Abstract
Emotion regulation decreases the processing of arousing stimuli, as indexed by the late positive potential (LPP), an electrocortical component that varies in amplitude with emotional arousal. Emotion regulation increases activity in the prefrontal areas associated with cognitive control, including the dosolateral prefrontal cortex (DLPFC). The present study manipulated working memory load, known to activate the DLPFC, and recorded the LPP elicited by aversive and neutral IAPS pictures presented during the retention interval. The LPP was larger on low-load compared to high-load trials, and on trials with aversive compared to neutral pictures. These LPP data suggest that emotional content and working memory load have opposing effects on attention to distracting stimuli. State anxiety was associated with reduced modulation of the LPP by working memory load. Results are discussed in terms of competition for attention between emotion and cognition and suggest a relationship between DLPFC activation and the allocation of attentional resources to distracting visual stimuli–a relationship that may be disrupted with increasing anxiety.
Similar content being viewed by others
The ability to regulate one’s emotional response is important for social functioning (Butler et al., 2003; Gross & John, 2003), physical health (Denollet, Pedersen, Vrints, & Conraads, 2006; Pedersen & Denollet, 2003), and subjective well-being (Gross & John, 2003). Deficits in emotion regulation may contribute to the development or maintenance of psychological disorders such as chronic anxiety (Borkovec, Alcaine, & Behar, 2004; Mennin, 2004; Salters-Pedneault, Roemer, Tull, Rucker, & Mennin, 2006). In an increasing number of studies, researchers have sought to better understand emotion regulation by studying neural correlates of interactions between emotion and executive function. Neuroimaging studies have consistently shown that emotion regulation decreases activity in the amygdala and other limbic structures. For example, amygdala activity is decreased when participants willfully down-regulate their response to aversive pictures using reappraisal (Ochsner et al., 2002; Ochsner, Bunge, Gross, & Gabrieli, 2004; Phan et al., 2005), reduce their response to sad films by adopting a detached perspective (Lévesque et al., 2003), or down-regulate response to sexually arousing films (Beauregard, Levesque, & Bourgouin, 2001).
Event-related potentials (ERPs) have also been used to index the neural correlates of emotion regulation. The late positive potential (LPP) is a positive deflection in the event-related brain potential evident over parietal recording sites by 300 ms after stimulus presentation. The LPP is larger for both aversive and appetitive pictures, compared to neutral; indeed, the LPP is even larger for neutral even for neutral stimuli that are relatively more arousing (e.g., those that contain people, as compared with those that do not; Weinberg & Hajcak, 2010). The LPP extends throughout the entire duration of picture presentation and indexes increased sustained attention to emotional stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Foti, Hajcak, & Dien, 2009; Hajcak, MacNamara, & Olvet, 2010b; Hajcak & Olvet, 2008; Schupp, Junghöfer, Weike, & Hamm, 2003). Like amygdala activity, the LPP is sensitive to a number of emotion regulation strategies (Hajcak & Nieuwenhuis, 2006; Krompinger, Moser, & Simons, 2008; Moser, Hajcak, Bukay, & Simons, 2006).
In addition to reducing hemodynamic and electrocortical indices of emotional processing, emotion regulation appears to increase activity in neural regions associated with executive control. For example, emotion regulation increases activity in a number of prefrontal regions, including the ventrolateral (Lévesque et al., 2003; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008), ventromedial (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007), dorsomedial (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Phan et al., 2005), and orbitofrontal (Banks et al., 2007; Eippert et al., 2007) cortices. Several studies have also shown increased activation in the dorsolateral prefrontal cortex (DLPFC) during emotion regulation (Beauregard et al., 2001; Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Lévesque et al., 2003; Ochsner et al., 2002; Ochsner & Gross, 2005; Ochsner et al., 2004; Phan et al., 2005). Moreover, activity in the DLPFC covaries with amygdala activity during reappraisal (Banks et al., 2007) and is associated with reduced self-reported negative emotion in emotion regulation paradigms (Phan et al., 2005). Although it is likely that no direct link between the DLPFC and the amygdala exists, the DLPFC may be reciprocally related to activation in limbic structures through connections that include the orbitofrontal cortex (Amaral & Price, 1984; Cavada, 2000; Porrino, Crane, & Goldman Rakic, 1981).
In terms of other cognitive domains, the DLFPC has been most frequently implicated in tasks that require working memory (D’Esposito, Postle, & Rypma, 2000; Miller & Cohen, 2001). A number of studies have also shown that activation of the DLPFC in the context of difficult cognitive tasks can reduce the neural response to emotional stimuli. Van Dillen and colleagues (Van Dillen, Heslenfeld, & Koole, 2009) required participants to view neutral and aversive International Affective Picture System (IAPS) pictures (Lang, Bradley, & Cuthbert 2005); following picture offset, participants performed complex or simple math. High-load math problems resulted in greater activation in the DLPFC and were associated with reduced ratings of negative affect and decreased amygdala and insula activity in response to aversive pictures. Covariation analysis indicated that greater DLPFC activation was related to less activity in limbic regions associated with the processing of emotional stimuli.
