the previous research using emotional stimuli, particularly on relational memory, to an overnight design to attempt to replicate and reconcile findings in the nap research. Several previous studies have examined the role of sleep on associative and relational memory using a nap study design, concluding that sleep is involved in memory consolidation of both direct associative and relational memories (Alger & Payne, 2016; Coutanche, Gianessi, Chanales, Willison, & Thompson-Schill, 2013; Lau et al., 2010; Lau, Alger, & Fishbein, 2011). In addition, studies using a similar face-object memory task as the present experiment found that sleep leads to better memory performance on both the direct associative memory test and the surprise relational memory task compared with wakefulness. This memory benefit was seen if the pairs contained entirely neutral elements (Lau et al., 2010) or a combination of neutral and negative elements (Alger & Payne, 2016). In line with these findings, we observed benefits of overnight sleep on both types of associative memory (direct and relational), independent of stimulus valence.
Despite the many studies demonstrating preferential consolidation of emotional stimuli following sleep (Chambers & Payne, 2014; Payne et al., 2012, 2015; Payne & Kensinger, 2018; Tempesta et al., 2018), we did not observe sleep-related benefits for emotional memory, in line with Lehmann et al. (2016) and Lewis and colleagues (2011). Given that our findings do not support our hypothesis that negative information would be treated preferentially over sleep, we offer a potential, albeit speculative, explanation. The prioritized binding theory, for example, suggests that the presence of negative stimuli enhances the binding of associative pairs into a more memorable representation, and thus negative-neutral elements are remembered better than neutral-neutral pairs (MacKay & Ahmetzanov, 2005). However, as theorized by Murray and Kensinger (2012), intentionally forming associations between neutral items is more effortful than the quicker binding when negative salience is involved. This greater effort results in a deeper level of encoding. Alger and Payne (2016) discussed this theory as a possible explanation underlying the unexpected preferential benefit to neutral memory during sleep, with deeper encoding leading to a higher likelihood of these pairs being reactivated during sleep-based processing (Tucker & Fishbein, 2008). While the current study did not demonstrate preferential consolidation of neutral over negative memories, it may be that stronger encoding of the neutral pictures offset the benefit of negative salience, resulting in similar benefits for both neutral and negative pairs. The ability to form relational memories, being highly correlated to direct associative memory, likely benefitted similarly from a night of sleep, compared with wake, with both negative and neutral relational associations facilitated during sleep. However, more work will be necessary to test this hypothesis directly.
Unexpectedly, we did not observe any sleep physiology correlations with memory performance. Based on previous theories and findings (Genzel et al., 2015; Hutchison & Rathore, 2015; Nishida et al., 2009; Wagner, Hallschmid, Rasch, & Born, 2006), we predicted a preservation of emotional memory associated with higher percentages of REM sleep. We did not detect any relationship between REM sleep and any memory measure, nor did we detect relationships with SWS, another stage frequently associated with declarative memory processes (Gais & Born, 2004; Lewis & Durrant, 2011; Payne et al., 2015; Tucker et al., 2006) and implicated in nap studies examining associative and relational memory.
Because this is the first overnight study to examine sleep-based consolidation of emotional associative memory, both direct and relational, it is difficult to say why we found no relationship between memory and sleep physiology. However, several studies also report behavioral benefits of sleep without determining a specific relationship with sleep architecture (Baran, Pace-Schott, Ericson, & Spencer, 2012; Cellini et al., 2016). It also is possible that analyses looking purely at sleep stages may be insufficient for measuring the true sleep-related factors influencing memory. Several studies have suggested that memory consolidation is likely the result of underlying cellular mechanisms or sleep architecture characteristics (Doran, 2003; Ladenbauer et al., 2017; Nishida et al., 2009; Plihal & Born, 1997). Future research conducting analyses looking at sleep characteristics, including sleep spindle analysis or slow-wave oscillations, rather than sleep stages alone, may provide more insight into the mechanistic role of sleep in associative and relational memory.
