Abstract
According to a recent hypothesis, the prefrontal cortex has been proposed as the site of emotional memory integration, because it is sensitive to the recognition of emotional contents. In the present research, we explored the role of the dorsolateral prefrontal cortex (DLPFC) in memory recognition processes for positive versus negative emotional stimuli when old (target) and new (distractor, either semantically related or unrelated to the target) stimuli were presented. The role of the DLPFC was analysed using an rTMS (repeated transcranial magnetic stimulation) paradigm that induced increased cortical activation of the left DLPFC. The subjects were required to perform a task that consisted of two experimental phases (i.e., an encoding and a recognition phase) in which the targets and the distractors were presented and recognition performance was measured. rTMS stimulation was provided over the left DLPFC during the recognition phase. We found that the rTMS stimulation affected the memory recognition of positive emotional material. Moreover, related and unrelated distractors were discarded better when they were positively valenced, and a more significant effect (i.e., increased performance) was produced in response to related distractors. This result suggests that the activation of the left DLPFC favours the memory recognition of positive emotional information, and that such activation is able to induce a more appropriate selective process to distinguish target from distractor stimuli in the presence of more complex processes (related distractors). The valence model of emotional cue processing may explain this increased performance by demonstrating the distinct role of the left hemisphere in the retrieval of positive emotional information.
Similar content being viewed by others
The present study explores the neural mechanisms of emotional memory recognition. Specifically, we intend to verify the effects of the prefrontal network on memory performance when subjects engage in memory recognition of emotional stimuli. Several studies have shown that the prefrontal cortex (PFC) plays a crucial role in the integration of different aspects of cognition, memory, and emotional regulation by managing the cognitive control over emotional stimuli and emotional behaviour (Hariri, Bookheimer, & Mazziotta, 2000; Kalish & Robins, 2006; Knight, Staines, Swick, & Chao, 1999; Miller & Cohen, 2001).
Neuropsychological and lesion studies have documented the involvement of the frontal lobes in recognition memory. Specifically, neuroimaging, transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS) research has shown that the dorsolateral prefrontal cortex (DLPFC) is involved in the recognition process (Javadi & Walsh, 2011; Sandrini, Cappa, Rossi, Rossini, & Miniussi, 2003; Turriziani, Smirni, Oliveri, Semeza, & Cipolotti, 2010). With regard to the contributions of specific brain areas in memory tasks, neuroimaging studies have shown increased activation of the DLPFC during tasks that require the organisation of information and the need to manage the relationships between memory cues. This process of manipulation promotes the strengthening of interitem associations, with a resulting enhancement of memory formation (Blumenfeld & Ranganath, 2006).
Using TMS, Manenti, Cotelli, Calabria, Maioli, and Miniussi (2010) provided new evidence of the involvement of prefrontal areas in memory processes. In another study, Turriziani et al. (2010) suggested that the DLPFC plays a critical role in recognition memory that is based on familiarity as well as recollection. Interestingly, another rTMS study showed bilateral involvement of the DLPFC in long-term memory processes, in both the encoding and recognition phases (Sandrini et al., 2003). Although several other studies have shown increased activation of the DLPFC during the manipulation of a cue, none have shown a correlation between the DLPFC and memory performance (Davachi, Maril, & Wagner, 2001). Thus, the relationship between the DLPFC and memory performance needs to be elucidated.
Only a limited number of studies have addressed the topic of where in the brain memories for emotional content could be represented, and those have specifically studied the prefrontal cortex (Balconi, Ferrari, & Amenta, 2010; Gray, Braver, & Raichle, 2002). This debate is still open, and a new approach that focuses on the interaction of emotions and memories has recently drawn attention. Models of the processing of emotional information have suggested that a network of interconnected neuroanatomical regions—including the amygdala, hippocampus, thalamus, and PFC—operates to process emotional information and emotional memories (Davis, 1992; LeDoux, Cicchetti, Xagoraris, & Romanski, 1990). This top-down control of the amygdala by the PFC allows for the cognitive modulation of emotional processes by frontal brain structures, and the PFC could be crucial for mechanisms underlying the regulation of emotion, such as the inhibition of emotional information or the regulation of specific control monitoring on interference effects (Hariri et al., 2000; Kalish & Robins, 2006; MacNamara, Ferri, & Hajcak, 2011; Sandrini et al., 2003).
An interference effect on memory processes may be produced when a distractor (an irrelevant cue) is semantically associated with a target stimulus and the distractor increases the probability of false recognitions (false memories) because of the relationship with the target (Stadler, Roediger, & McDermott, 1999). Recent studies have demonstrated that distractors may affect working memory (WM) performance and that DLPFC activity is related to the control of this disruptive effect. Specifically, the DLPFC may intervene to modulate and reduce interference by activating a selection process that allows the DLPFC to manage a cognitively challenging condition that requires increased effort for the cognitive system (Sandrini et al., 2003).