Along similar lines, an initial investigation by Erk and colleagues showed that increased working memory load reduced neural correlates of anticipating aversive IAPS (Erk, Abler, & Walter, 2006). A follow-up study examined the effects of a working memory task on the concurrent processing of IAPS pictures (Erk, Kleczar, & Walter, 2007). In this latter study, participants performed a working memory task interleaved with neutral, pleasant, or unpleasant pictures. High working memory load (i.e., participants were required to memorize six letters) led both to reductions in amygdala activation in response to unpleasant pictures and to reductions in ventral striatal activity in response to pleasant pictures. Similar results were found by McRae and colleagues (McRae et al., 2010).
If activation of the DLPFC results in reduced processing of arousing stimuli, this should also be evident in the LPP. One study demonstrated that direct stimulation of the DLPFC reduces the LPP. Hajcak and colleagues measured the LPP in five treatment-resistant depressed patients who had epidural stimulators implanted above Brodmann’s areas (BAs) 10 (i.e., frontopolar cortex) and 46 (i.e., DLPFC; Hajcak, Anderson, et al., 2010a). Participants, who were blind to activation condition, had smaller LPPs to aversive pictures when BA 46 was stimulated at 4 V, as compared with a sham condition. The LPP elicited by aversive pictures was not reduced when BA 10 was stimulated, in line with the notion that the DLPFC is involved in the down-regulation of the response to arousing stimuli.
Prior work suggests that distracting tasks may reduce the LPP elicited by task-irrelevant pictures. Participants performed a target detection task or a working memory task while simultaneously viewing emotional or neutral pictures presented in the background (Wangelin, Löw, McTeague, Bradley, & Lang, 2011). The picture-elicited LPP was smaller when participants performed these tasks, as compared with when they passively viewed the pictures. Working memory load was not modulated on a trial-to-trial basis, however, and results were analyzed across both distracting tasks. Therefore, no study to date has examined the effect of functional activation of the DLPFC on the LPP using a task designed to elicit differential levels of DLPFC activation across conditions. Although DLPFC activation was not measured in the present study, working memory load reliably elicits DLPFC activation (D’Esposito et al., 2000), and DLPFC activation increases with the number of items to be held in memory (Manoach et al., 1997). Hence, we reasoned that high-load, as compared with low-load, working memory trials would result in greater increases in DLPFC activation. We predicted that the LPP elicited by aversive pictures would be reduced under high working memory load, as compared with low working memory load (Erk et al., 2007; Van Dillen, Heslenfeld, & Koole, 2009). Conversely, we also predicted that aversive pictures would interfere with performance on the working memory task (Dolcos & McCarthy, 2006; Zanto & Gazzaley, 2009).
A secondary aim of the present study was to examine the role of individual differences in state anxiety on the processing of aversive and neutral pictures under working memory load. Anxiety has been linked to the decreased recruitment of the DLPFC during a demanding task involving aversive distractors (Bishop, Duncan, Brett, & Lawrence, 2004a)—a factor that may explain why state anxiety is associated with the increased processing of task-irrelevant and arousing stimuli (Bishop, Duncan, & Lawrence, 2004b; MacNamara & Hajcak, 2009). Hence, we reasoned that working memory load might result in less modulation of DLPFC activation for anxious individuals, leading to an attenuated effect of load on picture processing. In other words, we predicted that as state anxiety increased, working memory load would have less of an impact on the LPP.
Method
Participants
Forty-seven undergraduate students (23 female) participated in the study. Two participants had poor quality EEG recordings; therefore, 45 participants (21 female) were included in the EEG analyses. Behavioral data was missing for one participant due to a technical failure, and another participant had extremely poor performance in one condition (i.e., >5 standard deviations below the mean); hence, 45 participants (22 female) were included in the behavioral analyses. The state version of the State Trait Anxiety Inventory (STAI; Spielberger, 1983) was completed by 44 participants (22 female). The study was approved by the Stony Brook University Institutional Review Board, and all participants received course credit.
Stimulus materials
Sixty aversive pictures (e.g., threatening animals, people with guns) and 60 neutral pictures (e.g., household objects, neutral faces) were selected from the IAPS (Lang, Bradley, & Cuthbert, 2005).Footnote 1 Normative ratings indicated that the aversive pictures were less pleasant (valence M = 2.33, SD = 1.49) and more emotionally arousing (M = 6.32, SD = 2.21) than the neutral pictures (M = 4.99, SD = 1.22, and M = 3.11, SD = 1.95, respectively; higher numbers indicate more pleasant and higher arousal ratings).
Letter strings were created using a random letter generator (Reed, 2002). Vowels were not included in the strings; there were 60 two-consonant strings and 60 six-consonant stringsFootnote 2 (Ashcraft & Kirk, 2001).
Procedure
Immediately prior to the experiment, participants completed the state version of the STAI (Spielberger, 1983). This questionnaire consists of 20 items; responses are made on a scale ranging from 1 (not at all) to 4 (very much so). Responses can sum to a maximum of 80; greater scores indicate higher levels of state anxiety.