It is important to note, when discussing why the current study did not replicate the same relationships between sleep and memory as Alger & Payne’s nap study, that naps and overnight sleep are inherently different. Naps by definition are much shorter than overnight sleep and do not see the same 90-minute cycles that are characteristic of overnight sleep. In addition, sleep structure changes dramatically over a normal overnight sleep, with the earlier portion of the night having a higher percentage of SWS and the later portion having greater REM sleep. Neurohormones may interact with overnight sleep in a manner that is different during the day, thus differentially influencing memory processing. Emotional memory, in particular, may be sensitive to the late-night rise in cortisol, which is at its nadir early in the night and gradually increases throughout the night (Nadel, Payne, & Jacobs, 2002; Payne & Kensinger, 2018; Weitzman et al., 1971). A nap, then, cannot be viewed simply as a condensed version of overnight sleep. Furthermore, interactions between sleep stages have been proposed to play a significant role in memory consolidation (Batterink et al., 2017; Giuditta, 2014; Giuditta et al., 1995). The sequential hypothesis, for example, theorizes that the brain sorts new information and distinguishes the relevant memories during SWS; these new memory traces are then integrated into existing schemata during REM sleep (Giuditta, 2014; Giuditta et al., 1995). Although the specific mechanism of this process has yet to be discovered, recent studies using targeted memory reactivation (TMR) during SWS have suggested the importance of the SWS-REM interactions in memory consolidation (Batterink et al., 2017; Cairney et al., 2014), providing some support for the sequential hypothesis. This is an interesting theory that future studies should continue to explore.
It should be acknowledged that using a daytime wake/nighttime sleep experimental design comes with potential confounds, because participants go through encoding and retest at different times of day. However, the daytime wake/overnight sleep experimental design is standard in the field to look at the effects of normal overnight sleep (as opposed to sleep deprivation). Prior research suggests that sleep-related emotional memory benefits are not affected by time-of-day influences on encoding and test (Payne et al., 2008, Payne & Kensinger, 2010). Furthermore, the study’s results are largely similar to previous studies controlling for time of day effects (Alger & Payne, 2016), which can help to eliminate time-of-day concerns. In addition, in our study, differences in memory performance could not be attributed to any other group difference, including morningness-eveningness type, which were assessed prior to encoding.
Conclusions
We demonstrate a benefit of sleep on the consolidation of both associative and relational memory, with especial emphasis on its role in relational memory. While some of our emotional memory findings showed an unexpected pattern of results, overall, this study provides additional support for the importance of sleep for memory consolidation. More importantly, these findings add to the literature suggesting that emotional valence may not always lead to preferential consolidation, at least in the case of associative and relational memories. Furthermore, it adds information into the debate of the role of sleep physiology in memory consolidation, supporting the idea that overnight sleep may work in a distinct manner from sleep during naps when it comes to memory consolidation. As such, our findings suggest that while naps may be sufficient to achieve the same memory benefits seen in overnight sleep, future studies should continue to investigate the differences in underlying mechanisms.
References
Alger, S. E., Chambers, A. M., Cunningham, T., & Payne, J. D. (2015). The role of sleep in human declarative memory consolidation. In P. Meerlo, R. M. Benca, & T. Abel (Eds.), Sleep, neuronal plasticity and brain function (pp. 269–306). https://doi.org/10.1007/7854_2014_341
Alger, S. E., Chen, S., & Payne, J. D. (2018). Do different salience cues compete for dominance in memory over a daytime nap? Neurobiology of Learning and Memory. https://doi.org/10.1016/j.nlm.2018.06.005
Alger, S. E., & Payne, J. D. (2016). The differential effects of emotional salience on direct associative and relational memory during a nap. Cognitive, Affective, & Behavioral Neuroscience, 16(6), 1150–1163. https://doi.org/10.3758/s13415-016-0460-1
Baran, B., Pace-Schott, E. F., Ericson, C., & Spencer, R. M. C. (2012). Processing of emotional reactivity and emotional memory over sleep. Journal of Neuroscience, 32(3), 1035–1042. https://doi.org/10.1523/JNEUROSCI.2532-11.2012
Batterink, L. J., Westerberg, C. E., & Paller, K. A. (2017). Vocabulary learning benefits from REM after slow-wave sleep. Neurobiology of Learning and Memory, 144, 102–113. https://doi.org/10.1016/j.nlm.2017.07.001
Beck, A. T., Epstein, N., Brown, G., & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897.