In one study, Gray et al. (2002) proposed that the lateral prefrontal cortex (LPFC) may be identified as the site of memory and cognition integration, because the LPFC has been shown to be particularly sensitive to the activation of memory and emotion. With regard to emotional valence, other studies have found that the PFC is involved in emotional evaluation processes (Balconi & Pozzoli, 2007; Davidson & Irwin 1999), and the DLPFC has been implicated in emotional memories with a specific valence (Dolcos, Diaz-Granados, Wang, & McCarthy, 2008). In their fMRI study, Gray et al. found that the LPFC was the main cerebral region activated in response to the interaction between a memory task and the emotional valence of the stimulus, which predicted the subjects’ behavioural responses. Dolcos, LaBar, and Cabeza (2004) investigated the role of the PFC in memories with an emotional valence and concluded that the ability of emotion (specifically related to emotional arousal) to enhance memory formation is partly mediated by changes in PFC activity (left ventrolateral and dorsolateral PFC) and may involve the amplification of the WM operations mediated by LPFC regions (MacNamara et al., 2011; Mikles, Reuter-Lorenz, Beyer, & Fredrickson, 2008).
Unfortunately, the majority of previous studies have only considered long-term memory processes, without showing clear evidence of short-term or WM mechanisms operating in the retrieval of emotional information. In many cases, only the first step of the encoding process was explored, without an explicit analysis of the subsequent retrieval mechanisms. To summarise, we currently have only limited understanding of how prefrontal areas accomplish both emotional valence and memory functions in recognition. Thus, specific analyses of the potential effects of the DLPFC on the recognition process in cases in which the stimuli have emotional content should be considered, because prefrontal areas may affect emotional memories that elicit specific responses during the recognition phase.
Another critical point that is currently debated is the distinct contributions of the left versus the right DLPFC in recognition. Indeed, significant evidence has been reported in favour of both the left and the right DLPFC playing roles in retrieving emotional stimuli. Thus, a promising theory, called the valence model, was proposed to explain the relationship between emotional information processing and a frontal left/right hemispheric lateralisation effect. This theory suggests that withdrawal-related emotions are located to the right hemisphere, whereas approach-related emotions are biased to the left hemisphere (Balconi, Brambilla, & Falbo, 2009; Davidson, Abercrombie, Nitschke, & Putnam, 1999). Thus, the different effects of the left and right DLPFC on memory recognition may be due to the emotional valence of the stimuli and to the distinct contributions that the two hemispheres may have in manipulating stimuli from different emotional categories. In the present study, we intended to verify the impact of the stimulus valence and to investigate the mechanisms involved in discarding information that produce interference during recognition when a potential interference is produced in cases of stimulus acceptance/rejection (Carneiro et al., 2011). To accomplish this, we explored the recognition process in the presence of distractors (new stimuli) that were either semantically related or unrelated to the targets (old stimuli).
In the present research, we applied an activation TMS paradigm to the left DLPFC (LDLPFC) in order to analyse the contribution of the LDLPFC to the recognition of positive versus negative emotional information in conditions that required cognitive effort. We used a repetitive stimulation (rTMS) paradigm, which creates a “perturbation” and offers a unique opportunity to directly interfere with the functioning of a cortical area during the execution of a memory task. Thus, this paradigm can manipulate the causal relationships between neural activity and a subject’s performance (Miniussi, Ruzzoli, & Walsh, 2010). High-frequency electrical stimulation is known to induce long-term potentiation, whereas low-frequency stimulation induces long-term depression (Miniussi et al., 2008). The TMS method (high frequency, 5 Hz) was applied in order to induce increased activation of the left DLPFC (associated with approach emotion). An activation TMS paradigm was used to increase the cortical excitability of the left hemisphere in order to enhance the response to positive emotional cues and to produce better recognition of such cues (Balconi & Mazza, 2010; Davidson et al., 1999). In the present study, we took into account the underlying interhemispheric competition by adopting the valence hypothesis. In other words, we tried to obtain potentiation of the hemisphere that has been reported to restore positive emotions.
We initially tested the LDLPFC by performing a memory task in which old (previously encoded, targets) and new (previously not encoded, distractors) positive or negative emotional words had to be recognised. On the basis of the valence hypothesis, we hypothesised that repetitive TMS (rTMS) of the LDLPFC would produce significantly higher performance in memory recognition for the positively valenced stimuli. Enhanced performance was expected for both accuracy and response time (RT) measures.