Figure 1 depicts a sample trial from the task. Participants were told that they would be performing a task that involved memorizing letters. They were told that they would also see a picture on each trial but that their task was simply to memorize the letters, which they would be asked to recall at the end of each trial. They were asked to keep their eyes on the screen throughout the entire trial. Each trial began with a two- or six-letter string (Ashcraft & Kirk, 2001) that was displayed for 5,000 ms. Next, a white fixation cross was presented on a black background for 500–1,000 ms; this was followed by an aversive or neutral picture that was presented for 2,000 ms. Then, “What were the letters? (then press enter):” was presented in white on a black screen. Participants used the keyboard to enter the letters they had seen at the beginning of the trial; they were asked to enter the letters in the same order in which they had been presented and were told that they could use the delete key to correct any mistakes. Participants were instructed to use only one finger to enter the letters, to discourage them from placing their fingers on the keyboard as a memory aid. The trial ended when participants pressed the enter key. The intertrial interval varied randomly from 2,000 to 2,500 ms, during which time a white fixation cross was presented on a black background.
A depiction of a sample trial from the task. On each trial, participants viewed a letter string (two or six letters) for 5,000 ms. This was followed by a fixation cross that was presented for 500–1,000 ms, and then by an aversive or neutral IAPS picture that was presented for 2,000 ms. Then participants were asked to recall the letters they had seen at the beginning of the trial, in the same order as they had initially been presented. (Font size is not to scale)
Each picture was displayed in color and filled the monitor screen (which measured 48.26 cm, diagonally). Participants were seated approximately 60 cm from the screen, and the images occupied about 40° of visual angle horizontally and vertically. Each participant saw all pictures and all letter strings exactly one time. The pairing of pictures and letter strings was pseudorandom: There were 30 trials in which a two-letter string was followed by a neutral picture (low-load neutral); 30 trials on which a two-letter string was followed by an aversive picture (low-load aversive); 30 trials on which a six-letter string was followed by a neutral picture (high-load neutral); and 30 trials on which a six-letter string was followed by an aversive picture (high-load aversive). Trial types were intermixed, and the order of these trials was completely random. There were 120 trials in total, and a break was given after 60 trials. Participants performed 4 practice trials at the beginning of the experiment to familiarize themselves with the procedure.
Electroencephalographic recording and behavioral response
Continuous EEG was recorded using an elastic cap and the ActiveTwoBioSemi system (BioSemi, Amsterdam, Netherlands). Sixty-four electrode sites were used, based on the 10/20 system, as well as one electrode on each of the left and right mastoids. The electrooculogram (EOG) generated from eye blinks and eye movements was recorded from four facial electrodes: Vertical eye movements and blinks were measured with two electrodes placed approximately 1 cm above and below the right eye; horizontal eye movements were measured using two electrodes that were placed approximately 1 cm beyond the outer edge of each eye. The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio. The data were digitized at 24-bit resolution with a sampling rate of 512 Hz, using a low-pass fifth-order sinc filter with a half-power cutoff of 102.4 Hz. The voltage from each active electrode was referenced online with respect to a common mode sense active electrode producing a monopolar (nondifferential) channel. Offline analyses were performed using Brain Vision Analyzer software (Brain Products, Gilching, Germany). Data were rereferenced offline to the average of the two mastoids and band-pass filtered with low and high cutoffs of 0.01 and 30 Hz, respectively. The EEG was segmented for each trial beginning 200 ms prior to picture onset and continuing for 2,200 ms (i.e., the entire picture duration). Baseline correction was performed for each trial, using the 200 ms prior to picture onset.
Eye blink and ocular corrections were made using the method developed by Gratton, Coles, and Donchin (1983). Noisy data due to technical problems on isolated electrodes necessitated the removal of data from FP1, FP2, and FPz in one subject. Artifact analysis identified a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100-ms intervals. Trials were also inspected visually for any remaining artifacts, and data from individual channels containing artifacts were rejected on a trial-to-trial basis. The LPP was scored by averaging amplitudes from 400 to 1,000 ms and from 1,000 to 2,000 ms following picture onset, at six centroparietal sites where the LPP was maximal: CP1, CP2, CPz, P1, P2, and Pz (Hajcak, Dunning, & Foti, 2009; MacNamara & Hajcak, 2009, 2010).
Responses to the letter recall task were considered correct if and only if they contained the same letters as those that had been presented at the beginning of the trial, entered in the exact order in which they had originally been presented. The percentage of correct responses per condition was calculated as the number of correct trials divided by 30 trials in each condition.
The LPP voltage area and accuracy data on the letter recall task were evaluated with a 2 (working memory load: low, high) × 2 (picture type: neutral, aversive) repeated measures analysis of variance. Pearson’s correlations were performed between STAI state anxiety scores and the effect of picture type (aversive vs. neutral) and working memory load (low-load vs. high-load) on each of the LPP voltage area and accuracy data. Statistical analyses were performed using PASW (Version 18.0) General Linear Model software.
Results
Behavioral data
Overall, participants performed well on the letter recall task (M = 84.74% correct, SD = 12.4). Table 1 presents behavioral data according to condition.Footnote 3 As was expected, participants made significantly more errors on high-load than on low-load trials, F(1, 44) = 75.30, p < .0001, η p 2 = .63. In addition, participants made more errors on aversive than on neutral trials, F(1, 44) = 7.10, p < .05,η p 2 = .14. These effects were qualified by an interaction between load and picture type, F(1, 44) = 15.02, p < .05,η p 2 = .12, which indicated that the emotional content of aversive stimuli increased the negative impact of working memory load on performance.
