Abstract
In healthy humans, it has been shown that executive functions are associated with increased frontal-midline EEG theta activity and theta phase coupling between frontal and posterior brain regions. In individuals with schizophrenia, central executive functions are supposed to be heavily impaired. Given that theta phase coupling is causally involved in central executive functions, one would expect that patients with an executive function deficit should display abnormal EEG theta synchronization. We therefore investigated executive functioning in 21 healthy controls and 21 individuals with schizophrenia while they performed a visuospatial delayed match to sample task. The task required either high executive demands (manipulation of content in working memory [WM]) or low executive demands (retention of WM content). In addition, WM load (one vs. three items) was varied. Results indicated higher frontal theta activity for manipulation processes than for retention processes in patients with schizophrenia, as compared with healthy controls, independently of WM load. Furthermore, individuals with schizophrenia revealed a reduction in theta phase coupling during early stages of the delay period for retention, as well as for manipulation processes at high-WM loads. Deviations in theta phase coupling in individuals with schizophrenia were mainly characterized by aberrant fronto-posterior connections, but also by attenuated posterior connections during manipulation of high-WM load. To conclude, fronto-parietal theta coupling seems to be substantially involved in executive control, whereas frontal theta activity seems to reflect general task demands, such as deployment of attentional resources during WM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Working memory (WM) can be defined as an online system in the human brain that allows sensory representations or information from long-term memory to be actively kept in mind for a few seconds. These storage functions require control and coordination processes that are mastered by a modality-free central executive (Baddeley, 1996). A difficulty in investigating executive functions is its fractionation into many cognitive functions. For instance, increased demands on a central executive are caused by the manipulation of WM content, updating of information held in WM, temporal coding or sequencing of WM content, interference control, and attentional and monitoring processes (Barch & Smith, 2008; Smith & Jonides, 1999). In a more general manner, all these processes have mainly been associated with prefrontal structures, especially the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex, and anterior cingulate cortex (D’Esposito et al., 1995; Smith & Jonides, 1999), but also parietal brain areas (Collette et al., 1999). The successful implementation of central executive functions therefore seems to rely on the integration of widely distributed neuronal activity.
In healthy humans, it has been suggested that the integration of neuronal activity is based on synchronous oscillatory activity within, as well as between, dedicated frequency bands (Axmacher et al., 2010; Canolty et al., 2006; Demiralp et al., 2007; Hanslmayr et al., 2007; Lakatos et al., 2005; Sarnthein, Petsche, Rappelsberger, Shaw, & Von Stein, 1998; Sauseng et al., 2009). Low frequencies (e.g., theta, 4–7 Hz) are particularly suitable in enabling large-scale neuronal integration, whereas high frequencies (e.g., gamma, 30–70 Hz) have been proposed to be more supportive in local neuronal integration (von Stein & Sarnthein, 2000). Indeed, several studies support the involvement of synchronous theta activity in cognitive tasks requiring the integration of distant neuronal activity (Hanslmayr et al., 2007; Sarnthein et al., 1998; Sauseng, Hoppe, Klimesch, Gerloff, & Hummel, 2007; Wu, Chen, Li, Han, & Zhang, 2007). In addition, interregional theta (4–7 Hz) phase synchronization between frontal and posterior brain areas has been reported to reflect central executive demands during mental arithmetics (Mizuhara & Yamaguchi, 2007), task switching (Sauseng et al., 2006), and WM tasks with high executive demands (Sauseng, Klimesch, Schabus, & Doppelmayr, 2005). The functional role of interregional theta phase synchronization during WM has been recently proposed to be the integration and coordination of subnetworks involved in a specific WM process (Sauseng, Griesmayr, Freunberger, & Klimesch, 2010). All these findings make theta oscillations a prime candidate in implementing executive functions.
However, most studies investigating the neural signatures of central executive functions in WM report mere correlational associations, measuring brain activity during cognitive tasks loading highly on central executive functions. To provide evidence for a causal link between particular brain activation patterns and central executive functions, one possibility is to alter brain activity using medication or noninvasive brain stimulation. Another way is to compare neuronal activity between healthy subjects and patients who display a deficit in central executive functioning. The rationale for the latter is that exactly those neural signatures of executive functions suggested by associational studies in healthy subjects need to be abnormal in patients with executive dysfunction if there is indeed a causal link between neuronal activity and the cognitive function. There is a broad range of neurological and psychiatric disorders in which central executive functions are impaired, including dementia, acquired brain injury, depression, affective disorders, attention deficit hyperactivity disorder, and schizophrenia (Baddeley & Della Sala, 1998; Diamond, 2011; Tan, Callicott, & Weinberger, 2007). It is well-known that individuals with schizophrenia show substantial impairments particularly in tasks requiring executive functioning, with executive functions being far more obvious than general, more unspecific attenuation of memory functions (Reichenberg & Harvey, 2007). Furthermore, abnormal integration of brain processes on different spatial scales has been claimed to be fundamental for the illness (Friston, 1998; Uhlhaas et al., 2009; Uhlhaas & Singer, 2010). There is, for instance, growing evidence that individuals with schizophrenia show altered local, synchronous oscillatory activity in the theta, alpha, beta, and gamma frequency bands associated with perceptual binding/grouping (Spencer et al., 2004; Uhlhaas et al., 2006), WM (Haenschel et al., 2009; Haenschel, Uhlhaas & Singer 2007; Manoach, 2003), and cognitive control (Cho, Konecky, & Carter, 2006). WM and, particularly, executive functions rely on large-scale integration for proper functioning, and thus, deficits in any of these cognitive processes (as are expected in individuals with schizophrenia) should be manifested in aberrant theta connectivity. Although evidence of altered functional connectivity within a fronto-parietal network supporting WM has been reported (Kim et al., 2003), the specific role of theta oscillations for large-scale integration during executive functioning has not been investigated in individuals with schizophrenia so far.
The aim of the present study was to investigate the neural correlates of executive functioning in healthy controls and individuals with schizophrenia. On this account, a visuospatial delayed match to sample task was carried out. The task required either high executive demands (manipulation of WM content) or low executive demands (retention of WM content). In addition, WM load was varied by one versus three to-be-remembered items. On the basis of the above-mentioned findings, we focused on interregional EEG theta (4–7 Hz) phase coupling and local frontal theta activity.
Frontal theta activity is a salient feature during WM (Gevins, Smith, McEvoy, & Yu, 1997; Jensen & Tesche, 2002; Onton, Delorme, & Makeig, 2005) and has been proposed to be useful in studying executive functions in the frontal cortex (Tsujimoto, Shimazu, & Isomura, 2006). There is some debate as to whether frontal (midline) theta activity reflects mnemonic processes per se (Jensen & Tesche, 2002) or, rather, unspecific allocation of cognitive task resources (Onton et al., 2005; Sauseng et al., 2007). If frontal midline theta activity reflects mnemonic processes, patients with impaired memory functions, such as subjects with schizophrenia, will display decreased theta amplitudes, as compared with healthy controls. If frontal midline theta activity is a signature for memory-unspecific allocation of cognitive resources depending on task demand, individuals with schizophrenia for whom the memory task should be more difficult, are expected to exhibit stronger theta amplitudes than are healthy subjects.