In addition, we expected to observe enhanced positive memories after rTMS of the LDLPFC for both the target and distractor categories. Indeed, the salience effect induced by the stimulation of the LDLPFC should be observed for the recognition of previously encoded stimuli and the correct rejection of stimuli that were not previously encoded (on the basis of their positive valence). We distinguished between the two categories (i.e., target and distractor) in order to verify that the DLPFC control functions acted as expected for the selection of previously encoded stimuli and the discard of stimuli that had not previously been encoded. We wanted to verify that improved performance for positive stimuli would be observed for both of these functions (correct recognition and correct rejection), where the rejection-of-distraction procedure was considered to be the more effortful process.
In the present study, the distractor set was subdivided into two distinct categories: distractors that were not semantically associated with the targets (unrelated distractors; e.g., gun and face) and distractors that were semantically associated with the targets (related distractors; e.g., gun and pistol). The two categories were both positively and negatively valenced, and we expected that a higher percentage of the positively valenced distractors would be correctly recognised as novel (i.e., correctly rejected), as compared with the negatively valenced distractors, which could be due to the increased salience of the positive stimuli induced by the potentiation of the left hemisphere. Indeed, the potentiation of the LDLPFC may enhance the ability to distinguish the target from the distractor categories on the basis of their emotional valence, even when semantic interference is present. Specifically, we demonstrated that the possible interference effect when recalling related distractors may be reduced as a function of the stimulus valence (i.e., positively valenced related distractors may be more accurately recognised and discarded as new, as compared with positively valenced unrelated distractors). Thus, we postulated that the specific role of the LDLPFC in controlling more complex and effortful situations would favour the correct rejection of positively valenced related distractors in comparison with the correct rejection of positively valenced unrelated distractors.
Method
Subjects
The present study included 16 females and 11 males (21–37 years old). All of the subjects were right-handed and had normal or corrected-to-normal visual acuity. The exclusion criteria were a history of psychopathology for either the subjects or their immediate family. No payment was provided for participation in the present study. The subjects provided informed written consent for participating in the study, and the research was approved by the ethical committee of the institution at which the work was carried out.
Procedure
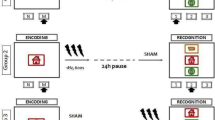
The subjects sat in a comfortable chair in front of a PC screen. The experimental paradigm consisted of an encoding phase and a recognition phase. In the encoding phase, the subjects were asked to memorise word lists during a specific time window (90 s) for a successive recognition phase, which was administered right after the encoding phase had ended (see Fig. 1 for the whole procedure). Thus, each encoding list was followed by a recognition list. In the recognition phase, words were randomly presented one at a time on the PC screen for 6 s, and the subjects were asked to decide whether they had previously viewed each word (for a similar procedure, see Carneiro et al., 2011). The subjects were asked to press one of two buttons on the mouse (the left bottom button if they recognised a word, and the right button if they did not recognise a word) as soon as possible after the presentation of the word on the screen. Response accuracy for both targets and distractors and RTs were recorded using E-Prime 2.0 software. The entire recognition phase was subdivided into three blocks, with an interblock interval of approximately 2 min.
Two distinct sets of materials were used, the first for the encoding phase and the second for the recognition phase. In the encoding phase, each list was presented on the PC screen and was counterbalanced across subjects. The words were Italian nouns (from four to seven letters long) of moderate frequency. All of the words that were included were counterbalanced with respect to word length and their abstract versus concrete contents (De Mauro, Mancini, Vedovelli, & Voghera, 1993). Each word was presented in black Arial font (16-point) on a white background. For the encoding phase, nine lists were used, and each list consisted of 20 words: Ten of the words had positive emotional valence, and the other ten words had negative emotional valence (e.g., smile, gun, anger, murder, freedom, help, fitness, and abuse). For the recognition phase, the stimulus materials were composed of a total of 450 stimuli, which were subdivided into nine lists (these were the same across the three stimulation conditions). Each recognition list was composed of 50 words, which were grouped into targets (20 words contained in the encoding lists) and distractors (30 words not contained in the encoding lists). Each category (target vs. distractor) was further divided into two equally distributed subgroups: words with negative emotional valence and words with positive emotional valence. Moreover, the distractors were also divided into two equally distributed subcategories: words that were semantically related (e.g., gun and pistol) and words that were not semantically related (e.g., gun and smile) to the old words.