LPP (400–1,000 ms)
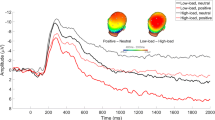
Table 1 presents the mean amplitudes for the earlier and later portions of the LPP in each of the four conditions. Figure 2 depicts the grand average waveforms at the centroparietal pooling for each of the four conditions, and Fig. 3 (top left) depicts the spatial distribution of voltage (scalp topographies) associated with aversive, as compared with neutral, pictures in the 400- to 1,000-ms window. As suggested by Fig. 3, the LPP was largest at centroparietal sites following the presentation of aversive, as compared with neutral, pictures (Cuthbert et al., 2000; Foti et al., 2009; Hajcak & Olvet, 2008; MacNamara & Hajcak, 2009; Schupp, Junghöfer, Weike, & Hamm, 2004; Weinberg & Hajcak, 2010). The impression from Figs. 2 and 3 (top left) was confirmed statistically: The earlier portion of the LPP was larger following aversive than following neutral pictures in the earlier window, F(1, 44) = 101.31, p < .0001, η p 2 = .70.
Grand average waveforms elicited by pictures in each of the four conditions at centroparietal pooling CP1, CP2, CPz, P1, P2, and Pz. Low-load trials were those on which a two-letter string was presented; high-load trials were those on which a six-letter string was presented. Each letter string was followed by either an aversive or a neutral picture
Top row: Voltage differences (scalp topographies) for aversive minus neutral pictures (collapsed across low-load and high-load trials), 400–1,000 ms after picture presentation (top left) and 1,000–2,000 ms after picture presentation (top right). Bottom row: Voltage differences (scalp topographies) for low-load minus high-load trials (collapsed across neutral and aversive pictures), 400–1,000 ms after picture presentation (bottom left) and 1,000–2,000 ms after picture presentation (bottom right; note the different scale used for the top and bottom rows)
Figure 3 (bottom left) depicts the spatial distribution of voltage differences for low-load minus high-load trials in the early window. As is suggested by Figs. 2 and 3 (bottom left), the LPP was larger on low-load versus high-load trials in the earlier window, F(1, 44) = 18.85, p < .0001, η p 2 = .30. The interaction between picture type and load did not reach significance (p > .09).
LPP (1,000-2,000 ms)
Figure 3 (top right) depicts the scalp topography of voltage differences for aversive, as compared with neutral, pictures in the later LPP window. Aversive, as compared with neutral, pictures elicited larger positive amplitudes in this window, F(1, 44) = 58.76, p < .0001, η p 2 = .57, as in previous work (Cuthbert et al., 2000; Hajcak & Olvet, 2008; MacNamara, Foti, & Hajcak, 2009). The voltage difference for low-load, as compared with high-load, trials in the later window of the LPP is depicted in Fig. 3 (bottom right). Again, load moderated the later portion of the LPP: Pictures presented on low-load trials elicited larger LPPs than did those presented on high-load trials, F(1, 44) = 6.44, p < .05, η p 2 = .13. The interaction between load and picture type did not reach significance (p > .53).
Correlations
Correlations were performed to determine whether the LPP was associated with accuracy on the recall task and to determine whether state anxiety was associated with the effect of picture type or working memory load on the LPP or accuracy data. Difference scores for the LPP were calculated by subtracting trials with neutral pictures from trials with aversive pictures, and subtracting high-load from low-load trials; similarly for accuracy data, trials with aversive pictures were subtracted from trials with neutral pictures, and high-load trials were subtracted from low-load trials. Pearson’s correlations were performed using these difference scores; Bonferroni corrections were used to control for multiple comparisons, and only significant correlations are reported below.
State anxiety scores ranged from 20 to 59 (M = 31.95, SD = 9.56); published norms for college-aged students are M = 36, SD = 10 (Spielberger, 1983). State anxiety predicted the LPP difference between low- and high-load trials in the 400- to 1,000-ms window, r(40) = −.50, p < .001, and in the 1,000- to 2,000-ms window, r(40) = −.45, p < .004, such that increased anxiety was associated with a smaller difference between LPP amplitudes on low-load, as compared with high-load, trials.
Figure 4 presents the LPP amplitude difference for low-load versus high-load trials (collapsed across picture type)as a function of state anxiety, in the 400- to 1,000-ms window (left) and the 1,000- to 2,000-ms window (right). For illustrative purposes, a median split was performed on the STAI scores. Figure 5 depicts grand average waveforms for low-load and high-load trials (collapsed across neutral and aversive pictures), for participants with low (left; n = 21) and high (right; n = 21) state anxiety scores. As is evident from Fig. 5, working memory load had less of an effect on the LPP for high-anxious participants. State anxiety did not correlate with the effect of picture type on the LPP in either the early or the late window (ps > .75). There were no significant correlations using difference scores for the LPP and accuracy data or for accuracy data and state anxiety scores (ps > .12). Furthermore, there were no significant correlations between state anxiety and the low-load minus high-load accuracy difference when considered separately for trials with aversive and neutral pictures (ps > .44).