Interregional, particularly fronto-parietal, theta phase synchronization, on the other hand, is suggested to be associated with central executive control of WM processes (e.g., Sauseng et al., 2010). It might be an electrophysiological signature of activity within a fronto-parietal control network (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008). Therefore, individuals with schizophrenia with a rather selective deficit in executive control of WM processes should display attenuated fronto-parietal theta phase synchronization.
Method
Subjects
Twenty-seven patients meeting ICD 10 criteria for schizophrenia participated in the study. Patients were recruited from the inpatient and outpatient facilities of the Christian-Doppler Klinik, Salzburg. They showed (1) no history of neurological illness, (2) no history of alcohol or substance abuse within the last month prior to testing, and (3) no symptoms of color blindness (the experimental task requires dissociation of red and green colors). Further exclusion criteria were alternating medication treatment and florid symptomatic. Six patients were excluded from data analysis due to the following reasons: Three out of 6 patients were not capable of finishing the task, and EEG signals of 3 further patients were too heavily contaminated by artifacts. Diagnostic subgroups of the remaining sample (n = 21; see Table 1 for demographical characteristics) were paranoid schizophrenia (n = 17), undifferentiated schizophrenia (n = 1), and schizoaffective disorder (n = 3). Severity of clinical symptoms was assessed with the Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987) by three trained psychiatrists, indicating an average total PANSS score of 77.19 ± 20.61. Average ratings were 18.95 (SD = 7.12) on the Positive subscale and 19.24 (SD = 8.11) on the Negative subscale. At the time of testing, patients received atypical (n = 15), typical (n = 2), and atypical as well as typical (n = 4) antipsychotics. In addition to antipsychotic treatment, 6 patients received antidepressants, and a further 3 patients received low doses of benzodiazepines. The mean chlorpromazine equivalent was 556.36 mg/day (SD = 371.63).
Twenty-one healthy control subjects were recruited by flyers, Internet advertisements, and advertisements in newspapers. They were accordingly matched for age, gender, handedness, and education (see Table 1). Control subjects completed the Brief Symptom Inventory (Franke, 2000), which reliably screens for clinically relevant psychological stress symptoms. In a second step, they were carefully screened with the structured clinical interview (SCI-I) in order to exclude mental disorders, past and present. Further exclusion criteria were (1) neurological diseases, (2) first-degree relatives suffering from psychiatric disorders, and (3) color blindness (see above).
Prior to testing, all subjects gave written informed consent, and after the experimental session, they were monetarily compensated for participation. The study was approved by the local ethics committee and conducted in agreement with the Declaration of Helsinki.
Experimental procedure
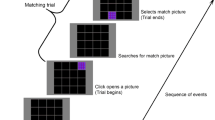
A 6 × 6 matrix with a visual angle of 9.2° × 9.2° (distance to computer screen: 0.8 m) was presented at the center of a computer screen (19-in. LCD monitor) using Presentation® 7.01 software (Neurobehavioral Systems). In this matrix, either one (load 1) or three (load 3) positions appeared in color for 700 ms (see Fig. 1). If the positions were shown in green, subjects had to maintain their locations in memory for a 2,000-ms delay period (retention condition). If matrix positions appeared in red, they had to be mirrored around a vertical gap in the matrix during a delay period, and the new positions had to be kept in memory (manipulation condition). Subsequently, a probe matrix was presented for 2,000 ms, with gray-colored positions representing the same (match) or different (nonmatch) locations, as in the encoding matrix. Subjects had to indicate by buttonpress whether they thought the probe item was matching the memory set or not. During the intertrial interval (jittered between 2,100 and 2,500 ms), a central fixation cross was presented. Two hundred twenty-four trials were run in total, resulting in 56 trials per experimental condition: manipulation load 1 (Man L1), manipulation load 3 (Man L3), retention load 1 (Ret L1), and retention load 3 (Ret L3). Half of the trials were match, the other half nonmatch. Conditions were presented in randomized order, and red and green matrix positions were isoluminant. In order to avoid color afterimages during the maintenance period, the matrix shown in the delay interval was filled with a structured gray/black pattern. A training block was carried out at the beginning of the experiment. Subjects were instructed to answer as correctly as possible. Due to response biases in Man L3, 1 subject of the schizophrenia sample and 2 subjects of the healthy control sample were excluded from further analysis. Therefore, the EEG analyses described in the EEG recording section refer to 20 subjects with schizophrenia and 19 healthy controls.
Example of the visuospatial delayed match to sample task with conditions “retention” and “manipulation” and varying memory load (one vs. three items). Subjects had either to retain the positions of the colored squares (a) or to mirror the squares around a vertical gap in the matrix (b). Note that match, as well as nonmatch, trials are shown
EEG recording
Scalp EEG was recorded from 28 Ag-AgCl ring electrodes (EasyCap) positioned according to the 10-20 system: Fp1, Fp2, F7, F3, Fz, F4, F8, FC3, FCz, FC4, T3, C3, Cz, C4, T4, CP5, CPz, CP6, T5, P3, Pz, P4, T6, PO3, PO4, O1, Oz, and O2. The recording reference was set on the tip of the nose, and the ground electrode was positioned on the forehead. For EEG analyses, data were digitally rereferenced to averaged earlobes. In order to control for vertical and horizontal eye movements, two additional electrodes were placed above and to the right of the right eye. The EEG signal was registered between 0.016 and 80 Hz, digitized with a sampling rate of 1000 Hz, and amplified with a BrainAmp MR+ amplifier (Brain Products). A notch-filter was set at 50 Hz, and impedances were kept below 15 kΩ.
Behavioral data analysis
Percentage of correct responses was used for behavioral data analysis. For statistical analysis, a repeated measures ANOVA with the between-subjects factor group (SzP, HC) and the within-subjects factors condition (retention, manipulation) and load (load 1, load 3) was run. Independent and paired-sample t-tests were used for post hoc comparisons, and false discovery rate (FDR) correction (Benjamini & Hochberg, 1995) was applied to control for multiple comparisons.