The familiarity of the words and their emotional valence were assessed for all of the stimulus materials before the experimental task by a group of 14 subjects (seven male and seven female, with a mean age of 26.8 ± 2.10 years). Familiarity was evaluated with a 4-point Likert scale, and all of the words in the study showed similarly high familiarity rates (M = 3.75, SD = 0.71). Words that showed low familiarity rates were excluded from the database. Emotional valence was also evaluated for each word with a 9-point Likert scale. The positive stimuli elicited highly positive valence scores (M = 8.61, SD = 0.23), whereas the negative stimuli elicited less positive valence scores (M = 2.18, SD = 0.30). Significant differences were found between the two categories in a repeated measures analysis of variance (ANOVA), F(1, 13) = 8.70, p < .001, η 2 = .46, since the positive stimuli showed a higher positive attribution than did the negative stimuli. The arousing power of each word had previously been tested in the preexperimental phase in order to allow for a balanced effect for arousal across the stimulus types. A repeated measures ANOVA showed no significant differences between the two categories, F(1, 13) = 1.09, p = .15, η 2 = .16. The mean arousal was considered high for both the positive and negative categories (M = 7.89, SD = 0.54, vs. M = 8.23, SD = 0.34, for the positive and negative categories, respectively). To directly test the semantic link between the target and distractor categories, pairs of semantically related and unrelated words were created for each recognition list (Davelaar, Haarmann, Goshen-Gottstein, & Usher, 2006; Stadler et al., 1999). The semantically unrelated word pairs were chosen on the basis of belonging to distant semantic categories and of a lack of association according to the association norms. Specifically, the association norms were drawn from the DPSS psycholinguistic database (Peressotti, Pisciarelli, & Job, 2002). Prior to the experimental phase, a group of judges evaluated the semantic proximity and relatedness (semantic associates) of each pair with a 9-point Likert scale. The judges (ten subjects: six male and four female, with a mean age of 26.2 ± 2.17 years) were required to consider the degree of the semantic association (“Do you think the two words are semantically related?”). In line with the adopted norms, the related and unrelated word categories obtained the following scores for semantic proximity: for semantically related words, M = 7.9, SD = 0.43; for semantically unrelated words, M = 2.1, SD = 0.29. A repeated measures ANOVA showed significant differences between the semantically related and unrelated categories, F(1, 9) = 1.10, p = .12, η 2 = .17.
TMS stimulation
rTMS was delivered using a Magstim Super Rapid2 magnetic simulator with a figure-8 coil (double wings with a 70-mm diameter). The subjects were asked to wear a cap on which the positions of all of the electrodes from the International 10–20 EEG system were reproduced (Jaspers, 1958). We applied rTMS (5-Hz frequency) at 100 % of the motor threshold on the LDLPFC (electrode F3, stimulating BA 9) immediately upon the appearance of each recognition word.
The approximate location of the LDLPFC was automatically identified on the subject’s scalp using the SofTaxic navigator system (Brainsight Magstim, SofTaxic Optic 2.0), which uses a set of digitised skull landmarks (nasion, inion, and two preauricular points) and approximately 50 scalp points, which are entered with a FASTRAK Polhemus digitiser system and an averaged stereotaxic magnetic resonance imaging (MRI) brain atlas in Talairach space (Talairach & Tournoux, 1988). The average Talairach coordinates in the Softaxic navigator system were transformed through a linear transformation to each subject’s scalp. The Talairach coordinates of the cortical sites underlying the coil locations were estimated on the basis of an MRI-constructed stereotaxic template (the accuracy was approximately 1 mm in Talairach space). This scan procedure suggested that TMS was applied over the DLPFC (Talairach coordinates –10, 40, 25, medial frontal gyrus).
To control the effect of the rTMS stimulation, we adopted two control conditions: the stimulation of a cortical control site (Cz), which is not supposed to be involved in memory processes, and a sham condition (no stimulation). During the sham condition, the same intensity and timing of stimulation was used, but the coil was held in such a manner that no magnetic stimulation reached the brain (i.e., the TMS coil was placed at a 45 º angle to the head, and the point of maximal activation was superficial as compared with active stimulation; George et al., 1997; Kimbrell et al., 1999; Wassermann, Wedegaertner, Ziemann, George, & Chen, 1998). The subjective sensation of coil–scalp contact and the discharge noise in the sham condition were similar to the sensations in the real stimulation phase. Single-pulse TMS was applied at increasing intensities to determine the individual motor threshold according to the standard procedure (Rossini et al., 1994). The motor threshold was defined as the lowest TMS intensity capable of evoking a muscle twitch in the contralateral hand in eight out of ten consecutive trials. All of the subjects received 90 trains of rTMS over the LDLPFC, 90 trains of rTMS over the control site (Cz vertex), and 90 trains of rTMS in the sham phase. The order of the stimulation conditions was randomly assigned and counterbalanced.
Two factorial repeated measures ANOVAs with three independent factors (Valence, positive/negative; Target/Distractor [TD] word; and Stimulation Condition, F3/control/sham) were applied on the dependent measures of accuracy—that is, correct recognition for targets [(total correct responses – false alarms)/(total occurrences for both categories)] and correct rejections for distractors [(correct rejections)/(total rejections)]—and RT (calculated according to the same formulas used for the previous accuracy measures).