For illustrative purposes, a median split was performed on STAI state anxiety scores. Grand average waveforms elicited by pictures on low-load and high-load trials (collapsed across neutral and aversive pictures), at centroparietal pooling CP1, CP2, CPz, P1, P2, and Pz are depicted for participants with low (left; n = 21) and high (right; n = 21) state anxiety scores
Discussion
Prior work showed that electrical stimulation of the DLPFC reduced the LPP elicited by aversive pictures; however, the systematic effects of functional activation of the DLPFC on the electrocortical processing of pictorial stimuli had not been explored previously. Thus, the present study sought to determine whether working memory load—known to activate the DLPFC—would decrease the LPP elicited by aversive and neutral pictures.
As was expected, participants made more errors on high-load than low-load trials. Participants were also less accurate on trials containing aversive, as compared with neutral, distracting pictures. Aversive pictures are more likely to capture attention than are neutral pictures (Öhman, Flykt, & Esteves, 2001), even when they are task-irrelevant (Bishop et al., 2004b; MacNamara & Hajcak, 2009; Vuilleumier, Armony, Driver, & Dolan, 2001). Therefore, aversive pictures may have competed more with task-relevant items for attention and working memory resources (see Weinberg & Hajcak, in press; Zanto & Gazzaley, 2009). The consumption of working memory resources by aversive stimuli should have been particularly detrimental to task performance when task load was high and working memory resources were already stretched to capacity; indeed, an interaction between working memory load and picture type revealed that this was the case.
Aversive, as compared with neutral, pictures elicited larger LPPs 400–1,000 and 1,000–2,000 ms after picture onset (Cuthbert et al., 2000; Foti & Hajcak, 2008; Foti et al., 2009; MacNamara et al., 2009; Pastor et al., 2008). Therefore, even though aversive pictures were irrelevant to the task, they were allocated greater attentional resources, indicated by an increased LPP (Hajcak, MacNamara, & Olvet, 2010b; Schupp, Flaisch, Stockburger, & Junghöfer, 2006). The LPP was also larger for low- than for high-load trials: High working memory load reduced the LPP elicited by aversive and neutral pictures 400–1,000 and 1,000–2,000 ms after picture onset. Prior work showed that distracting tasks reduced the picture-elicited LPP (Wangelin et al., 2011); however, no study had previously varied working memory load on a trial-to-trial basis.
The results are in line with fMRI work, in which working memory load and high-load math reduced picture-elicited amygdala activity (Erk et al., 2007; Kanske, Heissler, Schönfelder, Bongers, & Wessa, in press; McRae et al., 2010; Van Dillen et al., 2009) and build on the results of a recent electrical stimulation study that showed that physiological stimulation of the DLPFC reduces the LPP (Hajcak, Anderson, et al., 2010a). The DLPFC is thought to reduce interference from distractors in a variety of tasks (Miller & Cohen, 2001); moreover, the DLPFC has been linked specifically to the modulation of interference from arousing stimuli in working memory tasks (Dolcos & McCarthy, 2006; Johnson et al., 2005). Thus, it stands to reason that functional activation of the DLPFC in the high-load, as compared with the low-load, condition reduced the processing of distracting pictorial stimuli.
Although the interaction between working memory load and picture type trended toward significance in the present study (see Fig. 2), working memory load reduced the LPP elicited by both aversive and neutral pictures (see Wangelin et al., 2011, for similar results). In contrast, fMRI studies by Erk and colleagues (2007) and Van Dillen and Koole (2009) showed that working memory load moderated the processing of aversive, but not neutral, pictures. We have argued that the LPP does not simply index the difference between emotional and neutral stimuli; rather, the LPP is sensitive to the degree to which picture content is motivationally engaging. Indeed, stimulus salience modulates both the LPP (Foti & Hajcak, 2008) and the P300 (Rozenkrants & Polich, 2008; Johnston, Miller, & Burleson, 1986; Radilová, 1982). For instance, even neutral stimuli vary in arousal and the extent to which they detract from a primary task, and the LPP is sensitive to this variation (Weinberg & Hajcak, 2010, in press). Moreover, the LPP elicited by neutral pictures is subject to task-related attentional manipulations (MacNamara et al., 2009; Pastor et al., 2008; Schupp et al., 2007). In the present study, then, the moderation of the LPP elicited by aversive and neutral pictures suggests that the DLPFC regulates attention to distracting stimuli more generally, rather than to emotional stimuli in particular. Other frontal regions, such as the ventrolateral prefrontal cortex, may be involved in the inhibition of distraction from aversive stimuli in particular (i.e., subjective distraction during a working memory task, as rated by self-report; Dolcos & McCarthy, 2006).
As state anxiety increased, the effect of working memory load on the LPP was attenuated. Attentional control theory asserts that anxiety reduces the influence of a goal-directed attentional system, relative to that of a stimulus-driven attentional system (Derakshan & Eysenck, 2009; Eysenck, Derakshan, Santos, & Calvo, 2007). By varying working memory load and recording the LPP elicited by task-irrelevant aversive and neutral pictures, it was possible to index the balance of these two systems. In the context of attentional control theory, the negative correlation between state anxiety scores and the low-load minus high-load LPP difference suggests that the goal-directed attentional system exerted less of an influence on the stimulus-driven attentional system as state anxiety increased. Although more work is needed to determine the mechanisms responsible for this imbalance and their potential role in the development and maintenance of anxiety, one possibility is that among individuals who were more anxious, the DLPFC was recruited less efficiently in response to distracting stimuli (Bishop et al., 2004a).
