Abstract
Response conflicts play a prominent role in the flexible adaptation of behavior as they represent context-signals that indicate the necessity for the recruitment of cognitive control. Previous studies have highlighted the functional roles of the affectively aversive and arousing quality of the conflict signal in triggering the adaptation process. To further test this potential link with arousal, participants performed a response conflict task in two separate sessions with either transcutaneous vagus nerve stimulation (tVNS), which is assumed to activate the locus coeruleus-noradrenaline (LC-NE) system, or with neutral sham stimulation. In both sessions the N2 and P3 event-related potentials (ERP) were assessed. In line with previous findings, conflict interference, the N2 and P3 amplitude were reduced after conflict. Most importantly, this adaptation to conflict was enhanced under tVNS compared to sham stimulation for conflict interference and the N2 amplitude. No effect of tVNS on the P3 component was found. These findings suggest that tVNS increases behavioral and electrophysiological markers of adaptation to conflict. Results are discussed in the context of the potentially underlying LC-NE and other neuromodulatory (e.g., GABA) systems. The present findings add important pieces to the understanding of the neurophysiological mechanisms of conflict-triggered adjustment of cognitive control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control
Goal-directed adaptive behavior is based on mechanisms of cognitive control. Individuals flexibly engage in cognitive control to protect the current action goal from distraction (shielding), while at the same time relax goal shielding when the environment calls for integrative processing and goal updating (Cohen, McClure, & Yu, 2007; Goschke, 2013; Hommel, 2015; Miller & Cohen, 2001). According to the influential conflict monitoring theory, optimal balance between shielding and relaxation can be attained and regulated by registering signals, such as response conflicts, that indicate the need for the up-regulation of cognitive control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004). More precisely, response conflicts as measured in Stroop, Simon or Flanker tasks are detected by a conflict-monitoring module (e.g., the anterior cingulate cortex, ACC), which then signals to a control unit (e.g., dorsolateral prefrontal cortex, dlPFC) to recruit cognitive control in order to adjust and optimize subsequent behavior. Importantly, the conflict, that results from two simultaneously active response alternatives, serves as signal that initiates an up-regulation of goal shielding (e.g., increased attentional focus on task-relevant features), which facilitates the attentional selection in the subsequent trial (cf. Gratton, Coles, & Donchin, 1992). Consistent with these predictions, sequential modulations of the conflict effect, i.e., reduced conflict interference following conflict trials, have been interpreted as evidence for a conflict-triggered adjustment of cognitive control (for recent reviews see Duthoo, Abrahamse, Braem, Boehler, & Notebaert, 2014; Egner, 2007, 2017). Most research has since been dedicated to specifying the mechanistic processes that underlie and/or are involved in the adaptation to conflict (e.g., see Abrahamse, Braem, Notebaert, & Verguts, 2016; Braem, Abrahamse, Duthoo, & Notebaert, 2014; Duthoo et al., 2014; Egner, 2014, 2017).
Only recently, motivational and affective influences on conflict adaptation (for reviews see Dreisbach & Fischer, 2012b, 2016; van Steenbergen, 2015) as well as the functionality and the affective properties of the conflict signal itself have shifted into the focus of research (Botvinick, 2007; Dreisbach & Fischer, 2012a; Fritz & Dreisbach, 2013; Schouppe, De Houwer, Ridderinkhof, & Notebaert, 2012). It has been argued, for example, that the adaptation to conflict is motivated by the aversive quality of the conflict experience (Dreisbach & Fischer, 2012a, 2015, 2016; see also Inzlicht, Bartholow, & Hirsh, 2015). Empirical evidence supports this claim, demonstrating that negative mood enhances adaptation to conflict (Schuch & Koch, 2015; van Steenbergen, Band, & Hommel, 2010). More direct evidence shows that response conflicts are evaluated as aversive experiences (Braem et al., 2017; Dreisbach & Fischer, 2012a; Fritz & Dreisbach, 2013, 2015; Schouppe et al., 2015), motivate avoidance behavior (Dignath, Kiesel, & Eder, 2015; Schouppe et al., 2012) and the salience and thus effectiveness of the conflict signal in triggering a recruitment of control depends on the affective context in which the conflict signal is presented (Dreisbach, Reindl, & Fischer, 2017; Fritz, Fischer, & Dreisbach, 2015).
Although by now it has been widely established that conflict signals are registered as aversive emotional events (Dreisbach & Fischer, 2015; Inzlicht et al., 2015; van Steenbergen, 2015), it remains unclear to which extent negative affective valence, increased arousal, or both contribute to the adaptation to conflict. Similarly, the specification of the underlying neurotransmitter systems responsible for adjustments to affective conflict experience remains largely unclear (cf. van Steenbergen, 2015).
An important role has been attributed to the neuromodulatory neurotransmitter dopamine, as the activation of the ACC is strongly interconnected with dopaminergic function (Holroyd & Coles, 2002). The ACC is supposed to indirectly influence response selection pathways via midbrain dopaminergic neurons (Frank, 2005). In particular, aversive stimuli trigger phasic inhibition of dopaminergic neurons in the ventral tegmental area in rats (Ungless, Magill, & Bolam, 2004). Thus, dopamine is assumed to play a key role in signaling and resolving conflict (Botvinick, 2007; Holroyd & Coles, 2002), with response conflicts and thus, aversive events, inducing a phasic reduction in dopamine levels. Despite the strong evidence for the prominent role of the dopaminergic system in performance monitoring and behavioral adjustments (Holroyd & Coles, 2002), it is unlikely, however, that the dopaminergic system is the exclusive neuromodulator driving the adaptation to conflict (Jocham & Ullsperger, 2009). In a recent pharmacological approach, van Steenbergen and colleagues tested to which extent the mu-opioid system modulates the aversive arousal effect on adaptation to conflict (van Steenbergen, Weissman, Stein, Malcolm-Smith, & van Honk, 2017). With its high distribution of mu-opioid receptors in the ACC (Bush, Luu, & Posner, 2000), it was hypothesized that an active mu-opioid system may attenuate the aversive arousal response to conflicts. Blocking the mu-opioid system by administering the opioid antagonist naltrexone should therefore increase an aversive arousal response (van Steenbergen, 2015). Results showed increased post-error slowing but no effect on adaptation to conflict, which was interpreted as errors eliciting stronger aversive arousal than conflicts (van Steenbergen et al., 2017).
Furthermore, nuclei of the main neuromodulators in the midbrain (grouping dopaminergic neurons), in the basal forebrain (grouping cholinergic neurons), and in the brainstem (grouping noradrenergic neurons), share not only highly reciprocal connections but are also commonly regulated by the prefrontal cortex (see Aston-Jones & Cohen, 2005; Sara, 2009, for a detailed discussion). In particular, the involvement of the neurotransmitter norepinephrine (NE) might be of particular relevance as neurochemical basis for performance monitoring (Jocham & Ullsperger, 2009; Riba, Rodriguez-Fornells, Morte, Munte, & Barbanoj, 2005). Compared to the established significance of the dopaminergic system, however, the specific role of the locus coeruleus-norepinephrine (LC-NE) system in the adaptation to conflict, for example, remains mostly theoretical (Verguts & Notebaert, 2008, 2009) with to date inconclusive empirical support (see for example de Rover et al., 2015, for a failed attempt of demonstrating an impact of the ß-adrenergic system on conflict processing). However, accumulating findings, highlighting that heightened emotional arousal might reflect an important feature of conflict processing (e.g., van Steenbergen, 2015; van Steenbergen & Band, 2013), suggest a potential role of the noradrenergic LC arousal system in the adaptation to conflict.
The LC nucleus is a small assembly of noradrenergic neurons in the brain stem. It consists of widely distributed projections to the entire cerebral cortex, including the frontal cortex and reflects the brain’s source of NE (Aston-Jones & Cohen, 2005; Sara, 2009). LC activity is essential in behavioral and cortical arousal (Carter et al., 2010) and enhances the processing of motivationally relevant signals. In particular, the activation of LC neurons by salient and behaviorally significant stimuli induces a release of NE that facilitates sensory processing, cognitive flexibility, and executive function (Aston-Jones & Cohen, 2005; Cohen, Aston-Jones, & Gilzenrat, 2004; Sara, 2009; Sara & Bouret, 2012). In the context of adaptation to conflict, the adaptation by binding theory (Verguts & Notebaert, 2009), for example, argued that a conflict-induced phasic release of NE may function as a reinforcement signal that strengthens currently active (i.e., task-relevant) representations by means of Hebbian learning (Verguts & Notebaert, 2008, 2009). The arousal elicited by the conflict serves as binding glue to enhance the association between task-specific top-down states and currently active stimulus features (Verguts & Notebaert, 2009) or even more abstract task episodes (cf. Egner, 2014), which in consequence reduces interference in the subsequent trial.Footnote 1
Two main approaches have been pursued to provide evidence for a functional role of the neuromodulatory LC-NE system in the adaptation to conflict. A first strategy is to manipulate valence and/or arousal during conflict processing in order to modulate (e.g., attenuate or increase) the adaptation to conflict. Studies interspersing affective stimuli in a response conflict task to measure the impact on the adaptation to conflict, however, did either not differentiate between valence and arousal (Braem et al., 2013; Padmala, Bauer, & Pessoa, 2011) or came to opposite conclusions: Whereas Zeng and colleagues showed that heightened arousal levels but not valence facilitate adaptation to conflict (Zeng et al., 2016), others found the reversed pattern (van Steenbergen et al., 2010) or could not support the impact of affective picture stimuli on attentional selectivity and adaptation to conflict (Dignath, Janczyk, & Eder, 2017; van Steenbergen, Band, & Hommel, 2011).