In addition, two repeated measures ANOVAs were performed with Related/Unrelated (RU) distractor, Valence, and Stimulation Condition as independent variables applied, respectively, to the accuracy measure for distractors—that is, (correct rejections)/(total rejections)—and RTs (with only correct-rejection responses used). Type I errors associated with inhomogeneity of variance were controlled by decreasing the degrees of freedom using the Greenhouse–Geisser epsilon.
Results
For the RT measure, significant main effects were found for valence and TD, as well as a significant Valence × Condition interaction (Tables 1 and 2). No other effects were statistically significant.
First, positive stimuli were recognised more quickly than negative stimuli, and target stimuli were recognised more quickly than distractor stimuli (reduced RTs). Moreover, as is shown by the contrast effects (contrast effects for ANOVA) used to follow up on the significant interaction between valence and condition, RT values decreased in the presence of LDLPFC stimulation more for positive stimuli than for negative stimuli [F(1, 26) = 4.78, p = .020, η 2 = .35]. In addition, a consistent reduction of RTs was revealed for positive stimuli in the LDLPFC stimulation condition in comparison with the control [F(1, 26) = 4.51, p = .023, η 2 = .32] and sham [F(1, 26) = 4.39, p = .026, η 2 = .31] conditions (Fig. 2). The other paired comparisons were not significant (all ps ≥ .15). In contrast, the accuracy measure did not show significant differences as a function of the experimental variables (all ps ≥ .19).
The second set of repeated measures ANOVAs, with three independent factors (Valence, RU, and Condition), were applied to correct rejections [(correct rejections)/(total rejections)] and RTs (only for correct rejections) (Table 3).
For the RT measure, there were significant interactions for Valence × Condition [F(1, 52) = 8.97, p < .001, η 2 = .46], RU × Condition [F(1, 52) = 8.16, p < .001, η 2 = .45], and Valence × RU × Condition [F(1, 52) = 6.98, p = .010, η 2 = .43] (Table 4). No other effects were statistically significant (all ps ≥ .11).
Specifically, as is shown by the post-hoc contrast analyses, positive stimuli were processed more quickly (as shown by shorter RTs) than the negative stimuli [F(1, 26) = 8.90, p < .001, η 2 = .45] when TMS stimulation was performed on the LDLPFC. Moreover, faster RTs were found in response to related distractors than in response to unrelated distractors in the case of TMS stimulation to the LDLPFC [F(1, 26) = 8.90, p < .001, η 2 = .45] (see Fig. 3). Finally, contrast effects applied to the significant Valence × RU × Condition interaction revealed shorter RTs for related than for unrelated distractors in the LDLPFC stimulation condition for the positive stimulus category [F(1, 26) = 8.16, p < .001, η 2 = .43]. The other post-hoc comparisons were not significant (all ps ≥ .18).
In contrast, correct rejections did not show significant differences as a function of the experimental variables (all ps ≥ .22).
Discussion
Several interesting results of the present research need to be highlighted. We investigated the role of the LDLPFC in modulating memories in relation to the valence of emotional words (positive vs. negative) and found a greater TMS effect on the LDLPFC for positive than for negative stimuli. In addition, a similar pattern was shown for both the target and distractor categories in response to positive emotional cues, since they both showed a reduction of RTs. Moreover, we examined the effective impacts of LDLPFC stimulation and of stimulus valence on semantic interference effects in performing the task by comparing the results for related and unrelated distractors. Interestingly, the positively valenced related distractor category showed a greater decrease in RTs in response to LDLPFC TMS than did the unrelated distractor condition.
With respect to the cortical contribution of the PFC, the present results allowed us to confirm a significant role of the DLPFC in retrieving cues with a positive emotional valence. Specifically, we found a clear LDLPFC effect on the subjects’ performance when F3 (presumably, the medial frontal gyrus) was stimulated. This higher performance (measured by an RT reduction) was based on the specific emotional content of the linguistic stimuli, which was related to the valence effect. Through the present experimental evidence, an increased facilitation to retrieve the positive emotional cues was confirmed by the reduced RTs after stimulation of the LDLPFC. In contrast, negative-cue recognition was not influenced by the left frontal stimulation (no differences among the F3, Cz, and sham conditions).