EEG data analysis
For EEG data analysis, Brain Vision Analyzer 2.0 (Brain Products) and MATLAB 2008a (Math Works) were used. Data were first rereferenced offline to averaged ears and high-pass filtered with a low cutoff at 1 Hz, 48db/Oct (Butterworth Zero Phase IIR Filter, as implemented in Brain Vision Analyzer 2.0) to eliminate slow drifts in the EEG signal. Then ICA ocular correction (as implemented in Brain Vision Analyzer 2.0) was applied to remove horizontal and vertical eye artifacts. In addition, data were manually inspected for remaining artifacts. Before segmentation, Laplacian current source density (CSD) was applied to attenuate effects of volume conduction on interregional phase coupling (Trujillo, Peterson, Kaszniak, & Allen, 2005). CSD-transformed data were then segmented into 3,400-ms epochs, separately for each experimental condition. The 3,400-ms segments comprised 100 ms at the edges of the segments that were trimmed after filtering, a 500-ms pretrial baseline, a 700-ms presentation of the memory set, and a 2,000-ms delay interval. On average, this resulted in 45.6 (SD = 6.4) trials for Ret L1, 46.5 (SD = 7.1) trials for Ret L3, 44.1 (SD = 5.5) trials for Man L1, and 47.5 (SD = 5.8) trials for the Man L3 condition for the individuals with schizophrenia. Healthy controls, on average, showed 47.05 (SD = 4.9) trials for Ret L1, 46.53 (SD = 6.6) trials for Ret L3, 46.79 (SD = 6.2) trials for Man L1, and 46.47 (SD = 5.9) for Man L3. In terms of the number of analyzed trials, there were no significant differences between individuals with schizophrenia and healthy controls in any condition (all ps > .05). The analyses described below were exclusively applied to the 2,000-ms delay period.
Analyses of frontal-midline theta (4–7 Hz) event-related amplitude in- or decrease
To calculate amplitude estimates, single trials were first wavelet filtered between 1 and 30 Hz in 1-Hz steps (continuous wavelet demodulation using a 10-cycle Complex Morlet Wavelet; for the center frequency of the theta band, this resulted in a temporal resolution of 636 ms and a frequency resolution of 1 Hz). Single trials were then averaged for each condition separately, and event-related amplitude (ERA) increase/decrease was calculated. ERA increase/decrease is defined as the percentage of increase or decrease with respect to a predefined baseline ([activity period − baseline period]/baseline * 100). We extracted a baseline period from −500 to −200 ms prior to stimulus onset for ERA increase/decrease calculation. ERA increase/decrease estimates were then averaged within 4–7 Hz for four different time windows of 500 ms covering the 2,000–ms delay interval. Electrode Fz was selected for ERA increase/decrease analysis, since time-frequency plots and topographical maps showed clear amplitude maxima in this frequency band at the selected recording site (see Fig. 2). Previous studies also report FM theta activity being dominant at electrode Fz (Gevins et al., 1997; Onton et al., 2005; Sauseng et al., 2007). In addition to frontal theta ERA increase/decrease, theta ERA increase/decrease was calculated for a pooled set of posterior electrodes (P3, Pz, P4, Po3, PO4, O1, Oz, O2) to investigate the topographical specificity of frontal theta effects.
a Sustained frontal theta (4–7 Hz) event-related amplitude (ERA) increase/decrease at Fz is shown for healthy controls (left) and individuals with schizophrenia (right), averaged over all conditions. Vertical dashed lines mark the encoding interval from 0 to 700 ms, which is followed by the delay period from 700 to 2,700 ms; dashed horizontal lines mark frequency of interest for statistical analysis. b Topographical distribution of theta ERA increase/decrease is shown for healthy controls (left) and individuals with schizophrenia (right) for the 2,000-ms delay period
For statistical analysis, repeated measures ANOVAs with the between-subjects factor group (SzP, HC) and the within-subjects factors condition (retention, manipulation), load (load 1, load 3), and time (time window 1, time window 2, time window 3, time window 4) were run. ERA increase/decrease estimates were used as dependent variables. Greenhouse–Geisser correction was applied where necessary. Independent and paired-sample t-tests were used for post hoc comparisons, and FDR correction was applied to correct for multiple comparisons.
Interregional phase coupling analysis
To estimate interregional phase synchronization for the theta (4–7 Hz) frequency band, phase locking value (PLV) according to Lachaux, Rodriguez, Martinerie, and Varela (1999) was calculated (see Supplemental online material for depiction of analysis steps). PLV is a measure for intertrial variability of phase differences between signals from two electrode sites at a specific time bin. PLV can range from 0 to 1, with a value of 0 indicating completely random distribution of phase differences and a value of 1 indicating perfect stability of phase differences across trials. On the basis of continuous complex Morlet wavelet demodulation as described above, instantaneous phase angles of theta frequency (center frequency, 5.14 Hz; bandwidth, 4.63–5.66 Hz) were obtained for single trials. Thereafter, PLV was estimated for all possible electrode pairs (n = 378) for each time bin within the 2,000-ms delay period and each experimental condition. PLVs were then averaged into 500-ms time windows. The same procedure was carried out for a baseline period from −500 to −200 ms prior to stimulus onset. Phase coupling within the delay period was then compared with that of the baseline period by applying paired-sample t-tests. This was done for each of the 378 electrode pairs and for each time window, experimental condition, and group separately. In a first explorative analysis step, electrode pairs reaching a significance level of p < .05 were considered as relevant for further statistical analysis. For further statistical evaluation, the number of significant electrode pairs showing an increase or decrease, as compared with the baseline period, was compared between varying loads (Ret L1 vs. Ret L3, Man L1 vs. Man L3) and between conditions (Ret L1 vs. Man L1, Ret L3 vs. Man L3) in each experimental group by running McNemar tests (a chi-squared test procedure for repeated measures). This was done separately for each time window, resulting in 16 comparisons per experimental group. A further 16 comparisons were carried out for between-group analyses (SzP Ret L1 vs. HC Ret L1, SzP Ret L3 vs. HC Ret L3, SzP Man L1 vs. HC Man L1, SzP Man L3 vs. HC Man L3). Results from McNemar tests were corrected for multiple comparisons using FDR correction.
Results
Behavioral data
As Fig. 3a shows, overall performance (mean of correct responses) was better in healthy controls (M = 94.88 % ± 0.73 % SEM) than in individuals with schizophrenia (92.63 % ± 0.71 %). This was indicated by a significant main effect of the group factor, F(1, 37) = 4.88, p < .05. In general, performance was better for load 1 (97.58 % ± 0.36 %) as compared with load 3 (89.94 % ± 0.82 %) and for retention (97.04 % ± 0.44 %) as compared with manipulation [90.48 % ± 0.67 %; main effects for the factors of load, F(1, 37) = 104.18, p < .001, and condition, F(1, 37) = 167.41, p < .001]. Furthermore, a significant load × condition interaction, F(1, 37) = 177.68, p < .001, revealed a decline in performance for manipulation, as compared with retention, that was more pronounced for load 3 [84.17 % ± 1.02 % vs. 95.71 % ± 0.75 %; t(38) = 14.67, p = .000; p < .05 after correction for multiple comparisons] than for load 1 [96.79 % ± 0.55 % vs. 98.36 % ± 0.27 %; t(38) = 3.24, p = .002; p < .05 after correction for multiple comparisons]. Between-group results revealed a significant condition × group interaction, F(1, 37) = 6.55, p < .05. Post hoc t-tests indicated that patients with schizophrenia performed significantly worse in the manipulation condition (88.71 % ± 0.94 %), as compared with healthy controls [92.25 % ± 0.96 %; t(37) = −2.64, p = .021; p < .05 after correction for multiple comparisons], whereas no differences were found for the retention condition (96.56 % ± 0.61 % vs. 97.51 % ± 0.63 %), t(37) = −1.08, p =.228.