A second, more direct approach is the recording of physiological indices of arousal as marker of LC-NE activity in paradigms measuring adaptation to conflict. Empirical support in favor of a conflict-related arousal component can be taken from findings of increased skin conductance responses for conflict trials and error responses (Kobayashi, Yoshino, Takahashi, & Nomura, 2007) and from findings of adaptation effects after high-conflict trials that were associated with increased pre-stimulus pupil diameter (van Steenbergen & Band, 2013; Wendt, Kiesel, Geringswald, Purmann, & Fischer, 2014). At the same time, there are also reports of peripheral affect/arousal measures being insensitive to response conflicts in a selective attention task (Schacht, Dimigen, & Sommer, 2010).
In the present study we further extended these approaches by applying a new method, the transcutaneous vagus nerve stimulation (tVNS), which allows the non-invasive stimulation of the vagus nerve without using implanted stimulators (Van Leusden, Sellaro, & Colzato, 2015; Yuan & Silberstein, 2016). Among other activations in transmitter systems (see Van Leusden et al., 2015), stimulation of the vagus nerve afferents have been shown to particularly enhance NE release in the animal brain via LC activation (Dorr & Debonnel, 2006; Raedt et al., 2011; Roosevelt, Smith, Clough, Jensen, & Browning, 2006). Specifically, tVNS acts upon the auricular branch of the vagus nerve located medial of the tragus at the entry of the acoustic meatus (Kreuzer et al., 2012). By means of delivery of electric pulses, the stimulation of the auricular branch of the vagus nerve reaches the brain through direct projections to the nucleus of the solitary tract (NST; He et al., 2013; Liu et al., 2014; Nomura & Mizuno, 1984) and the LC (Van Bockstaele, Peoples, & Telegan, 1999). Recent neuroimaging studies confirmed the activation of this brainstem circuitry (NST and LC) following vagal stimulation (Dietrich et al., 2008; Frangos, Ellrich, & Komisaruk, 2015; Kraus et al., 2013). It has also been shown that VNS elevates resting pupil diameter, demonstrating its effects on the central autonomous nervous system (Desbeaumes Jodoin et al., 2015). Thus, altogether it is plausible to assume that VNS directly affects LC activity and results in the release of NE (cf. Colzato & Vonck, 2017; George & Aston-Jones, 2010; see McIntyre, McGaugh, & Williams, 2012, for a review). In addition to the activation of the LC-NE system, VNS can also, when applied for longer duration, increase GABA levels (Ben-Menachem et al., 1995). Due to its assumed effects on both the LC-NE and the GABAergic system, VNS has been successfully applied in the treatment of neurological disorders such as epilepsy (DeGiorgio et al., 2000; Englot, Chang, & Auguste, 2011). VNS can also modulate the activation of higher brain areas through the afferent projections from the LC-NE system to medial and cortical brain regions (Clayton & Williams, 2000; Mello-Carpes & Izquierdo, 2013; Sara & Bouret, 2012) such as the ACC, a region heavily interconnected with the LC-NE System (Arnsten & Goldman-Rakic, 1984; Aston-Jones & Cohen, 2005; Porrino & Goldman-Rakic, 1982). As part of the neural network involved in the modulation of the arousal response associated with effortful behavior (Critchley, 2009) and cognitive control (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004), the ACC, in interaction with the LC-NE system, plays a crucial role in the modulation of arousal states during stimulus-driven behavior (Ebitz & Platt, 2015; Joshi, Li, Kalwani, & Gold, 2016).
Applying tVNS in order to stimulate LC activity has been shown to facilitate a wide range of affective and cognitive functions, including emotion recognition (Colzato, Sellaro, & Beste, 2017; Sellaro, de Gelder, Finisguerra, & Colzato, 2018), flow experience (Colzato, Wolters, & Peifer, 2018), divergent thinking (Colzato, Ritter, & Steenbergen, 2018), associative memory (Jacobs, Riphagen, Razat, Wiese, & Sack, 2015), attentional processing (Ventura-Bort et al., submitted), and various processes associated with cognitive control. For example, tVNS has been shown to increase post-error slowing (Sellaro et al., 2015) to speed responses in a stop-change paradigm without affecting the stopping response time (Steenbergen et al., 2015) and to reduce the commission of false alarms under challenging conditions in a response inhibition paradigm (Beste et al., 2016). Furthermore, activation of the LC by implanted VNS devices have been reported to enhance response-inhibition mechanisms in a stop-signal task (Schevernels et al., 2016).
In the current study we extended prior findings by specifically investigating the influence of tVNS on the adaptation to conflict, behaviorally assessed in an adapted response conflict Simon task (Fischer, Plessow, Dreisbach, & Goschke, 2015; Plessow, Fischer, Kirschbaum, & Goschke, 2011). In parallel, we also recorded electrophysiological indices of cognitive control, such as the N2 and P3 component of the event-related potentials (ERPs; Clayson & Larson, 2011a; Larson, Clayson, & Baldwin, 2012).
The N2 is a negative deflection of the ERPs typically observed over fronto-central electrodes peaking between 250 and 350 ms after stimulus presentation (van Veen & Carter, 2002; Yeung, Botvinick, & Cohen, 2004). The N2, probably originating from the ACC, has been related to the detection of conflict (for a review see Larson, Clayson, & Clawson, 2014), as indicated by larger amplitudes on incongruent compared to congruent trials (van Veen & Carter, 2002; Yeung et al., 2004). Importantly, the N2 amplitude has also been shown to be sensitive to the ongoing adaptation of cognitive control. Thus, larger N2 amplitudes are evoked by conflict trials when preceded by non-conflict compared to conflict trials (Clayson & Larson, 2011a; Forster, Carter, Cohen, & Cho, 2011; Freitas, Banai, & Clark, 2009; Larson et al., 2012). Conflict-related stimuli also modulate the P3 component, a positive-going waveform (350–600 ms), which is more prominent over centro-parietal areas. The P3 has been associated with response inhibition (e.g., Albert, Lopez-Martin, Hinojosa, & Carretie, 2013) and attention allocation processes (Polich, 2007) and as with the N2, the P3 is also linked with adaptation to conflict (Clayson & Larson, 2011a).
Following the assumption that the neuromodulatory LC-NE system is involved in performance monitoring and conflict processing, stimulation of the LC-NE system by tVNS should impact markers of conflict-triggered adaptation. Because previous findings showed a link between the degree of the arousal response (as indicated by pupil dilatation) and the strength of the behavioral adaptation (Wendt et al., 2014), we predicted an increased adaptation to conflict under tVNS compared to sham stimulation. This is expressed in a stronger performance benefit following conflict (e.g., reduction of RTs in incompatible trials following conflict [iI] compared to incompatible trials following non-conflict [cI]) and a stronger reduction of the N2 and P3 components following conflict.
Methods
Participants
Twenty-one students from the University of Greifswald (18 female; Mage = 20.3 years, SD = 1.4 years) participated for course credits or financial compensation. Each individual provided written informed consent for a protocol approved by the Review Board of the German Psychological Society. All participants had normal or corrected-to-normal vision and all, except one participant, claimed right-handedness. Each participant underwent a phone-screening interview prior to the first experiment session. Participants fulfilling any of the following exclusion criteria were discarded prior to participation: Neurological or mental disorders, brain surgery, undergoing chronic or acute medication, pregnancy, migraine and/or epilepsy susceptibility, heart-related diseases, metal implants in the face or brain, implants or physical alterations in the ear.
Apparatus and procedure
We used a randomized, single-blinded, tVNS-sham, within-subject design. The experiment took place on two consecutive days, in which either tVNS or sham stimulation was administered to each participant, alternately. The order of stimulation condition (tVNS-sham; sham-tVNS) was counterbalanced across participants.
In both sessions, upon arrival and prior to the application of the stimulator, participants were seated in the experimental cabin, and heart rate, blood pressure, and salivary alpha-amylase were measured (pre). Afterwards, the stimulation electrodes were applied in the left ear. In order to individually adjust the stimulation intensity, in each session participants received increasing and decreasing series of 10-s stimulation trials, and rated the subjective sensation of the stimulation in a 10-point scale, ranging from nothing (0), light tingling (3), strong tingling (6), to painful (10). The increasing series of trials started from an intensity of 0.01 mA and increased by 0.01 mA on a trial-by-trial basis until participants reported a “tingling” sensation of 9. Before starting the decreasing series, the same intensity was repeated and then reduced trial by trial in 0.01 mA bins until a subjective sensation of 6 or below was experienced. This procedure was repeated a second time. The final stimulation intensity used for the experimental procedure was calculated based on the average of the four intensities rated as 8 (two from the increasing and two from the decreasing series).