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
Bennion, K. A., Payne, J. D., & Kensinger, E. A. (2015). Selective effects of sleep on emotional memory: What mechanisms are responsible? Translational Issues in Psychological Science, 1(1), 79–88. https://doi.org/10.1037/tps0000019
Bennion, K. A., Payne, J. D., & Kensinger, E. A. (2016). The impact of napping on memory for future-relevant stimuli: Prioritization among multiple salience cues. Behavioral Neuroscience, 130(3), 281–289. https://doi.org/10.1037/bne0000142
Bunsey, M., & Eichenbaum, H. (1996). Conservation of hippocampal memory function in rats and humans. Nature, 379(6562), 255–257. https://doi.org/10.1038/379255a0
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213.
Cai, D. J., Mednick, S. A., Harrison, E. M., Kanady, J. C., & Mednick, S. C. (2009). REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences of the United States of America, 106(25), 10130–10134. https://doi.org/10.1073/pnas.0900271106
Cairney, S. A., Durrant, S. J., Hulleman, J., & Lewis, P. A. (2014). Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep, 37(4), 701–707, 707A. https://doi.org/10.5665/sleep.3572
Cellini, N., Torre, J., Stegagno, L., & Sarlo, M. (2016). Sleep before and after learning promotes the consolidation of both neutral and emotional information regardless of REM presence. Neurobiology of Learning and Memory, 133, 136–144. https://doi.org/10.1016/j.nlm.2016.06.015
Chambers, A. M., & Payne, J. D. (2014). Laugh yourself to sleep: Memory consolidation for humorous information. Experimental Brain Research, 232(5), 1415–1427. https://doi.org/10.1007/s00221-013-3779-7
Coutanche, M. N., Gianessi, C. A., Chanales, A. J. H., Willison, K. W., & Thompson-Schill, S. L. (2013). The role of sleep in forming a memory representation of a two-dimensional space. Hippocampus, 23(12), 1189–1197. https://doi.org/10.1002/hipo.22157
Diekelmann, S., & Born, J. (2010). The memory function of sleep. Nature Reviews. Neuroscience, 11(2), 114–126. https://doi.org/10.1038/nrn2762
Doran, S. M. (2003). The dynamic topography of individual sleep spindles. Sleep Research Online, 5, 133–139.
Ellenbogen, J. M., Hu, P. T., Payne, J. D., Titone, D., & Walker, M. P. (2007). Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences of the United States of America, 104(18), 7723–7728. https://doi.org/10.1073/pnas.0700094104
Gais, S., & Born, J. (2004). Declarative memory consolidation: Mechanisms acting during human sleep. Learning & Memory, 11(6), 679–685. https://doi.org/10.1101/lm.80504
Genzel, L., Spoormaker, V. I., Konrad, B. N., & Dresler, M. (2015). The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiology of Learning and Memory, 122, 110–121. https://doi.org/10.1016/j.nlm.2015.01.008
Giuditta, A. (2014). Sleep memory processing: The sequential hypothesis. Frontiers in Systems Neuroscience, 8, 219. https://doi.org/10.3389/fnsys.2014.00219
Giuditta, A., Ambrosini, M. V., Montagnese, P., Mandile, P., Cotugno, M., Zucconi, G. G., & Vescia, S. (1995). The sequential hypothesis of the function of sleep. Behavioural Brain Research, 69(1), 157–166. https://doi.org/10.1016/0166-4328(95)00012-I
Goldstein, A. N., & Walker, M. P. (2014). The role of sleep in emotional brain function. Annual Review of Clinical Psychology, 10, 679–708. https://doi.org/10.1146/annurev-clinpsy-032813-153716
Groch, S., Wilhelm, I., Diekelmann, S., & Born, J. (2013). The role of REM sleep in the processing of emotional memories: Evidence from behavior and event-related potentials. Neurobiology of Learning and Memory, 99, 1–9. https://doi.org/10.1016/j.nlm.2012.10.006
Haeffel, G. J., Abramson, L. Y., Brazy, P. C., Shah, J. Y., Teachman, B. A., & Nosek, B. A. (2007). Explicit and implicit cognition: A preliminary test of a dual-process theory of cognitive vulnerability to depression. Behaviour Research and Therapy, 45(6), 1155–1167. https://doi.org/10.1016/j.brat.2006.09.003
Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., & Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10(4), 431–436.
Horne, J. A., & Ostberg, O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4(2), 97–110.