a Mean correct responses in percentages are shown separately in response to experimental conditions retention and manipulation and load 1 and load 3 for healthy controls (HC) and individuals with schizophrenia (SzP). Error bars represent standard errors of the means. b Frontal theta event-related amplitude (ERA) increase is shown for HC and SzP in response to retention and manipulation. Error bars represent standard errors of the means
Event-related amplitude increase/decrease
Frontal theta (4–7 Hz) ERA increase
The repeated measures ANOVA run on theta ERA increase at electrode site Fz revealed an increase in amplitude for manipulation as compared with retention, F(1, 37) = 8.08, p < .01, and for load 3 as compared with load 1, F(1, 37) = 4.81, p < .05, independently of the group factor. Furthermore, the main effect of the time factor, F(1.56, 57.84) = 38.61, p < .001, indicated that frontal theta ERA increase was strongest in time windows 1 and 2, followed by less ERA increase in time windows 3 and 4 [t1 vs. t3, t(38) = 3.25, p = .002; t1 vs. t4, t(38) = 7.32, p = .000; t2 vs. t3, t(38) = 4.16, p = .000; t2 vs. t4, t(38) = 9.37, p = .000; t3 vs. t4, t(38) = 11.5, p = .000; all ps < .05 after correction for multiple comparisons]. Moreover, a significant condition × time interaction, F(1.82, 67.34) = 5.72, p < .01, suggests higher ERA increase for manipulation than for retention in the later time windows of the delay period [t3, t(38) = −2.74, p = .009; t4, t(38) = −4.09, p = .000; all ps < .05 after correction for multiple comparisons]. In addition, frontal theta ERA increase was more pronounced for load 3 than for load 1 in time windows 2, 3, and 4 [t2, t(38) = −2.22, p = .034; t3, t(38) = −2.29, p = .027; t4, t(38) = −2.88, p = .007; all ps < .05 after correction for multiple comparisons], as indicated by a significant load × time interaction, F(1.70, 62.83) = 10.07, p < .001. Most important, however, as can be seen in Fig. 3b, individuals with schizophrenia showed a stronger frontal theta ERA increase for manipulation than for retention, t(19) = −2.95, p < .05, which was not the case for healthy controls. This effect was confirmed by a significant condition × group interaction, F(1, 37) = 4.24, p < .05. In general, patients with schizophrenia tended to show higher frontal theta ERA increase, as indicated by a marginally significant main effect of the group factor, F(1, 37) = 3.21, p = .08.
Posterior theta (4–7 Hz) ERA increase/decrease
For posterior theta, results revealed a decline in ERA over time in both groups, as indicated by a significant main effect of the time factor, F(1.46, 53.83) = 132.91, p < .001. A significant time × group interaction, F(3, 111) = 5.75, p < .05, indicates that specifically during time window 1, healthy controls showed higher theta ERA, as compared with individuals with schizophrenia. Furthermore, in healthy controls, a load-dependent increase in theta ERA can be observed, as compared with the patient group [load × group: F(1, 37) = 4.18, p < .05], which is more pronounced during late time windows [load × time × group: F(3, 111) = 4.00, p = .01].
Interregional theta phase coupling
Within group analysis, healthy controls
In Fig. 4a, the number of electrode pairs exhibiting significant increases in interregional theta phase coupling, as compared with a baseline period, is shown [t(37) > 2.03, p < .05, uncorrected for multiple comparison as exploratory analysis]. Theta phase coupling was most pronounced within time window 1 for Ret L3 and Man L3. Interestingly, all conditions showed a gradual decrease in theta phase coupling over time. McNemar tests comparing (number of electrode pairs with theta phase coupling significantly increased, as compared with baseline, for) loads 1 and 3 revealed significantly more theta phase coupling for Ret L3 than for Ret L1, as well as for Man L3 as compared with Man L1, within all four time windows (Ret L3 > Ret L1: time window 1, x 2 = 8.82, p = .003; time window 2, x 2 = 8.50, p = .004; time window 3, x 2 = 5.92, p = .014; time window 4, x 2 = 5.81, p = .016; Man L3 > Man L1: time window 1, x 2 = 15.28, p = .000; time window 2, x 2 = 39.45, p = .000; time window 3, x 2 = 16.57, p = .000; time window 4, x 2 = 16.70, p = .000; all ps < .05, after being corrected for multiple comparisons). Between conditions, manipulation versus retention results indicated significantly more electrode pairs with increased theta phase coupling for Man L3, as compared with Ret L3, within time windows 2 (x 2 = 10.08, p = .001; p < .05 after correction for multiple comparisons) and 3 (x 2 =6.11, p = .013; p < .05 after correction for multiple comparisons). No significant results were found for Man L1, as compared with Ret L1. With respect to the number of electrode pairs showing a decrease in theta phase coupling (as compared with a baseline period), no significant differences were found between conditions or loads.
The number of significant theta interactions for healthy controls (a) and individuals with schizophrenia (b) is shown for retention loads 1 and 3 (light gray bars) and manipulation loads 1 and 3 (dark gray bars) during the delay period. The delay period is separated into four time windows of 500 ms each. Significant differences between conditions are marked with asterisks. * p < .05, corrected for multiple comparison
Within group analysis, individuals with schizophrenia
In Fig. 4b, the number of electrode pairs exhibiting significant increases in interregional theta phase coupling, as compared with a baseline period [t(37) > 2.03, p < .05, uncorrected for multiple comparison as exploratory analysis] are shown for individuals with schizophrenia. Number of electrode pairs with increased theta phase coupling was highest within time window 1 of the delay period for Ret L1, Ret L3, and Man L1 and declined over time. For Man L3, however, it increased within the first 1,500 ms of the delay period. McNemar tests within conditions revealed significantly more coupled electrode pairs for Man L3 than for Man L1 within time windows 2 (x 2 = 14.79, p = .000; p < .05 after correction for multiple comparisons), 3 (x 2 = 18.95, p = .000; p < .05 after correction for multiple comparisons), and 4 (x 2 = 9.82, p = .002; p < .05 after correction for multiple comparisons), which was mainly characterized by an increase in frontal short-range connections (see Fig. 6b). No significant differences were found for Ret L3, as compared with Ret L1. Comparisons between manipulation and retention indicated significantly more theta phase coupled electrode pairs for Man L3 than for Ret L3 within time windows 2 (x 2 = 14.38, p = .000; p < .05 after correction for multiple comparisons) and 3 (x 2 = 15.02, p = .000; p < .05 after correction for multiple comparisons). No significant differences were found for Man L1, as compared with Ret L1. For the number of electrode pairs exhibiting a decrease in theta phase coupling (as compared with a baseline period), no significant differences were found between conditions or loads.