Subsequently, the electroencephalography (EEG) net was applied and participants performed two experimental tasks: a novelty oddball task (Venables, Patrick, Hall, & Bernat, 2011)Footnote 2 and a subsequent number version of the Simon task (Fischer, Dreisbach, & Goschke, 2008; Fischer, Plessow, Dreisbach, & Goschke, 2015), which lasted 28 and 8 min, respectively. The stimulation was continuously applied throughout both tasks. The order of tasks was kept constant, so that the Simon task consistently received 28 min of prior stimulation. After the experiment and the removal of the EEG and the stimulation electrodes, heart rate, blood pressure, and salivary alpha-amylase were measured again (post). Finally, using a seven-point scale participants were asked to report how much they experienced the following symptoms during the stimulation (from low “1” to high “7”): headache, nausea, dizziness, neck pain, muscle contractions in the neck, stinging sensations under the electrodes, skin irritation in the ear, fluctuation in concentration or feelings, and unpleasant feelings.
Transcutaneous vagus nerve stimulation
The tVNS stimulator consisted of two titan electrodes attached to a mount, which was located in the left auricle and wired to the stimulation unit (CMO2, Cerbomed, Erlangen, Germany). In the tVNS condition, the stimulator was placed in the left cymba conchae, an area innervated exclusively by the auricular branch of the vagus nerve (Ellrich, 2011; Peuker & Filler, 2002). Alike previous studies using tVNS (e.g., Kraus et al., 2007; Steenbergen et al., 2015), in the sham condition, the electrodes were positioned in the centre of the left ear lobe, an area known to be free of vagal innervation (Ellrich, 2011; Peuker & Filler, 2002). To ensure stimulation over the entire task performance, the stimulation was delivered continuously with a pulse width of 200–300 ms at 25 Hz.Footnote 3 Stimulus intensity of the tVNS was adjusted above the detection threshold and below the pain threshold (Ellrich, 2011). The average stimulation intensity used was 1.3 mA (0.4–3.3 mA) for active and 1.49 mA (0.6–4.8 mA) for sham condition. The stimulation intensity did not differ between both conditions (t[20] = 1.43, p = .16, d = .31). Because the right vagal nerve sends efferent projections to the heart, the stimulation in the current study was always applied to the left ear to avoid the possibility of cardiac side effects.
Conflict task
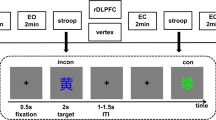
Participants performed a number version of the Simon task (Fischer, Dreisbach, & Goschke, 2008; Fischer et al., 2015) in which laterally presented target numbers were categorized as smaller (left key press) or larger than five (right key press). This version of the Simon task included eight stimuli, which allowed for the exclusion of identical stimulus repetitions from one trial to the next, to reduce the influence of low-level feature repetitions and to increase the involvement of cognitive control (cf. Egner, 2007, 2017). Response conflicts occurred when the required response side (e.g., right response to numbers larger than five) did not correspond with the location of the stimulus on the display (e.g., number 9 presented at the left). Performance differences between this incompatible condition and a compatible condition (i.e., stimulus location corresponds with response location) denoted the Simon effect. Each of the eight numbers was presented equally often in the compatible (e.g., large number on the right) and incompatible location (e.g., large number on the left). This resulted in a sequencing of 50% compatible and 50% incompatible trials, excluding the possibility of biased contingencies between stimulus and/or location and response (Schmidt, 2013). Conflict-triggered adjustments of cognitive control were indicated by the sequential modulation of the Simon effect and denoted a reduced Simon effect following incompatible trials as compared to a larger Simon effect following compatible trials.
The digits 1 to 9 (except 5) served as target stimuli and were presented in white against black on a 17-in. monitor. With a viewing distance of about 150 cm, target stimuli extended to a visual angle of about 0.38° horizontally and 0.76° vertically. They were presented 10 cm randomly to the left or right of fixation (plus sign), which resulted in an overall visual angle of 4.2°. Participants responded on a standard QWERTZ keyboard, using the left (Alt key) and right (Alt-Gr key) index finger to categorize digits as smaller or larger than five, respectively. Stimulus presentation and data recording were controlled by Presentation (Version 16.5; Neurobehavioral Systems, Inc., Albany, CA, USA).
A trial started with a fixation displayed for 1000 ms. The target stimulus was then presented for 200 ms and the fixation remained until a response was given (1,600 ms max). A blank screen was provided for 300 ms as feedback for correct responses, “zu langsam” (too slow) for misses and “falsch” (false) for erroneous responses. A random interval (steps of 100 ms between 100 and 1,000 ms) of a blank screen was presented prior to the start of the next trials and served to minimize a tendency for rhythmic responses. Following a block of 16 practice trials, participants performed a total of 192 experimental trials that were administered into three blocks of 64 trials each divided by short breaks.
Physiological measures
Heart rate and blood pressure (systolic and diastolic) were measured manually (Riva Rocci method) at two time points: immediately before the stimulation was applied (pre), and after the second experimental task was performed (post). Along with these physiological variables, salivary alpha-amylase (sAA) was also measured, which is often considered as a marker of endogenous noradrenergic activation (Chatterton, Vogelsong, Lu, Ellman, & Hudgens, 1996; but see also Nater & Rohleder, 2009, for a critical discussion). sAA was collected out of saliva samples using regular cotton Salivette sampling devices (Sarstedt, Nümbrecht, Germany). Participants were instructed to gently chew the swab in their mouth for 60 s. To guarantee the preservation of the saliva sample, they were stored at -20 °C before analyses were performed at the Dresden LabService GmbH using an enzyme kinetic method. Because extreme values of sAA levels identified by Dresden LabService GmbH might indicate technical problems (e.g., measurement errors), data from two participants were excluded from the analyses of the sAA measures.
Electrophysiological recording
EEG signals were recorded continuously from 257 electrodes using an Electrical Geodesics (EGI) high-density EEG system with NetStation software on a Macintosh computer. The EEG recording was digitized at a rate of 250 Hz, using vertex sensor (Cz) as recording reference. Scalp impedance for each sensor was kept below 30 kΩ, as recommended by manufacturer guidelines. All channels were band-pass filtered online from 0.1 to 100 Hz. Stimulus-synchronized epochs were extracted from 200 ms before to 1,200 ms after stimulus presentation, filtered at 20 Hz, and then submitted to the procedure proposed by Junghöfer and colleagues (Junghöfer, Elbert, Tucker, & Rockstroh, 2000), as implemented in the EMEGS software (Peyk, De Cesarei, & Junghöfer, 2011). This procedure uses statistical parameters of the data to exclude channels and trials that are contaminated with artifacts. Recording artifacts were first detected using the recording reference (i.e., Cz), and then global artifacts were identified using the average reference, which was used for all analyses. Subsequently, distinct sensors from particular trials were removed based on the distribution of their amplitude, standard deviation and gradient. Data at eliminated electrodes were replaced with a statistically weighted spherical spline interpolation from the full channel set (Junghöfer et al., 2000). In the present study, the electrode site Cz was used as the recording reference, and the average reference was calculated off-line, after artifact rejection, and used for all subsequent analyses. The MATLAB-based toolbox BioSig (Vidaurre, Sander, & Schlögl, 2011) was used for eye movement and blink artifacts corrections of the extracted epochs. This method is based on linear regression to reliably remove electrooculogram activity from the EEG (Schlögl et al., 2007).
For each participant, separated ERP averages were computed for each sensor and condition and were baseline corrected using the 200-ms window prior to stimulus presentation. Each condition was derived based on the CompatibilityN-1 (compatible, incompatible) and on the CompatibilityN (Compatible, Incompatible), resulting in four different conditions: cC, iC, cI, and iI (number of trials per condition: M = 28 [min= 16, max= 43], SD = 3).
Based on previous research showing that the N2 shows a centro-frontal distribution (Clayson & Larson, 2011a; Danielmeier, Wessel, Steinhauser, & Ullsperger, 2009; Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof, 2003), and on visual inspection, the N2 was extracted from a 240 to 388 ms time window, over centro-frontal brain regions (EGI Hydrocel sensors: 7, 8, 9, 16, 17, 45, 81, 132, 186, 198, and 257). For the P3, previous research identified maximal amplitudes in centro-parietal areas (Clayson & Larson, 2011a), thus in the present study mean amplitudes were extracted over a representative centro-parietal cluster (EGI Hydrocel sensors: 45, 80, 81, 90, 131, 132, and 257) in a 400 to 600 ms time window.