Previous studies revealed a significant role of the DLPFC in the encoding and recognition of emotional contents (Javadi & Walsh, 2011; Sandrini et al., 2003; Turriziani et al., 2010); however, the studies did not specifically examine the significance of specific emotional categories, such as the effects of positive versus negative cues on the recognition process. The approach–avoidance model of emotional information processing may explain the contribution of the left frontal area, specifically the DLPFC, in the retrieval of positive memories. In addition, the valence model of emotional processing may explain the reduced RTs by highlighting the distinct role that the left hemisphere has in emotional cue elaboration. The specificity of the left side for positive–approach emotions has been supported and discussed in previous studies (Balconi & Lucchiari, 2005; Balconi & Mazza, 2010; Davidson, 1995; Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997). Interestingly, neuroimaging, event-related potential, and electroencephalographic studies have demonstrated the existence of two different frontal cortical networks: one deputed to process negative, withdrawal emotions (the right hemisphere), and one deputed to process positive, approach emotions (the left hemisphere) (Balconi et al., 2009). This theory can help explain the pattern of data found in the present study. Specifically, it may be that when the cortical system that has been shown to recognise positive emotional cues is hyperactivated (the rTMS potentiation effect), subjects might have an unbalanced response to positive categories. In fact, in the present study, a facilitation effect was observed in response to positive cues as a consequence of stimulation of the left side, whereas no significant effect was seen with negative cues. Thus, we can conclude that cortical stimulation of the left, approach-related hemisphere may support faster recognition of positively valenced information without any direct effect on negatively valenced information.
These conclusions are in line with previous results with clinical (e.g., panic disorder and posttraumatic stress disorder) and subclinical (Heller & Nitschke, 1998; Schutter, van Honk, d’Alfonso, Postma, & de Haan, 2001; van Honk et al., 1999) samples that have led to the postulation of a “depressive” effect of low-frequency TMS (cortical depotentiation) on the right DLPFC, which induced a reduction of right prefrontal activity and produced an increased responsiveness to positive cues. In parallel, an interesting study in which a low-frequency (inhibitory) TMS paradigm was applied to the frontal left hemisphere revealed increased attention toward negative stimuli (d’Alfonso, van Honk, Hermans, Postma, & de Haan, 2000). These studies confirmed the specificity of the left and right hemispheres in processing positive and negative emotional cues, respectively—specializations that may influence the succeeding recognition mechanisms.
A similar impact of left frontal stimulation on the valence of stimuli was observed for both the target and the distractor categories. In general, however, we have observed a more direct impact of LDLPFC activation on subjects’ performance in relation to the efficiency of the process (i.e., a significant reduction in RTs) as compared with the accuracy measure (i.e., correct recognition and correct rejection for targets and distractors, respectively). Interestingly, both the target and the distractor categories showed an enhancement of the mechanisms responsible for the retrieval of emotional information in terms of a reduction in RTs, since the two categories did not statistically differ, but there was no significant increase in the accuracy of responding to positive emotional cues. This result may be due to the absolute high performance that was exhibited by the subjects (80 % of both correct responses and correct rejections) for all of the experimental conditions (i.e., the subjects completed the cognitive recognition task with a limited number of errors—both omissions and false alarms). In addition, the TMS stimulation effect may be more relevant for the efficiency of the cognitive system in producing a correct response. The central executive system could be responsible for this increased efficiency in cases of LDLPFC stimulation by consistently reducing the delay in performing the response task (Eysenck & Calvo, 1992).
A different impact of prefrontal stimulation was revealed for the two distractor categories (i.e., related and unrelated distractors). In fact, whereas all of the distractors benefited from the positive valence of the stimulus and demonstrated a significant improvement in the efficiency of the system to recognise the new stimuli (as was shown by the absence of differences between the target and distractor stimulus categories in obtaining decreasing RTs), this improvement was more significant for the semantically related distractor category than for the unrelated distractor after TMS stimulation. In the case of related distractors, when TMS was applied to the LDLPFC, the positive stimuli were very quickly discarded as new. Thus, these results provide direct evidence that the DLPFC is generally involved in mediating the effects of distractors, but that the results are dependent on the nature of the distractor. DLPFC activation may have acted to improve the general ability to recognise the positive cues by significantly affecting attention to their salience or valence.
Conclusions
The present results demonstrate that TMS is an important tool for the direct investigation of the functional role of a brain area in an ongoing cognitive process. Indeed, TMS offers a unique opportunity to directly interact with the functioning of a brain area and the related neural circuit during the execution of a defined task. Regarding memory, TMS may elucidate some mechanisms of memory for the recognition of emotional cues by demonstrating which brain areas are necessary for a specific aspect of performance.
Future research may further elucidate some important questions that the present research has identified. For example, future studies should test whether the effect of the LDLPFC in response to positive stimuli is primarily due to the valence hypothesis (i.e., to increased activation due to the approach attitudes supported by the left hemisphere) or to the main contribution of the LDLPFC to memory recognition mechanisms. In other words, future studies should attempt to provide more insight into the relevance and the specific contributions of the LDLPFC for positive emotion processing and memory recognition; however, these two factors should be considered separately. In addition, future studies could also perform experiments comparable to the one in the present study by inducing a cortical perturbation of the right DLPFC and adopting an inhibitory paradigm. Indeed, the increased memory performance with positive cues should be examined by reducing the cortical excitability of the right hemisphere, which has been shown to respond to aversive and potentially threatening information. Also, a TMS activation paradigm could be adopted to stimulate the right DLPFC, in order to demonstrate increased performance toward negative cues. Finally, the valence effect, which was supported by the present research, should be integrated by considering a more systematic comparison between the different levels of arousal induced by emotional stimuli. As in the previous research, the valence and arousal parameters may be integrated to obtain a complete view of the significance of the left and right hemispheres in responses to emotional cues.