According to attentional control theory (Eysenck et al., 2007), anxiety primarily affects the allocation of attentional resources toward task-irrelevant stimuli (referred to in terms of processing efficiency). Anxiety may also affect behavioral performance, albeit less consistently, because anxious individuals may be able to compensate for the effects of decreased processing efficiency on behavioral performance by exerting greater effort (Derakshan & Eysenck, 2009; Eysenck et al., 2007). In line with these predictions, state anxiety was associated with the decreased modulation of picture processing by working memory load (i.e., as indexed by the LPP); however, anxiety was not associated with differential behavioral performance (for similar results, see MacNamara & Hajcak, 2009). Recent work has used ERPs to index compensatory effort in anxiety (Ansari & Derakshan, 2011), and more studies of this kind may be useful in understanding why anxious individuals may, at times, perform on par with nonanxious individuals (Ikeda, Iwanaga, & Seiwa, 1996; MacNamara & Hajcak, 2009; Naveh-Benjamin, McKeachie, Lin, & Holinger, 1981; A. Richards, French, Keogh, & Carter, 2000).
Because worry is a verbal-linguistic phenomenon, some theorists have proposed that anxiety should primarily consume verbal working memory resources (Eysenck & Calvo, 1992), leaving visuospatial working memory unaffected (Ikeda et al., 1996; Rapee, 1993). However, other theorists have proposed that anxiety should primarily impact visuospatial working memory (Lavric, Rippon, & Gray, 2003)—possibly, because withdrawal-related affect and anxious arousal may compete with visuospatial working memory for right-hemispheric resources (e.g., Shackman et al., 2006). Therefore, future work may wish to examine whether a visuospatial working memory task would also reduce the LPP elicited by task-irrelevant pictures and, if so, whether anxiety would attenuate this effect (Van Dillen & Koole, 2007). In so doing, it may be important to differentiate between anxious arousal, which has been associated with greater right parietal activity and anxious apprehension, which is associated with worry and verbal ruminations and may be associated with increased left hemispheric activity (Heller, Nitschke, Etienne, & Miller, 1997; Nitschke, Heller, Palmieri, & Miller, 1999).
It is worth considering the similarities between working memory load and distraction, in which an individual thinks about something that is unrelated to the stimulus at hand (Gross, 1998). If a person’s attention is adequately occupied by unrelated thoughts, insufficient resources may remain for the processing of arousing material. In this way, high-load cognitive tasks—which place heavy demands on attentional resources—resemble distraction (Van Dillen & Koole, 2007). Alternatively, distraction may also increase working memory load by filling it with neutral contents drawn from long-term memory (i.e., in cases in which participants are asked to think about something unrelated to the arousing stimulus and emotionally neutral; Sheppes & Meiran, 2008). As compared with other emotion regulation techniques such as reappraisal, distraction has been linked to decreased memory for emotional events (Richards & Gross, 2006; Sheppes & Meiran, 2008), and emotion regulation effects may be less durable (Kross & Ayduk, 2008). Although memory for task-irrelevant pictures was not assessed in the present study, the reduction of the LPP in the high-load, as compared with low-load, condition suggests that pictures presented in the high-load condition may not have been encoded as well as pictures presented in the low-load condition (Dolcos & Cabeza, 2002). Future researchers may wish to examine this possibility directly by testing participants’ memory for task-irrelevant pictures presented under high and low working memory load. Moreover, future researchers might determine whether the regulatory effects of working memory load differ mechanistically from those of distraction (see McRae et al., 2010, for a comparison of working memory load and reappraisal).
It will also be important to investigate whether other mediating mechanisms, such as attentional allocation, could account for the regulatory effects of working memory load on picture processing. For example, variation in visual attention—that is, directing one’s gaze to more or less arousing picture regions—can modulate fMRI indices of picture processing (van Reekum et al., 2007). And indeed, the LPP elicited by aversive pictures is smaller when participants are instructed to look at nonarousing compared to arousing picture regions (Dunning & Hajcak, 2009; Hajcak et al., 2009). Along these lines, we are currently examining whether differences in the way participants visually explore pictures can explain the effects of working memory load on the LPP.
Notes
The IAPS pictures used were aversive (1050, 1300, 1300, 2730, 3010, 6570, 3170, 3350, 6230, 6250, 6312, 6370, 6550, 6821, 9040, 9253, 9410, 9414, 9433, 9920, 9921, 1120, 1304, 2120, 2130, 3001, 3015, 3016, 3051, 3053, 3059, 3068, 3100, 3102, 3168, 3213, 3400, 3530, 3500, 6231, 6510, 9413, 9420, 9427, 9425, 9584, 9635.1, 9902, 9910, 9911, 1201, 9265, 9250, 9252, 9075, 9050, 6571, 6560, 6313, 6263, 6243) and neutral (2038, 2026, 2026, 2107, 2190, 2393, 2411, 2570, 2840, 5534, 5740, 7000, 7004, 7009, 7025, 7130, 7140, 7217, 7224, 7491, 7595, 2383, 2384, 2357, 2210, 2200, 2191, 2102, 2039, 6150, 7002, 7003, 7010, 7018, 7026, 7037, 7038, 7050, 7175, 7234, 7493, 7500, 7512, 7546, 7590, 2745.1, 2495, 2400, 2397, 2396, 2446, 5500, 5731, 7019, 7020, 7056, 7100, 7150, 7180, 7496, 7504).