Results
Subjective ratings
Overall, subjective ratings indicated that the side effects of the stimulation were minimal (M = 1.92, SD = 0.6). T-comparisons showed no differences between stimulation conditions (all p’s > .203), except for the physical subjective experience of the stimulation, with higher ratings for the tVNS condition (stinging sensation under the electrodes: t[20] = 2.09, p = .049, d =0.46; skin irritation in the ear: t[20] = 3.56, p = .002, d =0.78). Data are presented in Table 1. These results indicate that participants only perceive physical differences between the sham and the tVNS stimulation, somewhat expected taking into account the stimulation intensity used. Verbal reports indicated that the sensations occurred predominantly in the beginning of the stimulation, suggesting rapid habituation. Differences in physical subjective stimulation experience between the tVNS and sham condition were not correlated with the behavioral or electrophysiological marker of conflict adaptation, all p’s > .315.
Physiological data
Physiological data are presented in Table 2. For salivary alpha amylase levels, the main effect of time was observed, F(1, 18) = 11.32, p = .003, ηp2 = .39, denoting an increase of the alpha amylase levels during task performance. Pre-post increases in alpha amylase levels were significant for tVNS, t(18) = 4.1, p < .001, d = 0.89, and approached significance in the sham stimulation, t(18) = 1.47, p = .082, d =0.32. As a consequence, the interaction between the factors Stimulation and Time point was not significant, F(1, 18) = 1.75, p = .202, ηp2 = .09. There was also no main effect of stimulation, F < 1, and no relation between the individual increase in sAA and the behavioral and electrophysiological marker of adaptation to conflict adaptation (r = -.353, p = .138 and r = .379, p = .109, respectively).
Given that only the right vagal nerve has efferent fibers to the heart, we expected no effects of stimulation on the cardiovascular system when applied to the left ear. Overall, we found a habituation of heart rate, F(1, 20) = 43.6, p < .001, ηp2 = .67, and systolic blood pressure, F(1, 20) = 5.74, p = .026, ηp2 = .22, from pre to post measurement. This reduction was not observed on diastolic blood pressure, F(1, 20) = 1.87, p = .186, ηp2 = .09. As expected, no effects of stimulation were observed (all p’s > .26), suggesting that physiological data did not differ prior to the experiment or after the experiment as a result of the stimulation.
Behavioral data
Prior to all analyses, the first trial of a block, trials with identical target repetitions, and post-error trials were excluded (15.5%). For RT analysis only, error trials (5.0%) and trials that deviated more than 2.5 standard deviations from the individual condition mean (1.9%) were additionally omitted. A repeated measures ANOVA with the within-subject factors CompatibilityN-1 (incompatible vs. compatible), CompatibilityN (incompatible vs. compatible), and Stimulation (tVNS vs. sham) was conducted on response times (RTs) and percent error (PE). RTs of incompatible and compatible trial transitions are presented in Table 3 separated for tVNS and sham condition. Figure 1 shows RTs for incompatible trials depending on previous compatibility and stimulation condition.
Response times (RTs, in ms) for Simon incompatible and Simon compatible trials in N depending on Simon compatibility of the previous trial (N-1), separately for tVNS and sham stimulation, respectively (left panel). The difference between cI-iI trials denotes the reduction of incompatible RTs following conflict and thus shows that the stronger adaptation to conflict under tVNS is essentially conflict related (right panel). Error bars represent standard errors of the means. C compatible, IC incompatible
RTs. A Simon effect was expressed in slower responses in incompatible (536 ms) than in compatible trials (522 ms), F(1, 20) = 15.32, p = .001, ηp2 = .43. Responses were also slowed by previous conflict (534 ms) compared to no previous conflict (524 ms), F(1, 20) = 9.72, p = .005, ηp2 = .33, denoting post-conflict slowing (Verguts, Notebaert, Kunde, & Wühr, 2011). A strong sequential modulation of the Simon effect was found in the interaction between CompatibilityN-1 and CompatibilityN, F(1, 20) = 97.01, p < .001, ηp2 = .83. That is, the Simon effect was strongly reduced after conflict (-15 ms) compared to following non-conflict trials (43 ms). Most importantly, however, the application of tVNS increased the sequential modulation of the Simon effect compared to sham stimulation. This is reflected in the significant three-way interaction between CompatibilityN-1, CompatibilityN, and Stimulation, F(1, 20) = 4.48, p = .047, ηp2 = .18. Crucially, this tVNS-based increase of the sequential modulation of the Simon effect was specifically related to conflict processing. An ANOVA on RTs for incompatible trials with the factors CompatibilityN-1 and Stimulation proved that the more efficient conflict processing following conflict (iI trials) compared to following non-conflict (cI trials) was more pronounced under tVNS than under sham stimulation, F(1, 20) = 8.07, p = .010, ηp2 = .29 (Fig. 1). No tVNS influence was observed on cC compared to iC trials (F < 1), suggesting that tVNS impacts specifically on conflict-related processing and less so on processing non-conflict trials. Furthermore, tVNS reduced post-conflict slowing, F(1, 20) = 4.54, p = .046, ηp2 = .19. A post-conflict slowing of 15 ms in the sham condition was reduced to 6 ms under tVNS. Stimulation did not yield an overall effect on RTs, F(1, 20) = 1.15, p = .297, ηp2 = .05.
To minimize further influences of feature repetitions in the present result pattern, we investigated the contribution of response repetitions versus response alternations. Most importantly, neither the sequential modulation of the Simon effect, nor the effect of tVNS on the sequential modulation of the Simon effect were affected by the factor Response transition (repetition versus alternation), F(1, 20) = 1.90, p = .183, ηp2 = .09, and F(1, 20) = 0.83, p = .372, ηp2 = .04, respectively. In fact, the important finding of faster responses in incompatible trials following conflict (versus non-conflict) under tVNS compared to sham was confirmed for both response repetitions, F(1, 20) = 5.43, p = .030, ηp2 = .21 as well as for response alternations, F(1, 20) = 5.18, p = .034, ηp2 = .21.
PE. Participants committed 5.0% errors. The Simon effect with higher error rates for incompatible (5.7%) compared to compatible trials (4.4%) did not reach significance, F(1, 20) = 2.03, p = .169, ηp2 = .09. Yet, there was a significant sequential modulation of the Simon effect, F(1, 20) = 33.21, p < .001, ηp2 = .62. No other effects were significant, all p’s > .284.
Electrophysiological data
ERP analyses were conducted only over correct trials. Following RT analyses, and to provide a good fit between behavioral performance and brain activity, the first trial of a block, trials with identical target repetitions, and post-error trials were excluded from analyses. Repeated measures ANOVAs with the within-subject factors CompatibilityN-1 (incompatible versus compatible), CompatibilityN (incompatible versus compatible), and Stimulation (tVNS versus sham) were conducted on the mean amplitudes of the N2 and P3 components. Data are presented in Fig. 2.
(A) N2 amplitudes for Simon incompatible trials in N depending on Simon compatibility of the previous trial (N-1), separately for tVNS and sham stimulation, respectively. (B) P3 amplitudes for Simon incompatible trials in N depending on Simon compatibility of the previous trial (N-1), separately for tVNS and sham stimulation, respectively
N2. Although no general sequential modulation effects were observed (CompatibilityN-1 × CompatibilityN interaction: F < 1), a close to significant three-way interaction indicated that the sequential modulation effects on N2 amplitudes were influenced by the stimulation condition, F (1, 20) = 4.22, p = .053, ηp2 = .17. Neither any main effect nor other interactions reached significance, all p’s > .21.
Separate subsequent ANOVAs for the tVNS and sham conditions with the factors CompatibilityN-1 and CompatibilityN revealed that for the tVNS condition, in the absence of a main effect of CompatibilityN-1, F (1, 20) = 2.63, p = .121, ηp2 = .12, and CompatibilityN, F < 1, sequential modulation effects were observed, F(1, 20) = 5.79, p = .026, ηp2 = .23. In the sham condition, a main effect of CompatibilityN was observed, F (1,20) = 4.56, p = .045, ηp2 = .17, showing larger N2 amplitudes for incompatible than compatible trials; however, neither a CompatibilityN-1, nor interaction effects were significant, all F’s < 1.
Most importantly, and like behavioral performance, the impact of tVNS on the sequential modulation was specifically linked to incompatible trials. An ANOVA on incompatible trials only with the factors CompatibilityN-1 and Stimulation suggested that the experience of a previous conflict elicited a reduced N2 in upcoming conflict trials especially under tVNS. This effect, however, only approached significance, F(1, 20) = 3.39, p = .080, ηp2 = .15. The reduction of N2 for incompatible trials following conflict (compared to following non-conflict) was found specifically in the tVNS stimulation, t(20) = 2.47, p = .022, d =0.54, but not in the sham stimulation, t < 1.
P3. A main effect of CompatibilityN-1 approached significance, F(1, 20) = 3.49, p = .078, ηp2 = .15, indicating a larger P3 after incompatible trials than after compatible trials. A main effect of CompatibilityN was also observed, F(1, 20) = 6.99, p = .016, ηp2 = .26, showing larger P3 amplitudes for current incompatible than compatible trials. Most importantly, a CompatibilityN-1 × CompatibilityN interaction was observed, F(1, 20) = 22.7, p < .001, ηp2 = .53, indicating sequential modulation effects. Concretely, incompatible trials produced a significantly reduced P3 when were preceded by a conflict, t(20) = -3.31, p = .003, d =.72. Unlike for the N2, tVNS did not modulate the sequential modulation effects on the P3 amplitudes, all p’s > .12.