References
Balconi, M., Brambilla, E., & Falbo, L. (2009). BIS/BAS, cortical oscillations and coherence in response to emotional cues. Brain Research Bulletin, 80, 151–158.
Balconi, M., Ferrari, C., & Amenta, S. (2010). Dorsolateral Prefrontal Cortex involvement in retrieval. An rTMS study on stress-related words. Neuropsychological Trends, 8, 100–103.
Balconi, M., & Lucchiari, C. (2005). In the face of emotions: Event-related potentials in supraliminal and subliminal facial expression recognition. Genetic, Social, and General Psychology Monographs, 131, 41–69. doi:10.3200/MONO.131.1.41-69
Balconi, M., & Mazza, G. (2010). Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Laterality: Asymmetries of Body, Brain and Cognition, 15, 361–384. doi:10.1080/13576500902886056
Balconi, M., & Pozzoli, U. (2007). Event-related oscillations (EROs) and event-related potentials (ERPs) comparison in facial expression recognition. Journal of Neuropsychology, 1, 283–294.
Blumenfeld, R. S., & Ranganath, C. (2006). Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. Journal of Neuroscience, 26, 916–925. doi:10.1523/JNEUROSCI.2353-05.2006
Carneiro, P., Fernandez, A., Diez, E., Garcia-Marques, L., Ramos, T., & Ferreira, M. B. (2011). “Identify-to-reject”: A specific strategy to avoid false memories in the DRM paradigm. Memory & Cognition, 40, 252–265. doi:10.3758/s13421-011-0152-6
d’Alfonso, A. A., van Honk, J., Hermans, E., Postma, A., & de Haan, E. H. (2000). Laterality effects in selective attention to threat after repetitive transcranial magnetic stimulation at the prefrontal cortex in female subjects. Neuroscience Letters, 280, 195–198.
Davachi, L., Maril, A., & Wagner, A. D. (2001). When keeping in mind supports later bringing to mind: neural markers of phonological rehearsal predict subsequent remembering. Journal of Cognitive Neuroscience, 13, 1059–1070.
Davelaar, E. J., Haarmann, H. J., Goshen-Gottstein, Y., & Usher, M. (2006). Semantic similarity dissociates short- from long-term recency effects: Testing a neurocomputational model of list memory. Memory & Cognition, 34, 323–334. doi:10.3758/BF03193410
Davidson, R. J. (1995). Cerebral asymmetry, emotion and affective style. In R. J. Davidson & K. Hugdahl (Eds.), Brain asymmetry (pp. 361–387). Cambridge: MIT Press.
Davidson, R. J., Abercrombie, H., Nitschke, J. B., & Putnam, K. (1999). Regional brain function, emotion and disorders of emotion. Current Opinion in Neurobiology, 9, 228–234.
Davidson, R., & Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3, 11–21.
Davis, M. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–375. doi:10.1146/annurev.ne.15.030192.002033
Dolcos, F., Diaz-Granados, P., Wang, L., & McCarthy, G. (2008). Opposing influences of emotional and non-emotional distracters upon sustained prefrontal cortex activity during a delayed-response working memory task. Neuropsychologia, 46, 326–335. doi:10.1016/j.neuropsychologia.2007.07.010
Dolcos, F., LaBar, K. S., & Cabeza, R. (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events brain sites sensitive to amygdalar modulatory influences. Neuron, 42, 855–863.
Eysenck, M. W., & Calvo, M. G. (1992). Anxiety and performance: The processing efficiency theory. Cognition & Emotion, 6, 409–434. doi:10.1080/02699939208409696
George, M. S., Wassermann, E. M., Kimbrell, T. A., Little, J. T., Williams, W. E., Danielson, A. L., . . . Post, R. M. (1997). Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. American Journal of Psychiatry, 154, 1752–1756.
Gray, J. R., Braver, T. S., & Raichle, M. E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Science, 99, 4115–4120.
Hariri, A., Bookheimer, S., & Mazziotta, J. (2000). Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport, 11, 43–48.
Harmon-Jones, E., & Allen, J. J. B. (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology, 106, 159–163. doi:10.1037/0021-843X.106.1.159
Heller, W., & Nitschke, J. B. (1998). The puzzle of regional brain activity in depression and anxiety: The importance of subtypes and comorbidity. Illinois Research, 12, 421–447.
van Honk, J., Tuiten, A., Verbaten, R., van den Hout, M., Koppeschaar, H., Thijssen, J., & de Haan, E. (1999). Correlations among salivary testosterone, mood, and selective attention to threat in humans. Hormones and Behavior, 36, 17–24. doi:10.1006/hbeh.1999.1521
Jaspers, H. H. (1958). The ten–twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology, 10, 367–380.