The authors would be happy to provide a complete list of the letter strings upon request.
Average reaction time per condition was determined as the time taken by participants to enter the letters and press Enter, on correct trials only. Table 1 presents the average reaction times (for correct trials) according to condition (overall M = 4.38 s, SD = 1.11). As was expected, participants were significantly slower on high-load than on low-load trials, F(1, 44) = 373.24, p < .0001, η p 2 = .90. There was no effect of picture type (p > .20) on reaction time, nor did the interaction between load and picture type reach significance (p > .81).
References
Amaral, D. G., & Price, J. L. (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology, 230, 465–496.
Ansari, T. L., & Derakshan, N. (2011). The neural correlates of cognitive effort in anxiety: Effects on processing efficiency. Biological Psychology, 86, 337–348.
Ashcraft, M. H., & Kirk, E. P. (2001). The relationships among working memory, math anxiety, and performance. Journal of Experimental Psychology: General, 130, 224–237.
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2, 303–312.
Beauregard, M., Levesque, J., & Bourgouin, P. (2001). Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience, 21, 161–166.
Bishop, S. J., Duncan, J., Brett, M., & Lawrence, A. D. (2004a). Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience, 7, 184–188.
Bishop, S. J., Duncan, J., & Lawrence, A. D. (2004b). State anxiety modulation of the amygdala Response to unattended threat-related stimuli. Journal of Neuroscience, 24, 10364–10368.
Borkovec, T. D., Alcaine, O. M., & Behar, E. (2004). Avoidance theory of worry and generaiized anxiety disorder. In R. Heimberg, C. Turk, & D. Mennin (Eds.), Generalized anxiety disorder: Advances in research and practice (pp. 77–108). New York: Guilford.
Butler, E. A., Egloff, B., Wilhelm, F. H., Smith, N. C., Erickson, E. A., & Gross, J. J. (2003). The social consequences of expressive suppression. Emotion, 3, 48–67.
Cavada, C. (2000). The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex, 10, 220–242.
Cuthbert, B. N., Schupp, H. T., Bradley, M. M., Birbaumer, N., & Lang, P. J. (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111.
Denollet, J., Pedersen, S. S., Vrints, C. J., & Conraads, V. M. (2006). Usefulness of type D personality in predicting five-year cardiac events above and beyond concurrent symptoms of stress in patients with coronary heart disease. American Journal of Cardiology, 97, 970–973.
Derakshan, N., & Eysenck, M. W. (2009). Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist, 14, 168–176.
D'Esposito, M., Postle, B. R., & Rypma, B. (2000). Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research, 133, 3–11.
Dolcos, F., & Cabeza, R. (2002). Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience, 2, 252–263.
Dolcos, F., & McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience, 26, 2072–2079.
Dunning, J. P., & Hajcak, G. (2009). See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology, 46, 28–33.
Eippert, F., Veit, R., Weiskopf, N., Erb, M., Birbaumer, N., & Anders, S. (2007). Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping, 28, 409–423.
Erk, S., Abler, B., & Walter, H. (2006). Cognitive modulation of emotion anticipation. European Journal of Neuroscience, 24, 1227–1236.
Erk, S., Kleczar, A., & Walter, H. (2007). Valence-specific regulation effects in a working memory task with emotional context. NeuroImage, 37, 623–632.
Eysenck, M. W., & Calvo, M. G. (1992). Anxiety and performance: The processing efficiency theory. Cognition and Emotion, 6, 409–434.
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353.
Foti, D., & Hajcak, G. (2008). Deconstructing reappraisal: Descriptions preceding arousing pictures modulates the subsequent neural response. Journal of Cognitive Neuroscience, 20, 977–988.
Foti, D., Hajcak, G., & Dien, J. (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46, 521–530.
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484.
Gross, J. J. (1998). The emerging field of emotion regulation: An integrative review. Review of General Psychology, 2, 271–299.
Gross, J. J., & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362.
Hajcak, G., Anderson, B. S., Arana, A., Borckardt, J., Takacs, I., George, M. S., et al. (2010a). Dorsolateral prefrontal cortex stimulation modulates electrocortical measures of visual attention: Evidence from direct bilateral epidural cortical stimulation in treatment-resistant mood disorder. Neuroscience, 170, 281–288.
Hajcak, G., Dunning, J. P., & Foti, D. (2009). Motivated and controlled attention to emotion: Time-course of the late positive potential. Clinical Neurophysiology, 120, 505–510.
Hajcak, G., MacNamara, A., & Olvet, D. M. (2010b). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35, 129–155.
Hajcak, G., & Nieuwenhuis, S. (2006). Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience, 6, 291–297.