Discussion
Performance adjustments are often triggered by internal or external signals such as the experience of response conflicts. In this line, previous work has emphasized the affectively aversive quality of conflict signals in triggering the recruitment of cognitive control. The aim of the present study was to test whether concurrent transcutaneous vagus nerve stimulation, which likely activates the locus coeruleus-noradrenaline system, affects behavioral and electrophysiological markers of the recruitment of cognitive control. For this, the N2 and P3 components of the EEG were recorded while participants performed a response conflict task, for which the sequential modulation of the conflict effect (i.e., adaptation to conflict) was taken as evidence for the conflict-triggered recruitment of cognitive control.
First of all, the administered version of the Simon conflict task (Fischer et al., 2008; Fischer et al., 2015; Plessow et al., 2011) produced a reliable sequential modulation of the conflict effect in behavioral and electrophysiological measures, confirming previous work with different conflict tasks (e.g., Eriksen flanker task, Clayson & Larson, 2011a; Forster et al., 2011). This is important as it demonstrates the sensitivity of these behavioral and electrophysiological measures to conflict adaptation despite different types of conflict and conflict processing in Simon and Eriksen flanker paradigms (see Hommel, 2011, for a discussion of conflict processing in different conflict tasks).
Most importantly, tVNS stimulation also impacted on both the behavioral and the electrophysiological measures of conflict-triggered recruitment of cognitive control. In particular, the sequential modulation of the Simon effect was more pronounced under tVNS than under sham stimulation in RTs. In addition to behavioral measures, this influence of tVNS was virtually mirrored in the corresponding N2 amplitudes. Of particular importance, the impact of tVNS on the sequential modulation of the Simon effect was essentially conflict related. tVNS compared to sham stimulation particularly facilitated conflict processing in trials following previous conflict experience. That is, the reduction of RTs and N2 amplitudes in iI compared to cI trials was more pronounced under tVNS than sham stimulation (see Figs. 1 and 2).
The P3 component was also affected in the present conflict task. In line with previous studies, we found larger P3 for conflict compared to non-conflict trials and a sequential modulation of the P3 amplitude, indicating that the P3 amplitude during conflict monitoring seems to reflect the degree of conflict to be resolved. As suggested by Clayson and Larson (Clayson & Larson, 2011a, 2011b), the P3 during conflict monitoring seems to be associated with the allocation of the attentional resources to the current stimulus and more linked to the P3a, rather than to the P3b (Clayson & Larson, 2011b). Different neurotransmitter systems are involved in generating the P3a and P3b. Whereas the NE system is related to the P3b, the P3a seems to be mediated by the dopaminergic system (Polich, 2007), which is probably the reason why tVNS did not impact the P3(a) in our study (see also Ventura-Bort et al., submitted).
Although the impact of tVNS on conflict-triggered recruitment of cognitive control is straight forward, the mechanisms underlying this modulation is to date not entirely understood. Based on the notion that the LC is the primary source of NE in the brain and that the LC is directly innervated by the vagus nerve and rapidly enhances noradrenaline release (compared to other transmitters) following stimulation in animals (Dorr & Debonnel, 2006; Raedt et al., 2011), it is reasonable to assume that the observed changes in conflict adaptation are predominantly modulated via this neural pathway.
The assumption of a noradrenergic basis of the tVNS influence on conflict adaptation is in line with the adaptation by binding model (Verguts & Notebaert, 2008, 2009). NE is assumed to act as “binding glue” strengthening the association between top-down states and currently active task characteristics and thus, reducing the extent of subsequent conflict (Egner, 2014; Verguts & Notebaert, 2009). In this framework, a potential increase of the LC-NE activity by tVNS might enhance this effect. Less conflict triggers less ACC activity, which in turn results in a stronger reduction of conflict interference and N2 amplitudes in tVNS as observed in sham stimulation (Clayson & Larson, 2011a). An enhanced adaptation to conflict under tVNS suggests that previous strategies of inserting affective (IAPS) picture stimuli (Dignath et al., 2017) or implementing alerting signals (Böckler, Alpay, & Stürmer, 2011; Fischer, Plessow, & Kiesel, 2010) might be less effective in impacting on markers of conflict adaptation than the application of tVNS.
Instead of strengthening task-relevant representations as adaptation mechanism to conflict (Verguts & Notebaert, 2008, 2009), tVNS might also target the affective quality of the conflict signal. Recent research has provided evidence that conflict signals are registered as aversive events and suggested that the experienced aversiveness triggers the adaptation process (Dreisbach & Fischer, 2012a, 2015, 2016; Inzlicht et al., 2015). Therefore, under the premise of tVNS activating the LC-NE system, an enhanced adaptation to conflict might also be the result of an increased aversiveness of the conflict signal, instead of a facilitated functioning of the adaptation mechanisms.
While the present study showed a clear impact of tVNS on behavioral and electrophysiological parameters of conflict adaptation, future research will now be needed to identify the underlying working mechanisms in more detail. In this respect, it should also be noted that although there was a significant increase of sAA levels in tVNS but not in sham stimulation, an interaction confirming a stronger increase after stimulation failed significance and we did not find an associated increase in conflict adaptation.Footnote 4 However, this observation does not conflict with our main argument. Although elevated alpha amylase levels are often used as marker of activation of central noradrenergic system (for recent findings, see Warren, van den Brink, Nieuwenhuis, & Bosch, 2017), the reliability of this marker has not yet been sufficiently proven (e.g., Bosch, Veerman, de Geus, & Proctor, 2011; Nater & Rohleder, 2009).
Therefore, additional variables (e.g., pupil dilatation) are clearly warranted to study the involvement of NE in conflict adaption in a more complete fashion. Furthermore, it is conceivable that in addition to NE other transmitter systems might also be activated by tVNS (Van Leusden et al., 2015), especially as performance in tasks sensitive to GABA or to a concomitant influence of GABA and NE have been modulated by the application of tVNS (e.g., Beste et al., 2016; Steenbergen et al., 2015).
Together, the present findings provided novel evidence of tVNS increasing behavioral and electrophysiological markers of conflict adaptation. While it seems reasonable to interpret the present findings as evidence for an involvement of the LC-NE system in the conflict-triggered adjustment of cognitive control, subsequent research is clearly warranted to provide further support for this conclusion (e.g., by using different stimulation protocols, other conflict tasks, and further additional markers of NE activity). The present research thus provides fertile grounds for future studies determining the underlying neuromodulatory mechanisms in the adaptation to conflict.
Author Note
We thank Miriam Grüning and Nilab Kaderie for assistance in data collection.
Notes
Note: the adaptation by binding model has been proposed to explain conflict adaptation by means of arousal mediated learning mechanisms that enhance the association between task-demand units and specific stimulus features. Recent conceptions, however, have extended this view and argued for more general associations between top-down states and task contexts (Bugg & Crump, 2012) or episodes (Egner, 2014).
Data from the novelty oddball task are not part of the present study and will be reported elsewhere (Ventura-Bort et al., submitted).
Please note that this procedure of continuous stimulation differs to other stimulation protocols applying a 30 sec ON and 30 sec OFF procedure.
References
Abrahamse, E., Braem, S., Notebaert, W., & Verguts, T. (2016). Grounding cognitive control in associative learning. Psychol Bull, 142(7), 693-728. doi:https://doi.org/10.1037/bul0000047
Albert, J., Lopez-Martin, S., Hinojosa, J. A., & Carretie, L. (2013). Spatiotemporal characterization of response inhibition. Neuroimage, 76(1), 272-281. doi:https://doi.org/10.1016/j.neuroimage.2013.03.011
Arnsten, A. F. T., & Goldman-Rakic, P. S. (1984). Selective prefrontal cortical projections to the region of the locus Coeruleus and Raphe Nuclei in the Rhesus-monkey. Brain Research, 306(1-2), 9-18. doi:https://doi.org/10.1016/0006-8993(84)90351-2
Aston-Jones, G., & Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403-450. doi:https://doi.org/10.1146/annurev.neuro.28.061604.135709
Ben-Menachem, E., Hamberger, A., Hedner, T., Hammond, E. J., Uthman, B. M., Slater, J., . . . et al. (1995). Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res, 20(3), 221-227.
Beste, C., Steenbergen, L., Sellaro, R., Grigoriadou, S., Zhang, R., Chmielewski, W., . . . Colzato, L. S. (2016). Effects of Concomitant Stimulation of the GABAergic and Norepinephrine System on Inhibitory Control - A Study Using Transcutaneous Vagus Nerve Stimulation. Brain Stimulation. doi:https://doi.org/10.1016/j.brs.2016.07.004
Böckler, A., Alpay, G., & Stürmer, B. (2011). Accessory stimuli affect the emergence of conflict, not conflict control. Experimental Psychology, 58(2), 102-109. doi:947X2L7124654048 [pii]10.1027/1618-3169/a000073
Bosch, J. A., Veerman, E. C., de Geus, E. J., & Proctor, G. B. (2011). alpha-Amylase as a reliable and convenient measure of sympathetic activity: don't start salivating just yet! Psychoneuroendocrinology, 36(4), 449-453. doi:https://doi.org/10.1016/j.psyneuen.2010.12.019
Botvinick, M. (2007). Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive, Affective and Behavioral Neuroscience, 7(4), 356-366.