Javadi, A. H., & Walsh, V. (2011). Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimulation. doi:10.1016/j.brs.2011.06.007
Kalish, Y., & Robins, G. (2006). Psychological predispositions and network structure: The relationship between individual predispositions, structural holes and network closure. Social Networks, 28, 56–84.
Kimbrell, T. A., Little, J. T., Dunn, R. T., Frye, M. A., Greenberg, B. D., Wassermann, E. M., . . . Post, R. M. (1999). Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biological Psychiatry, 46, 1603–1613. doi:10.1016/S0006-3223(99)00195-X
Knight, R. T., Staines, W. R., Swick, D., & Chao, L. L. (1999). Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica, 101, 159–178. doi:10.1016/S0001-6918(99)00004-9
LeDoux, J. E., Cicchetti, P., Xagoraris, A., & Romanski, L. M. (1990). The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. Journal of Neuroscience, 10, 1062–1069.
MacNamara, A., Ferri, J., & Hajcak, G. (2011). Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cognitive, Affective, & Behavioral Neuroscience, 11, 321–331. doi:10.3758/s13415-011-0036-z
Manenti, R., Cotelli, M., Calabria, M., Maioli, C., & Miniussi, C. (2010). The role of the dorsolateral prefrontal cortex in retrieval from long-term memory depends on strategies: A repetitive transcranial magnetic stimulation study. Neuroscience, 166, 501–507. doi:10.1016/j.neuroscience.2009.12.037
De Mauro, T., Mancini, F., Vedovelli, M., & Voghera, M. (1993). Lessico di fluenza dell’italiano parlato. Rome: Estaslibri.
Mikles, J. A., Reuter-Lorenz, P. A., Beyer, J. A., & Fredrickson, B. L. (2008). Emotion and working memory: Evidence for domain-specific processes for affective maintenance. Emotion, 8, 256–266.
Miller, E. K., & Cohen, D. J. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
Miniussi, C., Cappa, S. F., Cohen, L. G., Floel, A., Fregni, F., Nitsche, M. A., . . . Walsh, V. (2008). Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimulation, 1, 326–336. doi:10.1016/j.brs.2008.07.002
Miniussi, C., Ruzzoli, M., & Walsh, V. (2010). The mechanism of transcranial magnetic stimulation in cognition. Cortex, 46, 128–130.
Peressotti, F., Pesciarelli, F., & Job, R. (2002). Le associazioni verbali PD-DPSS: Norme per 294 parole. Giornale Italiano di Psicologia, 1, 153–172.
Rossini, P. M., Barker, A. T., Berardelli, A., Caramia, M. D., Caruso, G., Cracco, R. Q., . . . Tomberg, C. (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application: Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91, 79–92. doi:10.1016/0013-4694(94)90029-9
Sandrini, M., Cappa, S. F., Rossi, S., Rossini, P. M., & Miniussi, C. (2003). The role of prefrontal cortex in verbal episodic memory: rTMS evidence. Journal of Cognitive Neuroscience, 15, 855–916.
Schutter, D., van Honk, J., d’Alfonso, A., Postma, A., & de Haan, E. (2001). Effects of slow rTMS at the right dorsolateral prefrontal cortex on EEG asymmetry and mood. NeuroReport, 12, 445–447.
Stadler, M. A., Roediger, H. L., III, & McDermott, K. B. (1999). Norms for word lists that create false memories. Memory & Cognition, 27, 494–500. doi:10.3758/BF03211543
Sutton, S. K., & Davidson, R. J. (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science, 8, 204–210. doi:10.1111/j.1467-9280.1997.tb00413.x
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system. An approach to cerebral imaging. Stuttgart: Thieme.
Turriziani, P., Smirni, D., Oliveri, M., Semeza, C., & Cipolotti, L. (2010). The role of the prefrontal cortex in familiarity and recollection processes during verbal and non-verbal recognition memory: An rTMS study. NeuroImage, 52, 348–357.
Wassermann, E. M., Wedegaertner, F. R., Ziemann, U., George, M. S., & Chen, R. (1998). Crossed reduction of human motor cortex excitability by 1-Hz transcranial magnetic stimulation. Neuroscience Letters, 250, 141–144.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balconi, M., Ferrari, C. rTMS stimulation on left DLPFC increases the correct recognition of memories for emotional target and distractor words. Cogn Affect Behav Neurosci 12, 589–598 (2012). https://doi.org/10.3758/s13415-012-0090-1
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-012-0090-1