Between-group analysis and relation between theta phase coherence and task performance
As can be seen in Figs. 4 and 5, significantly fewer electrode pairs exhibited increased theta phase coupling in individuals with schizophrenia, as compared with healthy controls, for Man L3 and Ret L3 during time window 1 (confirmed by McNemar tests; Man L3, x 2 = 9.56, p = .002; Ret L3, x 2 = 14.02, p = .000; all ps < .05 after correction for multiple comparisons). No significant differences were found for Man L1 and Ret L1. Furthermore, we found no significant differences between groups with regard to conditions for adjacent time windows. For the number of electrode pairs exhibiting a decrease in theta phase coupling (as compared with a baseline period), no significant differences were found between groups.
Theta phase coupling within time window 1 of the delay period is shown for manipulation and retention with respect to load 3 (a). Significant theta phase coupling increase in healthy controls (HC) and individuals with schizophrenia (SzP) and phase coupling that is shared by both groups are color coded. Each cell represents one electrode pair; the areas marked in red represent networks of interest: a frontal-posterior network and a posterior network. A theta phase coupling ratio between the averaged theta phase synchronization across electrode pairs with higher PLV in the HC, as compared with SzP, divided by average phase coupling within the theta network stronger in SzP, as compared with HC, was correlated with task accuracy (b). The higher the PLV ratio, the more typical the connectivity pattern is to HCs; the lower the ratio, the more typical theta phase coupling is to SzPs. The PLV ratio obtained in the Man L3, as well as in the Ret L3, condition significantly correlates with task performance in Man L3, but not with performance on pure retention of three items (Ret L3)
To investigate whether theta phase synchronization showing group differences was associated with task performance, averaged phase coherence values across previously identified electrode pairs of interest were correlated with percentages of correct responses. In the first time window for Man L3 and Ret L3, a PLV ratio (PLV averaged over all electrode pairs exhibiting significantly higher phase coherence increase in the healthy controls, as compared with individuals with schizophrenia [green cells in Fig. 5a or left connectivity map below scatterplots in Fig. 5b], divided by PLV averaged over all electrode pairs exhibiting significantly higher phase coherence increase in the individuals with schizophrenia, as compared with healthy controls [blue cells in Fig. 5a or right connectivity map below scatterplots in Fig. 5b]) was calculated. This ratio being high indicates a connectivity pattern more typical for healthy controls during the task. If this PLV ratio is low, the theta coherence pattern is exhibiting more features typical for individuals with schizophrenia. As is depicted in Fig. 5b, percentage of correct responses in Man L3 (the condition for which task accuracy differed significantly between groups; see above) significantly correlated with the PLV ratio obtained in Man L3 (r = .27, p = .049, one-tailed), but also in Ret L3 (r = .33, p = .021, one-tailed). Task accuracy in Ret L3, on the other hand, did not exhibit any significant association with PLV ratio obtained in either condition (PLV ratio in Ret L3, r = .24, p = .070, one-tailed; PLV ratio in Man L3, r = .09, p = .285, one-tailed).
Effect of chlorpromazine equivalent on task performance and theta amplitude
Spearman correlations were calculated between chlorpromazine equivalent and task accuracy, as well as response times for each experimental condition separately. Task accuracy was not significantly related to chlorpromazine equivalent in any experimental condition (all ρs < .22, ps > .36), nor was response time (all ρs < .21, ps > .39). Spearman correlations between chlorpromazine equivalent and frontal midline theta activity for each experimental condition and each time window separately indicate that there is no consistent relation between theta activity and chlorpromazine equivalent in our data set. There were, however, some significant correlations—mainly for pure retention (R1 during time windows 2 [ρ = .54, p = .015], 3 [ρ = .76, p = .000], and 4 [ρ = .50, p = .025]; R3 during time windows 2 [ρ = .45, p = .049] and 4 [ρ = .59, p = .006]; M4 during time window 4 [ρ = .54, p = .014])—from which only one (R1 time window 3) survives correction for multiple testing. This suggests that theta activity was not consistently influenced by medication in the schizophrenia sample and that medication cannot account for the obtained effects described above.
Discussion
In the present study, we investigated the role of frontal theta activity and interregional phase synchronization at theta frequency for executive functioning in healthy controls and patients with impaired central executive functions—that is, individuals suffering from schizophrenia. Subjects had to perform a visuospatial delayed match to sample task that required either high (manipulation of visuospatial information) or low (retention of visuospatial information) executive demands. In addition, memory load was varied by the number of items presented in the memory set.
As was expected, behavioral data revealed impaired manipulation processes in individuals with schizophrenia, as compared with healthy controls, while pure retention processes seemed to be intact in the patient group. This is well in line with a variety of studies showing that individuals with schizophrenia are mainly impaired in cognitive tasks requiring executive processes (e.g., Kim, Glahn, Nuechterlein, & Cannon, 2004), whereas mixed evidence has been reported for memory storage deficits (Junghee & Sohee, 2005).
The study revealed sustained oscillatory theta activity during the delay period at frontal electrode sites, with maximal activity at electrode Fz for healthy controls and individuals with schizophrenia. Previously, it has been proposed that sustained frontal theta activity might be involved in memory retention per se (Jensen & Tesche, 2002). However, our findings do not indicate frontal theta activity to be a substrate of memory maintenance. Instead, the present results point toward an unspecific, general role of frontal theta activity, such as allocation of general cognitive resources. Naturally, an increase in memory load as in Jensen and Tesche’s study would require enhanced allocation of such general cognitive resources as well, possibly explaining this inconsistency between previous work (Jensen & Tesche, 2002) and the present results. This is backed up by the finding that individuals with schizophrenia showed a stronger increase in frontal theta activity for the manipulation of information, as compared with pure retention, which was not the case in healthy controls. The increase in frontal theta activity during manipulation might account for increased deployment of attentional resources that are required because of difficulties in handling manipulation processes. Therefore, increased frontal theta activity during manipulation in individuals with schizophrenia might be a by-product of an executive deficit, rather than the representation of this impairment per se. This interpretation is well in line with studies showing that frontal theta activity represents sustained attentional processes related to the task (Gevins et al., 1997; Onton et al., 2005; Sauseng et al., 2007). An alternative explanation is that during manipulation of WM content, processing capacity in individuals with schizophrenia is close to its limit. This could lead to neuronal overactivation in patients, as compared with healthy controls, which is underpinned by the fact that healthy controls did not show higher increase in frontal theta activity during manipulation, as compared with retention (see Fig. 3b). This indicates that processing capacity limits are not exceeded in this group. Several fMRI studies report similar effects within the prefrontal cortex (hyper- vs. hypoactivation of DLPFC; Callicott et al., 2003; Cannon et al., 2005; Manoach, 2003).