Hu, P., Stylos-Allan, M., & Walker, M. P. (2006). Sleep facilitates consolidation of emotional declarative memory. Psychological Science, 17(10), 891–898. https://doi.org/10.1111/j.1467-9280.2006.01799.x
Hutchison, I. C., & Rathore, S. (2015). The role of REM sleep theta activity in emotional memory. Frontiers in Psychology, 6. https://doi.org/10.3389/fpsyg.2015.01439
Kensinger, E. A. (2009). What factors need to be considered to understand emotional memories? Emotion Review: Journal of the International Society for Research on Emotion, 1(2), 120–121. https://doi.org/10.1177/1754073908100436
Ladenbauer, J., Ladenbauer, J., Külzow, N., Boor, R. de, Avramova, E., Grittner, U., & Flöel, A. (2017). Promoting sleep oscillations and their functional coupling by transcranial stimulation enhances memory consolidation in mild cognitive impairment. Journal of Neuroscience, 0260–17. https://doi.org/10.1523/JNEUROSCI.0260-17.2017
Lau, H., Tucker, M. A., & Fishbein, W. (2010). Daytime napping: Effects on human direct associative and relational memory. Neurobiology of Learning and Memory, 93(4), 554–560. https://doi.org/10.1016/j.nlm.2010.02.003
Lau, H., Alger, S. E., & Fishbein, W. (2011). Relational memory: A daytime nap facilitates the abstraction of general concepts. PLOS ONE, 6(11), e27139. https://doi.org/10.1371/journal.pone.0027139
Lehmann, M., Seifritz, E., & Rasch, B. (2016). Sleep benefits emotional and neutral associative memories equally. Somnologie, 20(1), 47–53. https://doi.org/10.1007/s11818-015-0034-4
Lewis, P. A., & Durrant, S. J. (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Sciences, 15(8), 343–351. https://doi.org/10.1016/j.tics.2011.06.004
Lipinska, G., Stuart, B., Thomas, K. G. F., Baldwin, D. S., & Bolinger, E. (2019). Preferential consolidation of emotional memory during sleep: A meta-analysis. Frontiers in Psychology, 10. https://doi.org/10.3389/fpsyg.2019.01014
MacKay, D. G., & Ahmetzanov, M. V. (2005). Emotion, memory, and attention in the taboo Stroop paradigm. Psychological Science, 16(1), 25–32. https://doi.org/10.1111/j.0956-7976.2005.00776.x
Murray, B. D., & Kensinger, E. A. (2012). The effects of emotion and encoding strategy on associative memory. Memory & Cognition, 40(7), 1056–1069. https://doi.org/10.3758/s13421-012-0215-3
Nadel, L., Payne, J. D., & Jacobs, W. J. (2002). The relationship between episodic memory and context: Clues from memory errors made while under stress. Physiological Research, 51(SUPPL. 1). Retrieved from https://arizona.pure.elsevier.com/en/publications/the-relationship-between-episodic-memory-and-context-clues-from-m
Nishida, M., Pearsall, J., Buckner, R. L., & Walker, M. P. (2009). REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex (New York, NY), 19(5), 1158–1166. https://doi.org/10.1093/cercor/bhn155
Payne, J. D. (2019). Stress and sleep interact to selectively consolidate and transform negative emotional memories: Implications for Clinical Treatment. In: Neuroscience of Enduring Change: Implications for Psychotherapy. Oxford: Oxford University Press. in press.