Botvinick, M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624-652.
Botvinick, M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Science, 8(12), 539-546. https://doi.org/10.1016/j.tics.2004.10.003
Braem, S., Abrahamse, E. L., Duthoo, W., & Notebaert, W. (2014). What determines the specificity of conflict adaptation? A review, critical analysis, and proposed synthesis. Frontiers in Psychology, 5. doi:https://doi.org/10.3389/fpsyg.2014.01134
Braem, S., King, J. A., Korb, F. M., Krebs, R. M., Notebaert, W., & Egner, T. (2013). Affective modulation of cognitive control is determined by performance-contingency and mediated by ventromedial prefrontal and cingulate cortex. J Neurosci, 33(43), 16961-16970. doi:https://doi.org/10.1523/JNEUROSCI.1208-13.2013
Braem, S., King, J. A., Korb, F. M., Krebs, R. M., Notebaert, W., & Egner, T. (2017). The Role of Anterior Cingulate Cortex in the Affective Evaluation of Conflict. Journal of Cognitive Neuroscience, 29(1), 137-149. doi:https://doi.org/10.1162/jocn_a_01023
Bugg, J. M., & Crump, M. J. (2012). In support of a distinction between voluntary and stimulus-driven control: A review of the literature on proportion congruent effects. Frontiers in Psychology, 3:367. doi:https://doi.org/10.3389/fpsyg.2012.00367
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci, 4(6), 215-222.
Carter, M. E., Yizhar, O., Chikahisa, S., Nguyen, H., Adamantidis, A., Nishino, S., . . . de Lecea, L. (2010). Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci, 13(12), 1526-1533. doi:https://doi.org/10.1038/nn.2682
Chatterton, R. T., Jr., Vogelsong, K. M., Lu, Y. C., Ellman, A. B., & Hudgens, G. A. (1996). Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol, 16(4), 433-448.
Clayson, P. E., & Larson, M. J. (2011a). Conflict adaptation and sequential trial effects: support for the conflict monitoring theory. Neuropsychologia, 49(7), 1953-1961. doi:https://doi.org/10.1016/j.neuropsychologia.2011.03.023
Clayson, P. E., & Larson, M. J. (2011b). Effects of repetition priming on electrophysiological and behavioral indices of conflict adaptation and cognitive control. Psychophysiology, 48(12), 1621-1630. doi:https://doi.org/10.1111/j.1469-8986.2011.01265.x
Clayton, E. C., & Williams, C. L. (2000). Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behavioural Brain Research, 112(1-2), 151-158. doi:https://doi.org/10.1016/S0166-4328(00)00178-9
Cohen, J. D., Aston-Jones, G., & Gilzenrat, M. S. (2004). A system-level perspective on attention and cognitive control: Guided activation, adaptive gating, conflict monitoring, and exploitation vs. exploration. In M. I. Posner (Ed.), Cognitive neuroscience of attention (pp. 71-90). New York: Guilford Press.
Cohen, J. D., McClure, S. M., & Yu, A. J. (2007). Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1481), 933-942. doi:https://doi.org/10.1098/rstb.2007.2098
Colzato, L. S., Ritter, S. M., & Steenbergen, L. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia, 111, 72-76. doi:https://doi.org/10.1016/j.neuropsychologia.2018.01.003
Colzato, L. S., Sellaro, R., & Beste, C. (2017). Darwin revisited: The vagus nerve is a causal element in controlling recognition of other's emotions. Cortex, 92, 95-102. doi:https://doi.org/10.1016/j.cortex.2017.03.017
Colzato, L. S., & Vonck, K. (2017). Transcutaneous vagus and trigeminal nerve stimulation. In L. Colzato (Ed.), Theory-driven approaches to cognitive enhancement: Springer International Publishing.
Colzato, L. S., Wolters, G., & Peifer, C. (2018). Transcutaneous vagus nerve stimulation (tVNS) modulates flow experience. Exp Brain Res, 236(1), 253-257. doi:https://doi.org/10.1007/s00221-017-5123-0
Critchley, H. D. (2009). Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology, 73(2), 88-94. doi:https://doi.org/10.1016/j.ijpsycho.2009.01.012
Danielmeier, C., Wessel, J. R., Steinhauser, M., & Ullsperger, M. (2009). Modulation of the error-related negativity by response conflict. Psychophysiology, 46(6), 1288-1298. doi:https://doi.org/10.1111/j.1469-8986.2009.00860.x
de Rover, M., Brown, S. B., Band, G. P., Giltay, E. J., van Noorden, M. S., van der Wee, N. J., & Nieuwenhuis, S. (2015). Beta receptor-mediated modulation of the oddball P3 but not error-related ERP components in humans. Psychopharmacology (Berl), 232(17), 3161-3172. doi:https://doi.org/10.1007/s00213-015-3966-2
DeGiorgio, C. M., Schachter, S. C., Handforth, A., Salinsky, M., Thompson, J., Uthman, B., . . . Heck, C. (2000). Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia, 41(9), 1195-1200.
Desbeaumes Jodoin, V., Lesperance, P., Nguyen, D. K., Fournier-Gosselin, M. P., Richer, F., & Centre Hospitalier de l'Universite de Montreal, Canada. (2015). Effects of vagus nerve stimulation on pupillary function. Int J Psychophysiol, 98(3 Pt 1), 455-459. doi:https://doi.org/10.1016/j.ijpsycho.2015.10.001
Dietrich, S., Smith, J., Scherzinger, C., Hofmann-Preiss, K., Freitag, T., Eisenkolb, A., & Ringler, R. (2008). A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed Tech (Berl), 53(3), 104-111. doi:https://doi.org/10.1515/BMT.2008.022
Dignath, D., Janczyk, M., & Eder, A. B. (2017). Phasic valence and arousal do not influence post-conflict adjustments in the Simon task. Acta Psychol (Amst), 174, 31-39. doi:https://doi.org/10.1016/j.actpsy.2017.01.004
Dignath, D., Kiesel, A., & Eder, A. B. (2015). Flexible conflict management: conflict avoidance and conflict adjustment in reactive cognitive control. J Exp Psychol Learn Mem Cogn, 41(4), 975-988. doi:https://doi.org/10.1037/xlm0000089
Dorr, A. E., & Debonnel, G. (2006). Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. Journal of Pharmacology and Experimental Therapeutics, 318(2), 890-898. doi:https://doi.org/10.1124/jpet.106.104166
Dreisbach, G., & Fischer, R. (2012a). Conflicts as aversive signals. Brain and Cognition, 78(2), 94-98. doi:https://doi.org/10.1016/j.bandc.2011.12.003
Dreisbach, G., & Fischer, R. (2012b). The role of affect and reward in the conflict-triggered adjustment of cognitive control. Frontiers in Human Neuroscience, 6, 342. doi:https://doi.org/10.3389/fnhum.2012.00342
Dreisbach, G., & Fischer, R. (2015). Conflicts as Aversive Signals for Control Adaptation. Current Directions in Psychological Science, 24(4), 255-260. doi:https://doi.org/10.1177/0963721415569569
Dreisbach, G., & Fischer, R. (2016). Conflicts as aversive signals: Motivation for control adaptation in the service of affect regulation. In Todd Braver (Ed.), Motivation and cognitive control. New York: Psychology Press.
Dreisbach, G., Reindl, A.-L., & Fischer, R. (2017). Conflcit and disfluency as aversive signals: Context-specific processing adjustments are modulated by affective location associations. . Psychological Research, online first. doi:https://doi.org/10.1007/s00426-016-0822-x
Duthoo, W., Abrahamse, E. L., Braem, S., Boehler, C. N., & Notebaert, W. (2014). The heterogeneous world of congruency sequence effects: an update. Frontiers in Psychology, 5. doi:https://doi.org/10.3389/Fpsyg.2014.01001
Ebitz, R. B., & Platt, M. L. (2015). Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron, 85(3), 628-640. doi:https://doi.org/10.1016/j.neuron.2014.12.053
Egner, T. (2007). Congruency sequence effects and cognitive control. Cognitive, Affective & Behavioral Neuroscience, 7(4), 380-390.
Egner, T. (2014). Creatures of habit (and control): a multi-level learning perspective on the modulation of congruency effects. Front Psychol, 5, 1247. doi:https://doi.org/10.3389/fpsyg.2014.01247
Egner, T. (2017). Conflict adaptation: Past, present, and future of the congruence sequence effect as an index of cognitive control. In T. Egner (Ed.), The Wiley handbook of cognitive control (pp. 64-78). Oxford: Wiley-Blackwell.