Results on interregional theta phase coupling indicated reduced synchronization during manipulation and retention processes at highest WM load (load 3) within the first 500 ms of the delay period in individuals with schizophrenia, as compared with healthy controls. A general reduction in functional connectivity is well in line with several theoretical models considering schizophrenia as disconnection syndrome (for reviews, see Friston, 1998; Uhlhaas & Singer, 2010), but also with empirical studies showing reduced functional connectivity in individuals with schizophrenia in several cognitive domains and EEG frequency bands (Ford, Mathalon, Whitfield, Faustman, & Roth, 2002; Micheloyannis et al., 2006; Uhlhaas et al., 2006). Results from the present study particularly highlight theta synchronization between prefrontal and posterior recording sites during processing of information requiring high executive control in healthy subjects—a pattern that is clearly reduced in the patient sample (see Fig. 5). This is well supported by several neuroimaging studies indicating an involvement of frontal and posterior brain areas in WM (Postle, Stern, Rosen, & Corkin, 2000; Smith & Jonides, 1998). Furthermore, a tightly organized posterior theta network seems to play a major role during manipulation of WM contents in healthy controls, as compared with individuals with schizophrenia. These findings are in good agreement with those of Sauseng et al. (2006) indicating an increase in fronto-parietal theta connectivity and increases in a posterior theta network associated with increased demands on central executive functioning. Moreover, a study by Champod and Petrides (2007) showed the posterior parietal cortex being particularly involved in the rearrangement of items held in WM, whereas activity of the DLPFC predominantly represents monitoring of information in WM.
Importantly, in the present study, strong posterior theta coupling in healthy subjects was observed throughout the entire delay period (see Fig. 6d), but only for the most demanding condition—namely, manipulation of three items. Pure retention of three positions in WM, however, did result in comparably strong posterior theta connectivity for the first 500 ms only (Fig. 6c). And apparently, as discussed above, individuals with schizophrenia did not exhibit this strong posterior phase coupling at theta frequency at all. Manipulation at memory load 3 requires a range of parallel cognitive processes: (1) maintenance of the encoded material, (2) dispersing the original multiitem memory trace, (3) performing the actual mental rotation of spatial positions (Cohen & Miles, 2000), (4) binding the new mental representations together, and (5) finally retaining the new multiitem WM representation. It has been suggested that binding mechanisms in WM are particularly strongly reflected by theta band activity (Sauseng et al., 2010). It is therefore likely that posterior theta phase coupling observed in the present study reflects exactly these binding-related processes that are supposed to be disrupted in individuals with schizophrenia (Foucher, Lacambre, Pham, Giersch, & Elliott, 2007; Tschacher & Bergomi, 2011; van Assche & Giersch, 2011).
Topographical distribution of theta phase coupling (5 Hz) is shown for healthy controls (left) and individuals with schizophrenia (right) separately for each experimental condition. In addition, topographical plots are depicted for four different 500-ms time windows (see a: t1, t2, t3, t4) covering the entire delay period
Attenuated posterior theta phase coupling in individuals with schizophrenia (as depicted in Fig. 5) could, in theory, also be explained by encoding deficits (Badcock, Badcock, Read, & Jablensky, 2008; Haenschel et al., 2007a; Hartman, Steketee, Silva, Lanning, & McCann, 2002)—particularly, since the most pronounced group differences are found within the first 500 ms of the delay period and temporal precision of filters in the theta frequency band is low. Another observation pointing toward a possible overlap between encoding deficits in individuals with schizophrenia and the obtained results is that a significant group difference for both the manipulation and pure retention of three items in WM has been found in the first time window. However, inefficient encoding in individuals with schizophrenia should have a similar impact on manipulation and retention processes of three items. And this pattern is clearly not found in the within-group analysis in the patient sample, where there is a deviant evolution of the connectivity pattern for the manipulation condition. The above-described findings rather speak against sole encoding deficits in individuals with schizophrenia, since retention and manipulation processes are affected differently in the patient group with respect to posterior theta phase coupling, but also with respect to behavioral performance. It is noteworthy that performance on manipulation trials in individuals with schizophrenia was significantly worse than that on retention trials, independently of WM load. The finding that there are significant group differences not only for manipulation, but also for retention of three items in the first time window could as well be explained by the fact that even pure retention of three items can substantially stress executive control in individuals with schizophrenia and healthy controls (although less than in the manipulation condition, as suggested by the behavioral results). This interpretation is supported by the fact that the ratio between theta PLV in a network predominately displayed by healthy controls against that more exhibited by individuals with schizophrenia in either Man L3 or Ret L3 was correlated only with task accuracy in Man L3, and not in Ret L3. Note that task performance was significantly different between groups in Man L3, the condition best reflecting central executive functions and, consequently, also best describing a central executive deficit in individuals with schizophrenia. The similarity of theta PLV shared between Man L3 and Ret L3 seems to be due to central executive demands in both conditions, and consequently, task performance in Man L3 correlates with both PLV ratios. If theta PLV group differences in the first time window were due to encoding deficits in the patient sample, one would also expect task accuracy in Ret L3 to correlate with PLV ratios, which is not the case. Although it cannot be excluded that encoding deficits in individuals with schizophrenia contribute to effects of interregional phase synchronization in the first time window, it is highly unlikely that they are the main factor responsible for these findings. Moreover, later effects and differences in the topography of theta phase coherence cannot be explained by possible encoding deficits in the patient group.
Another alternative interpretation could be that healthy subjects display stronger theta amplitude at posterior recording sites, possibly biasing theta phase coupling between them. However, it needs to be considered that in healthy controls, posterior theta amplitude showed a memory-load-dependent increase independently of experimental condition. Posterior theta phase coupling, on the other hand, exhibits a sustained increase in healthy controls only during manipulation of three items, and not during pure retention. These divergent patterns make it implausible that the findings on posterior theta phase coupling were biased by posterior theta amplitude.
Contrasting attenuated posterior theta synchronization, individuals with schizophrenia exhibit stronger fronto-frontal theta coupling during conditions high on executive demand (see Fig. 5), as compared with healthy controls. This hyperfrontality is supposed to reflect a compensatory but inefficient strategy (Callicott et al., 2003) overcoming a loss of posterior theta synchronization. Instead, what seems to be crucial for prefrontal control over posterior brain areas and with that efficient coordination and monitoring of parallel cognitive processes is fronto-posterior theta coupling (Sauseng et al., 2010). And exactly this kind of long-range connectivity at theta frequency, which is observed in healthy controls, is attenuated in individuals with schizophrenia.
The present results indicate theta oscillations to be involved in central executive functions of WM on a local, as well as on an interregional, scale: Frontal midline theta amplitude and interregional theta phase synchronization are both responding to the experimental conditions. Most important, however, individuals with schizophrenia who display a behavioral deficit in control of WM exhibit altered activation patterns for local (frontal midline), as well as interregional, theta oscillations. This suggests a causal relation between these brain rhythmical phenomena and central executive functions in WM. Particularly in the schizophrenia sample, an overlap between effects on local and interregional theta activity has been observed: As compared with healthy subjects, patients displayed stronger frontal midline theta amplitude, as well as increased fronto-frontal theta phase coherence. This clearly points toward hyperfrontality (Callicott et al., 2003) in individuals with schizophrenia and also suggests that the patients allocated more cognitive resources toward the task than did healthy subjects. On the other hand, a reduction of fronto-parietal theta synchronization was obtained for individuals with schizophrenia, as compared with healthy subjects, making long-range theta coherence a key signature of central executive functions in visual WM (see also Sauseng et al., 2010).