Payne, J., Ellenbogen, J. M., Walker, M., & Stickgold, R. (2008). The role of sleep in memory consolidation. In Learning and memory: A comprehensive reference (pp. 663–685). https://doi.org/10.1016/B978-012370509-9.00181-9
Payne, J. D. (2011). Learning, memory, and sleep in humans. Sleep Medicine Clinics, 6(1), 15–30. https://doi.org/10.1016/j.jsmc.2010.12.005
Payne, J. D., Chambers, A. M., & Kensinger, E. A. (2012). Sleep promotes lasting changes in selective memory for emotional scenes. Frontiers in Integrative Neuroscience, 6. https://doi.org/10.3389/fnint.2012.00108
Payne, J. D., & Kensinger, E. A. (2010). Sleep’s role in the consolidation of emotional episodic memories. Current Directions in Psychological Science, 19(5), 290–295. https://doi.org/10.1177/0963721410383978
Payne, J. D, & Kensinger, E. A. (2018). Stress, sleep, and the selective consolidation of emotional memories. Current Opinion in Behavioral Sciences, 19, 36–43. https://doi.org/10.1016/j.cobeha.2017.09.006
Payne, J. D., Kensinger, E. A., Wamsley, E. J., Spreng, R. N., Alger, S. E., Gibler, K., … Stickgold, R. (2015). Napping and the selective consolidation of negative aspects of scenes. Emotion (Washington, D.C.), 15(2), 176–186. https://doi.org/10.1037/a0038683
Payne, J. D., Stickgold, R., Swanberg, K., & Kensinger, E. A. (2008). Sleep preferentially enhances memory for emotional components of scenes. Psychological Science, 19(8), 781–788. https://doi.org/10.1111/j.1467-9280.2008.02157.x
Plihal, W., & Born, J. (1997). Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience, 9(4), 534–547. https://doi.org/10.1162/jocn.1997.9.4.534
Preston, A. R., & Eichenbaum, H. (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology : CB, 23(17), R764–R773. https://doi.org/10.1016/j.cub.2013.05.041
Preston, A. R., Shrager, Y., Dudukovic, N. M., & Gabrieli, J. D. E. (2004). Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus, 14(2), 148–152. https://doi.org/10.1002/hipo.20009
Ravassard, P., Hamieh, A. M., Joseph, M. A., Fraize, N., Libourel, P.-A., Lebarillier, L., … Salin, P.-A. (2016). REM sleep-dependent bidirectional regulation of hippocampal-based emotional memory and LTP. Cerebral Cortex, 26(4), 1488–1500. https://doi.org/10.1093/cercor/bhu310
Rechtschaffen, A., & Kales, A. (1968). A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Allan Rechtschaffen and Anthony Kales, editors. - NLM Catalog - NCBI. Retrieved January 28, 2018, from https://www.ncbi.nlm.nih.gov/nlmcatalog/?term=Rechtschaffen%20A,%20Kales%20A%20%281968%29
Spielberger, C. D. (2010). State-trait anxiety inventory. In The Corsini Encyclopedia of Psychology. https://doi.org/10.1002/9780470479216.corpsy0943
Stickgold, R., & Walker, M. P. (2013). Sleep-dependent memory triage: Evolving generalization through selective processing. Nature Neuroscience, 16(2), 139–145. https://doi.org/10.1038/nn.3303
Talamini, L. M., Nieuwenhuis, I. L. C., Takashima, A., & Jensen, O. (2008). Sleep directly following learning benefits consolidation of spatial associative memory. Learning & Memory, 15(4), 233–237. https://doi.org/10.1101/lm.771608
Tempesta, D., Socci, V., De Gennaro, L., & Ferrara, M. (2018). Sleep and emotional processing. Sleep Medicine Reviews, 40, 183–195. https://doi.org/10.1016/j.smrv.2017.12.005
Tucker, M. A., & Fishbein, W. (2008). Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep, 31(2), 197–203. https://doi.org/10.1093/sleep/31.2.197
Tucker, M. A., Hirota, Y., Wamsley, E. J., Lau, H., Chaklader, A., & Fishbein, W. (2006). A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory, 86(2), 241–247. https://doi.org/10.1016/j.nlm.2006.03.005
van der Helm, E., & Walker, M. P. (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin, 135(5), 731–748. https://doi.org/10.1037/a0016570
Wagner, U., Hallschmid, M., Rasch, B., & Born, J. (2006). Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry, 60(7), 788–790. https://doi.org/10.1016/j.biopsych.2006.03.061
Walker, M. P. (2009). The role of slow wave sleep in memory processing. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 5(2 Suppl), S20-26.
Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070.
Weitzman, E. D., Fukushima, D., Nogeire, C., Roffwarg, H., Gallagher, T. F., & Hellman, L. (1971). Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. The Journal of Clinical Endocrinology and Metabolism, 33(1), 14–22. https://doi.org/10.1210/jcem-33-1-14
Zhang, Z., & Yuan, K.-H. (2018). Practical statistical power analysis using Webpower and R (eds). Granger, IN: ISDSA Press.
Acknowledgments
The authors thank Hannah Kenneally for her invaluable contributions to this study.
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Open Practice Statement
The data and materials for the experiment can be made available upon request, and the experiment was not preregistered.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huguet, M., Payne, J.D., Kim, S.Y. et al. Overnight sleep benefits both neutral and negative direct associative and relational memory. Cogn Affect Behav Neurosci 19, 1391–1403 (2019). https://doi.org/10.3758/s13415-019-00746-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-019-00746-8