Ellrich, J. (2011). Transcutaneous vagus nerve stimulation. European Neurological Review, 6(4), 254-256. doi:https://doi.org/10.17925/ENR.2011.06.04.254
Englot, D. J., Chang, E. F., & Auguste, K. I. (2011). Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg, 115(6), 1248-1255. doi:https://doi.org/10.3171/2011.7.JNS11977
Fischer, R., Dreisbach, G., & Goschke, T. (2008). Context-sensitive adjustments of cognitive control: conflict-adaptation effects are modulated by processing demands of the ongoing task. Journal of Experimental Psychology: Learning, Memory and Cognition, 34(3), 712-718. doi:https://doi.org/10.1037/0278-7393.34.3.712
Fischer, R., Plessow, F., Dreisbach, G., & Goschke, T. (2015). Individual differences in the context-dependent recruitment of cognitive control: Evidence from action versus state orientation. J Pers, 83(5), 575-583. doi:https://doi.org/10.1111/jopy.12140
Fischer, R., Plessow, F., & Kiesel, A. (2010). Auditory warning signals affect mechanisms of response selection: evidence from a Simon task. Experimental Psychology, 57(2), 89-97. doi:https://doi.org/10.1027/1618-3169/a000012
Forster, S. E., Carter, C. S., Cohen, J. D., & Cho, R. Y. (2011). Parametric manipulation of the conflict signal and control-state adaptation. Journal of Cognitive Neuroscience, 23(4), 923-935. doi:https://doi.org/10.1162/jocn.2010.21458
Frangos, E., Ellrich, J., & Komisaruk, B. R. (2015). Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimulation, 8(3), 624-636. doi:https://doi.org/10.1016/j.brs.2014.11.018
Frank, M. J. (2005). Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci, 17(1), 51-72. doi:https://doi.org/10.1162/0898929052880093
Freitas, A. L., Banai, R., & Clark, S. L. (2009). When cognitive control is calibrated: Event-related potential correlates of adapting to information-processing conflict despite erroneous response preparation. Psychophysiology, 46(6), 1226-1233. doi:https://doi.org/10.1111/j.1469-8986.2009.00864.x
Fritz, J., & Dreisbach, G. (2013). Conflicts as aversive signals: Conflict priming increases negative judgments for neutral stimuli. Cogn Affect Behav Neurosci, 13(2), 311-317. doi:https://doi.org/10.3758/s13415-012-0147-1
Fritz, J., & Dreisbach, G. (2015). The time course of the aversive conflict signal. Exp Psychol, 62(1), 30-39. doi:https://doi.org/10.1027/1618-3169/a000271
Fritz, J., Fischer, R., & Dreisbach, G. (2015). The influence of negative stimulus features on conflict adaption: Evidence from fluency of processing. Front Psychol, 6, 185. doi:https://doi.org/10.3389/fpsyg.2015.00185
George, M. S., & Aston-Jones, G. (2010). Noninvasive techniques for probing neurocircuitry and treating illness: Vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology, 35(1), 301-316. doi:https://doi.org/10.1038/npp.2009.87
Goschke, T. (2013). Volition in action: Intentions, control dilemmas and the dynamic regulation of cognitive intentional control. In W. Prinz, A. Beisert, & A. Herwig (Eds.), Action science: Foundations of an emerging discipline. Cambridge, MA: MIT Press.
Gratton, G., Coles, M. G., & Donchin, E. (1992). Optimizing the use of information: strategic control of activation of responses. Journal of Experimental Psychology: General, 121(4), 480-506.
He, W., Jing, X. H., Zhu, B., Zhu, X. L., Li, L., Bai, W. Z., & Ben, H. (2013). The auriculo-vagal afferent pathway and its role in seizure suppression in rats. BMC Neurosci, 14, 85. doi:https://doi.org/10.1186/1471-2202-14-85
Holroyd, C. B., & Coles, M. G. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679-709.
Hommel, B. (2011). The Simon effect as tool and heuristic. Acta Psychol (Amst), 136(2), 189-202. doi:https://doi.org/10.1016/j.actpsy.2010.04.011S0001-6918(10)00083-1
Hommel, B. (2015). Between Persistence and Flexibility: The Yin and Yang of Action Control. In J. Elliot Andrew (Ed.), Advances in Motivation Science (Vol. Volume 2, pp. 33-67): Elsevier.
Inzlicht, M., Bartholow, B. D., & Hirsh, J. B. (2015). Emotional foundations of cognitive control. Trends in Cognitive Sciences, 19(3), 126-132. doi:https://doi.org/10.1016/j.tics.2015.01.004
Jacobs, H. I., Riphagen, J. M., Razat, C. M., Wiese, S., & Sack, A. T. (2015). Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiology of Aging, 36(5), 1860-1867. doi:https://doi.org/10.1016/j.neurobiolaging.2015.02.023
Jocham, G., & Ullsperger, M. (2009). Neuropharmacology of performance monitoring. Neuroscience and Biobehavioral Reviews, 33(1), 48-60. doi:https://doi.org/10.1016/j.neubiorev.2008.08.011
Joshi, S., Li, Y., Kalwani, R. M., & Gold, J. I. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89(1), 221-234. doi:https://doi.org/10.1016/j.neuron.2015.11.028
Junghöfer, M., Elbert, T., Tucker, D. M., & Rockstroh, B. (2000). Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology, 37(4), 523-532.
Kobayashi, N., Yoshino, A., Takahashi, Y., & Nomura, S. (2007). Autonomic arousal in cognitive conflict resolution. Autonomic Neuroscience-Basic & Clinical, 132(1-2), 70-75.
Kraus, T., Hosl, K., Kiess, O., Schanze, A., Kornhuber, J., & Forster, C. (2007). BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna), 114(11), 1485-1493. doi:https://doi.org/10.1007/s00702-007-0755-z
Kraus, T., Kiess, O., Hosl, K., Terekhin, P., Kornhuber, J., & Forster, C. (2013). CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal - a pilot study. Brain Stimulation, 6(5), 798-804. doi:https://doi.org/10.1016/j.brs.2013.01.011
Kreuzer, P. M., Landgrebe, M., Husser, O., Resch, M., Schecklmann, M., Geisreiter, F., . . . Langguth, B. (2012). Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry, 3, 70. doi:https://doi.org/10.3389/fpsyt.2012.00070
Larson, M. J., Clayson, P. E., & Baldwin, S. A. (2012). Performance monitoring following conflict: Internal adjustments in cognitive control? Neuropsychologia, 50(3), 426-433. doi:https://doi.org/10.1016/j.neuropsychologia.2011.12.021
Larson, M. J., Clayson, P. E., & Clawson, A. (2014). Making sense of all the conflict: A theoretical review and critique of conflict-related ERPs. International Journal of Psychophysiology, 93(3), 283-297. doi:https://doi.org/10.1016/j.ijpsycho.2014.06.007
Liu, K., Gao, X. Y., Li, L., Ben, H., Qin, Q. G., Zhao, Y. X., & Zhu, B. (2014). Neurons in the nucleus tractus solitarius mediate the acupuncture analgesia in visceral pain rats. Autonomic Neuroscience-Basic & Clinical, 186, 91-94. doi:https://doi.org/10.1016/j.autneu.2014.08.004
McIntyre, C. K., McGaugh, J. L., & Williams, C. L. (2012). Interacting brain systems modulate memory consolidation. Neuroscience & Biobehavioral Reviews, 36(7), 1750-1762. doi:https://doi.org/10.1016/j.neubiorev.2011.11.001
Mello-Carpes, P. B., & Izquierdo, I. (2013). The Nucleus of the Solitary Tract -> Nucleus Paragigantocellularis -> Locus Coeruleus -> CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem, 100, 56-63. doi:https://doi.org/10.1016/j.nlm.2012.12.002
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review: Neuroscience, 24, 167-202. doi:https://doi.org/10.1146/annurev.neuro.24.1.16724/1/167 [pii]
Nater, U. M., & Rohleder, N. (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34(4), 486-496. doi:https://doi.org/10.1016/j.psyneuen.2009.01.014
Nieuwenhuis, S., Yeung, N., van den Wildenberg, W., & Ridderinkhof, K. R. (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci, 3(1), 17-26.
Nomura, S., & Mizuno, N. (1984). Central distribution of primary afferent fibers in the Arnold's nerve (the auricular branch of the vagus nerve): a transganglionic HRP study in the cat. Brain Res, 292(2), 199-205.