Conclusion
Our data indicate that the amount of theta phase synchronization is differently involved in WM processes dependent on the level of executive demand that is required by the task. Besides a distinctive fronto-posterior theta network, demands on a posterior theta network seem to be crucial for manipulation processes at high WM load in healthy controls. In contrast, local frontal theta activity mediates the deployment of attentional resources and, thus, is associated with general task demands. By comparing these oscillatory brain activity patterns in the theta frequency range in healthy controls with the ones in individuals with schizophrenia, who show impairments in central executive functioning, we argue that our results indicate not only a correlation, but also a causal link between these activation patterns and central executive functions in WM. Moreover, the obtained results provide further evidence for schizophrenia as a brain disconnectivity disorder, but beyond desynchronization of fast oscillatory brain activity and beyond mere perceptual binding deficits.
References
Axmacher, N., Henseler, M. M., Jensen, O., Weinreich, I., Elger, C. E., & Fell, J. (2010). Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 107, 1–6. doi:10.1073/pnas.0911531107
Badcock, J. C., Badcock, D. R., Read, C., & Jablensky, A. (2008). Examining encoding imprecision in spatial working memory in schizophrenia. Schizophrenia Research, 100(1), 144–152.
Baddeley, A. (1996). The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America, 93(24), 13468–13472.
Baddeley, A., & Della Sala, S. (1998). Working memory and executive control. In A. C. Roberts, T. W. Robbins, & L. Weiskrantz (Eds.), The prefrontal cortex (pp. 9–21). Oxford: Oxford University Press.
Barch, D. M., & Smith, E. (2008). The cognitive neuroscience of working memory: Relevance to CNTRICS and Schizophrenia. Biological Psychiatry, 64(1), 11–17. doi:10.1016/j.biopsych.2008.03.003
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57, 289–300.
Callicott, J. H., Mattay, V. S., Verchinski, B. A., Marenco, S., Egan, M. F., & Weinberger, D. R. (2003). Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. American Journal of Psychiatry, 160, 2209–2215.
Cannon, T. D., Glahn, D. C., Kim, J., van Erp, T., Karlsgodt, K., Cohen, J. D., … Shirinyan, D. (2005). Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Archives of General Psychiatry, 62, 1071–1080.
Canolty, R. T., Edwards, E., Dalal, S. S., Soltani, M., Nagarajan, S. S., Kirsch, H. E., … Knight, R. T. (2006). High gamma power is phase-locked to theta oscillations in human neocortex. Science, 313(5793), 1626–1628. doi:10.1126/science.1128115
Champod, A. S., & Petrides, M. (2007). Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proceedings of the National Academy of Sciences of the United States of America, 104(37), 14837–14842. doi:10.1073/pnas.0607101104
Cho, R. Y., Konecky, R. O., & Carter, C. S. (2006). Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 103(52), 19878–19883. doi:10.1073/pnas.0609440103
Cohen, I., & Miles, R. (2000). Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. The Journal of Physiology, 524(2), 485–502. doi:10.1111/j.1469-7793.2000.00485.x
Collette, F., Salmon, E., Van der Linden, M., Chicherio, C., Belleville, S., Degueldre, C., … Franck, G. (1999). Regional brain activity during tasks devoted to the central executive of working memory. Cognitive Brain Research, 7(3), 411–417. doi:10.1016/S0926-6410(98)00045-7
Demiralp, T., Bayraktaroglu, Z., Lenz, D., Junge, S., Busch, N. A., Maess, B., … Herrmann, C. S. (2007). Gamma amplitudes are coupled to theta phase in human EEG during visual perception. International Journal of Psychophysiology, 64(1), 24–30. doi:10.1016/j.ijpsycho.2006.07.005
D’Esposito, M., Detre, J. A., Alsop, D. C., Shin, R. K., Atlas, S., & Grossman, M. (1995). The neural basis of the central executive system of working memory. Nature, 378(6554), 279–281. doi:10.1038/378279a0
Diamond, A. (2011). Biological and social influences on cognitive control processes dependent on prefrontal cortex. Progress in Brain Research, 189, 319–339.
Dosenbach, N. U., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105.
Ford, J. M., Mathalon, D. H., Whitfield, S., Faustman, W. O., & Roth, W. T. (2002). Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biological Psychiatry, 51(6), 485–492.
Foucher, J. R., Lacambre, M., Pham, B. T., Giersch, A., & Elliott, M. A. (2007). Low time resolution in schizophrenia: Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophrenia Research, 97(1–3), 118–127. doi:10.1016/j.schres.2007.08.013
Franke, G. H. (2000). BSI. Brief Symptom Inventory von L. R. Derogatis - Deutsche Version. Beltz: Göttingen.
Friston, K. J. (1998). The disconnection hypothesis. Schizophrenia Research, 30(2), 115–125. doi:10.1016/S0920-9964(97)00140-0
Gevins, A., Smith, M. E., McEvoy, L., & Yu, D. (1997). High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex, 7(4), 374–385. doi:10.1093/cercor/7.4.374
Haenschel, C., Bittner, R. A., Haertling, F., Rotarska-Jagiela, A., Maurer, K., Singer, W., & Linden, D. E. J. (2007a). Contribution of impaired early-stage visual processing to working memory dysfunctions in adolescents with schizophrenia. Archives of General Psychiatry, 64, 1229–1240.
Haenschel, C., Bittner, R. A., Waltz, J., Haertling, F., Wibral, M., Singer, W., … Rodriguez, E. (2009). Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. The Journal of Neuroscience, 29(30), 9481–9489. doi:10.1523/jneurosci.1428-09.2009
Haenschel, C., Uhlhaas, P. J., & Singer, W. (2007b). Synchronous oscillatory activity and working memory in schizophrenia. Pharmacopsychiatry, 40, S54–S61.
Hanslmayr, S., Pastötter, B., Bäuml, K.-H., Gruber, S., Wimber, M., & Klimesch, W. (2007). The electrophysiological dynamics of interference during the stroop task. Journal of Cognitive Neuroscience, 20(2), 215–225. doi:10.1162/jocn.2008.20020
Hartman, M., Steketee, M. C., Silva, S., Lanning, K., & McCann, H. (2002). Working memory and schizophrenia: Evidence for slowed encoding. Schizophrenia Research, 59, 99–113.
Jensen, O., & Tesche, C. D. (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399. doi:10.1046/j.1460-9568.2002.01975.x
Junghee, L., & Sohee, P. (2005). Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology, 114(4), 599–611.
Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. doi:10.1093/schbul/13.2.261
Kim, J., Glahn, D. C., Nuechterlein, K. H., & Cannon, T. D. (2004). Maintenance and manipulation of information in schizophrenia: Further evidence for impairment in the central executive component of working memory. Schizophrenia Research, 68(2–3), 173–187. doi:10.1016/S0920-9964(03)00150-6
Kim, J.-J., Kwon, J. S., Park, H. J., Youn, T., Kang, D. H. K., Lee, D. S., & Lee, M. C. (2003). Functional Disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: A [15O]H2O PET study. American Journal of Psychiatry, 160, 919–923.
Lachaux, J.-P., Rodriguez, E., Martinerie, J., & Varela, F. J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8(4), 194–208. doi:10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C
Lakatos, P., Shah, A. S., Knuth, K. H., Ulbert, I., Karmos, G., & Schroeder, C. E. (2005). An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. Journal of Neurophysiology, 94(3), 1904–1911. doi:10.1152/jn.00263.2005
Manoach, D. S. (2003). Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophrenia Research, 60, 285–298.
Micheloyannis, S., Pachou, E., Stam, C. J., Breakspear, M., Bitsios, P., Vourkas, M., … Zervakis, M. (2006). Small-world networks and disturbed functional connectivity in schizophrenia. Schizophrenia Research, 87(1–3), 60–66. doi:10.1016/j.schres.2006.06.028
Mizuhara, H., & Yamaguchi, Y. (2007). Human cortical circuits for central executive function emerge by theta phase synchronization. NeuroImage, 36(1), 232–244. doi:10.1016/j.neuroimage.2007.02.026
Möller, H.-J., Müller, W. E., & Volz, H.-P. (2000). Psychopharmakotherapie. Ein Leitfaden für Klinik und Praxis. Stuttgart: Kohlhammer.
Onton, J., Delorme, A., & Makeig, S. (2005). Frontal midline EEG dynamics during working memory. NeuroImage, 27(2), 341–356. doi:10.1016/j.neuroimage.2005.04.014
Postle, B. R., Stern, C. E., Rosen, B. R., & Corkin, S. (2000). An fMRI investigation of cortical contributions to spatial and nonspatial visual working memory. NeuroImage, 11(5), 409–423. doi:10.1006/nimg.2000.0570
Reichenberg, A., & Harvey, P. D. (2007). Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological Bulletin, 133, 833–858.
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., & Von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proceedings of the National Academy of Sciences of the United States of America, 95(12), 7092–7096.
Sauseng, P., Griesmayr, B., Freunberger, R., & Klimesch, W. (2010). Control mechanisms in working memory: A possible function of EEG theta oscillations. Neuroscience and Biobehavioral Reviews, 34(7), 1015–1022. doi:10.1016/j.neubiorev.2009.12.006
Sauseng, P., Hoppe, J., Klimesch, W., Gerloff, C., & Hummel, F. C. (2007). Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. European Journal of Neuroscience, 25(2), 587–593. doi:10.1111/j.1460-9568.2006.05286.x
Sauseng, P., Klimesch, W., Freunberger, R., Pecherstorfer, T., Hanslmayr, S., & Doppelmayr, M. (2006). Relevance of EEG alpha and theta oscillations during task switching. Experimental Brain Research, 170(3), 295–301. doi:10.1007/s00221-005-0211-y
Sauseng, P., Klimesch, W., Heise, K. F., Gruber, W. R., Holz, E., Karim, A. A., … Hummel, F. C. (2009). Brain oscillatory substrates of visual short-term memory capacity. Current Biology, 19(21), 1846–1852. doi:10.1016/j.cub.2009.08.062
Sauseng, P., Klimesch, W., Schabus, M., & Doppelmayr, M. (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57(2), 97–103. doi:10.1016/j.ijpsycho.2005.03.018
Smith, E. E., & Jonides, J. (1998). Neuroimaging analyses of human working memory. Proceedings of the National Academy of Sciences of the United States of America, 95(20), 12061–12068. doi:10.1073/pnas.95.20.12061
Smith, E. E., & Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science, 283(5408), 1657–1661. doi:10.1126/science.283.5408.1657
Spencer, K. M., Nestor, P. G., Perlmutter, R., Niznikiewicz, M. A., Klump, M. C., Frumin, M., … McCarley, R. W. (2004). Neural synchrony indexes disordered perception and cognition in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 101(49), 17288–17293. doi: 10.1073/pnas.0406074101
Tan, H. Y., Callicott, J. H., & Weinberger, D. R. (2007). Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cerebral Cortex, 17, 171–181.
Trujillo, L. T., Peterson, M. A., Kaszniak, A. W., & Allen, J. J. B. (2005). EEG phase synchrony differences across visual perception conditions may depend on recording and analysis methods. Clinical Neurophysiology, 116(1), 172–189.
Tschacher, W., & Bergomi, C. (2011). Cognitive binding in schizophrenia: Weakened integration of temporal intersensory information. Schizophrenia Bulletin, 37(suppl 2), S13–S22. doi:10.1093/schbul/sbr074
Tsujimoto, T., Shimazu, H., & Isomura, Y. (2006). Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. Journal of Neurophysiology, 95(5), 2987–3000. doi:10.1152/jn.00730.2005
Uhlhaas, P. J., Linden, D. E. J., Singer, W., Haenschel, C., Lindner, M., Maurer, K., & Rodriguez, E. (2006). Dysfunctional long-range coordination of neural activity during gestalt perception in schizophrenia. The Journal of Neuroscience, 26(31), 8168–8175. doi:10.1523/jneurosci.2002-06.2006
Uhlhaas, P. J., Roux, F., Singer, W., Haenschel, C., Sireteanu, R., & Rodriguez, E. (2009). The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proceedings of the National Academy of Sciences of the United States of America, 106(24), 9866–9871. doi:10.1073/pnas.0900390106
Uhlhaas, P. J., & Singer, W. (2010). Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews Neuroscience, 11(2), 100–113. doi:10.1038/nrn2774. http://www.nature.com/nrn/journal/v11/n2/suppinfo/nrn2774_S1.html.
van Assche, M., & Giersch, A. (2011). Visual organization processes in schizophrenia. Schizophrenia Bulletin, 37(2), 394–404. doi:10.1093/schbul/sbp084
von Stein, A., & Sarnthein, J. (2000). Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. International Journal of Psychophysiology, 38(3), 301–313. doi:10.1016/S0167-8760(00)00172-0
Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry, 64, 663–667.
Wu, X., Chen, X., Li, Z., Han, S., & Zhang, D. (2007). Binding of verbal and spatial information in human working memory involves large-scale neural synchronization at theta frequency. NeuroImage, 35(4), 1654–1662. doi:10.1016/j.neuroimage.2007.02.011
Acknowledgments
B.G. was recipient of a Doc fFORTE fellowship by the Austrian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 85 kb)
Rights and permissions
About this article
Cite this article
Griesmayr, B., Berger, B., Stelzig-Schoeler, R. et al. EEG theta phase coupling during executive control of visual working memory investigated in individuals with schizophrenia and in healthy controls. Cogn Affect Behav Neurosci 14, 1340–1355 (2014). https://doi.org/10.3758/s13415-014-0272-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-014-0272-0