Padmala, S., Bauer, A., & Pessoa, L. (2011). Negative emotion impairs conflict-driven executive control. Frontiers in Psychology, 2, 192. doi:https://doi.org/10.3389/fpsyg.2011.00192
Peuker, E. T., & Filler, T. J. (2002). The nerve supply of the human auricle. Clin Anat, 15(1), 35-37. doi:https://doi.org/10.1002/ca.1089
Peyk, P., De Cesarei, A., & Junghöfer, M. (2011). Electromagnetic encephalography software: Overview and integration with other EEG/MEG toolboxes. Computational Intelligence and Neuroscience. doi:https://doi.org/10.1155/2011/861705
Plessow, F., Fischer, R., Kirschbaum, C., & Goschke, T. (2011). Inflexibly focused under stress: Acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. Journal of Cognitive Neuroscience, 23(11), 3218-3227. doi:https://doi.org/10.1162/jocn_a_00024
Polich, J. (2007). Updating p300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128-2148. doi:https://doi.org/10.1016/j.clinph.2007.04.019
Porrino, L. J., & Goldman-Rakic, P. S. (1982). Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol, 205(1), 63-76. doi:https://doi.org/10.1002/cne.902050107
Raedt, R., Clinckers, R., Mollet, L., Vonck, K., El Tahry, R., Wyckhuys, T., . . . Meurs, A. (2011). Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem, 117(3), 461-469. doi:https://doi.org/10.1111/j.1471-4159.2011.07214.x
Riba, J., Rodriguez-Fornells, A., Morte, A., Munte, T. F., & Barbanoj, M. J. (2005). Noradrenergic stimulation enhances human action monitoring. J Neurosci, 25(17), 4370-4374. doi:https://doi.org/10.1523/JNEUROSCI.4437-04.2005
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., & Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science, 306(5695), 443-447. doi:https://doi.org/10.1126/science.1100301
Roosevelt, R. W., Smith, D. C., Clough, R. W., Jensen, R. A., & Browning, R. A. (2006). Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res, 1119(1), 124-132. doi:https://doi.org/10.1016/j.brainres.2006.08.048
Sara, S. J. (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 10(3), 211-223. doi:https://doi.org/10.1038/nrn2573
Sara, S. J., & Bouret, S. (2012). Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron, 76(1), 130-141. doi:https://doi.org/10.1016/j.neuron.2012.09.011
Schacht, A., Dimigen, O., & Sommer, W. (2010). Emotions in cognitive conflicts are not aversive but are task specific. Cognitive Affective & Behavioral Neuroscience, 10(3), 349-356. doi:https://doi.org/10.3758/Cabn.10.3.349
Schevernels, H., van Bochove, M. E., De Taeye, L., Bombeke, K., Vonck, K., Van Roost, D., . . . Boehler, C. N. (2016). The effect of vagus nerve stimulation on response inhibition. Epilepsy Behav, 64(Pt A), 171-179. doi:https://doi.org/10.1016/j.yebeh.2016.09.014
Schlögl, A., Keinrath, C., Zimmermann, D., Scherer, R., Leeb, R., & Pfurtscheller, G. (2007). A fully automated correction method of EOG artifacts in EEG recordings. Clin Neurophysiol, 118(1), 98-104. doi:https://doi.org/10.1016/j.clinph.2006.09.003
Schmidt, J. R. (2013). Questioning conflict adaptation: proportion congruent and Gratton effects reconsidered. Psychonomic Bulletin & Review, 20(4), 615-630.
Schouppe, N., Braem, S., De Houwer, J., Silvetti, M., Verguts, T., Ridderinkhof, K. R., & Notebaert, W. (2015). No pain, no gain: the affective valence of congruency conditions changes following a successful response. Cognitive Affective & Behavioral Neuroscience, 15(1), 251-261. doi:https://doi.org/10.3758/s13415-014-0318-3
Schouppe, N., De Houwer, J., Ridderinkhof, K. R., & Notebaert, W. (2012). Conflict: run! Reduced Stroop interference with avoidance responses. Quarterly Journal of Experimental Psychology, 65(6), 1052-1058. doi:https://doi.org/10.1080/17470218.2012.685080
Schuch, S., & Koch, I. (2015). Mood states influence cognitive control: the case of conflict adaptation. Psychol Res, 79(5), 759-772. doi:https://doi.org/10.1007/s00426-014-0602-4
Sellaro, R., de Gelder, B., Finisguerra, A., & Colzato, L. S. (2018). Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex, 99, 213-223. doi:https://doi.org/10.1016/j.cortex.2017.11.007
Sellaro, R., van Leusden, J. W., Tona, K. D., Verkuil, B., Nieuwenhuis, S., & Colzato, L. S. (2015). Transcutaneous vagus nerve stimulation enhances post-error slowing. J Cogn Neurosci, 27(11), 2126-2132. doi:https://doi.org/10.1162/jocn_a_00851
Steenbergen, L., Sellaro, R., Stock, A. K., Verkuil, B., Beste, C., & Colzato, L. S. (2015). Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. Eur Neuropsychopharmacol, 25(6), 773-778. doi:https://doi.org/10.1016/j.euroneuro.2015.03.015
Ungless, M. A., Magill, P. J., & Bolam, J. P. (2004). Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science, 303(5666), 2040-2042. doi:https://doi.org/10.1126/science.1093360
Van Bockstaele, E. J., Peoples, J., & Telegan, P. (1999). Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: Evidence for a monosynaptic pathway. J Comp Neurol, 412(3), 410-428.
Van Leusden, J. W., Sellaro, R., & Colzato, L. S. (2015). Transcutaneous Vagal Nerve Stimulation (tVNS): A new neuromodulation tool in healthy humans? Front Psychol, 6, 102. doi:https://doi.org/10.3389/fpsyg.2015.00102
van Steenbergen, H. (2015). Affective modulation of cognitive control: A biobehavioral perspective. In G. H. E. Gendolla, M. Tops, & S. L. Koole (Eds.), Handbook of biobehavioral approaches to self-regulation (pp. 89-107). New York: Springer.
van Steenbergen, H., & Band, G. P. (2013). Pupil dilation in the Simon task as a marker of conflict processing. Front Hum Neurosci, 7, 215. doi:https://doi.org/10.3389/fnhum.2013.00215
van Steenbergen, H., Band, G. P., & Hommel, B. (2010). In the mood for adaptation: how affect regulates conflict-driven control. Psychological Science, 21(11), 1629-1634. doi:https://doi.org/10.1177/0956797610385951
van Steenbergen, H., Band, G. P., & Hommel, B. (2011). Threat but not arousal narrows attention: evidence from pupil dilation and saccade control. Front Psychol, 2, 281. doi:https://doi.org/10.3389/fpsyg.2011.00281
van Steenbergen, H., Weissman, D. H., Stein, D. J., Malcolm-Smith, S., & van Honk, J. (2017). More pain, more gain: Blocking the opioid system boosts adaptive cognitive control. Psychoneuroendocrinology, 80, 99-103. doi:https://doi.org/10.1016/j.psyneuen.2017.03.002
van Veen, V., & Carter, C. S. (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav, 77(4-5), 477-482. doi:https://doi.org/10.1016/S0031-9384(02)00930-7
Venables, N. C., Patrick, C. J., Hall, J. R., & Bernat, E. M. (2011). Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biol Psychol, 86(3), 279-288. doi:https://doi.org/10.1016/j.biopsycho.2010.12.009
Ventura-Bort, C., Wirkner, J., Genheimer, H., Wendt, J., Hamm, A. O., & Weymar, M. (submitted). Effects of transcutaneous vagus nerve stimulation (tVNS) on the P300 and alpha-amylase level: A pilot study.
Verguts, T., & Notebaert, W. (2008). Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychological Review, 115(2), 518-525. doi:https://doi.org/10.1037/0033-295x.115.2.518
Verguts, T., & Notebaert, W. (2009). Adaptation by binding: A learning account of cognitive control. Trends in Cognitive Science, 13(6), 252-257. doi:https://doi.org/10.1016/j.tics.2009.02.007
Verguts, T., Notebaert, W., Kunde, W., & Wühr, P. (2011). Post-conflict slowing: Cognitive adaptation after conflict processing. Psychonomic Bulletin & Review, 18(1), 76-82. doi:https://doi.org/10.3758/s13423-010-0016-2
Vidaurre, C., Sander, T. H., & Schlögl, A. (2011). BioSig: the free and open source software library for biomedical signal processing. Comput Intell Neurosci, 2011, 935364. doi:https://doi.org/10.1155/2011/935364
Warren, C. M., van den Brink, R. L., Nieuwenhuis, S., & Bosch, J. A. (2017). Norepinephrine transporter blocker atomoxetine increases salivary alpha amylase. Psychoneuroendocrinology, 78, 233-236. doi:https://doi.org/10.1016/j.psyneuen.2017.01.029
Wendt, M., Kiesel, A., Geringswald, F., Purmann, S., & Fischer, R. (2014). Attentional adjustment to conflict strength: evidence from the effects of manipulating flanker-target SOA on response times and prestimulus pupil size. Experimental Psychology, 61(1), 55-67.
Yeung, N., Botvinick, M. M., & Cohen, J. D. (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931-959. doi:https://doi.org/10.1037/0033-295X.111.4.939
Yuan, H., & Silberstein, S. D. (2016). Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache, 56(2), 259-266. doi:https://doi.org/10.1111/head.12650
Zeng, Q., Qi, S., Li, M., Yao, S., Ding, C., & Yang, D. (2016). Enhanced conflict-driven cognitive control by emotional arousal, not by valence. Cogn Emot, 1-14. doi:https://doi.org/10.1080/02699931.2016.1189882
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, R., Ventura-Bort, C., Hamm, A. et al. Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cogn Affect Behav Neurosci 18, 680–693 (2018). https://doi.org/10.3758/s13415-018-0596-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0596-2