Hajcak, G., & Olvet, D. M. (2008). The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion, 8, 250–255.
Hariri, A. R., Mattay, V. S., Tessitore, A., Fera, F., & Weinberger, D. R. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53, 494–501.
Heller, W., Nitschke, J. B., Etienne, M. A., & Miller, G. A. (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106, 376–385.
Ikeda, M., Iwanaga, M., & Seiwa, H. (1996). Test anxiety and working memory system. Perceptual and Motor Skills, 82, 1223–1231.
Johnson, M. K., Raye, C. L., Mitchell, K. J., Greene, E. J., Cunningham, W. A., & Sanislow, C. A. (2005). Using fMRI to investigate a component process of reflection: Prefrontal correlates of refreshing a just-activated representation. Cognitive, Affective, & Behavioral Neuroscience, 5, 339–361.
Johnston, V. S., Miller, D. R., & Burleson, M. H. (1986). Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology, 23, 684–694.
Johnstone, T., van Reekum, C. M., Urry, H. L., Kalin, N. H., & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience, 27, 8877–8884.
Kanske, P., Heissler, J., Schönfelder, S., Bongers, A., & Wessa, M. (in press). How to regulate emotion? Neural networks for reappraisal and distraction. Cerebral Cortex.
Krompinger, J. W., Moser, J. S., & Simons, R. F. (2008). Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion, 8, 132–137.
Kross, E., & Ayduk, O. (2008). Facilitating adaptive emotional analysis: Distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Personality and Social Psychology Bulletin, 34, 924–938.
Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (2005). International affective picture system (IAPS): Affective ratings of pictures and instruction manual (Tech. Rep. A-6). Gainesville: University of Florida.
Lavric, A., Rippon, G., & Gray, J. R. (2003). Threat-evoked anxiety disrupts spatial working memory performance: an attentional account. Cognitive Therapy and Research, 27, 489–504.
Lévesque, J., Eugène, F., Joanette, Y., Paquette, V., Mensour, B., Beaudoin, G., et al. (2003). Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry, 53, 502–510.
MacNamara, A., Foti, D., & Hajcak, G. (2009). Tell me about it: Neural activity elicited by emotional stimuli and preceding descriptions. Emotion, 9, 531–543.
MacNamara, A., & Hajcak, G. (2009). Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia, 47, 2975–2980.
MacNamara, A., & Hajcak, G. (2010). Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety, 27, 234–243.
Manoach, D. S., Schlaug, G., Siewert, B., Darby, D. G., Bly, B. M., Benfield, A., et al. (1997). Prefrontal cortex fMRI signal changes are correlated with working memory load. NeuroReport, 8, 545–549.
McRae, K., Hughes, B., Chopra, S., Gabrieli, J. D. E., Gross, J. J., & Ochsner, K. N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22, 248–262.
Mennin, D. S. (2004). Emotion regulation therapy for generalized anxiety disorder. Clinical Psychology & Psychotherapy, 11, 17–29.
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Neuroscience, 24, 167–202.
Moser, J. S., Hajcak, G., Bukay, E., & Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology, 43, 292–296.
Naveh-Benjamin, M., McKeachie, W. J., Lin, Y. G., & Holinger, D. P. (1981). Test anxiety: Deficits in information processing. Journal of Educational Psychology, 73, 816–824.
Nitschke, J. B., Heller, W., Palmieri, P. A., & Miller, G. A. (1999). Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology, 36, 628–637.
Ochsner, K. N., Bunge, S. A., Gross, J. J., & Gabrieli, J. D. E. (2002). Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14, 1215–1229.
Ochsner, K. N., & Gross, J. J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249.
Ochsner, K. N., Ray, R. D., Cooper, J. C., Robertson, E. R., Chopra, S., Gabrieli, J. D. E., et al. (2004). For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage, 23, 483–499.
Öhman, A., Flykt, A., & Esteves, F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130, 466–478.
Pastor, M. C., Bradley, M. M., Löw, A., Versace, F., Moltó, J., & Lang, P. J. (2008). Affective picture perception: Emotion, context, and the late positive potential. Brain Research, 1189, 145–151.
Pedersen, S. S., & Denollet, J. (2003). Type D personality, cardiac events, and impaired quality of life: A review. European Journal of Cardiovascular Prevention & Rehabilitation, 10, 241–248.
Phan, K. L., Fitzgerald, D. A., Nathan, P. J., Moore, G. J., Uhde, T. W., & Tancer, M. E. (2005). Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry, 57, 210–219.
Porrino, L. J., Crane, A. M., & Goldman Rakic, P. S. (1981). Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. Journal of Comparative Neurology, 198, 121–136.
Radilová, J. (1982). The late positive component of visual evoked response sensitive to emotional factors. Activitas Nervosa Superior, Suppl., 3(Pt. 2), 334–337.
Rapee, R. M. (1993). The utilisation of working memory by worry. Behaviour Research and Therapy, 31, 617–620.
Reed, D. (2002). Random letter sequence generator. Retrieved from http://www.dave-reed.com/Nifty/randSeq.html.
Richards, A., French, C. C., Keogh, E., & Carter, C. (2000). Test-anxiety, inferential reasoning and working memory load. Anxiety, Stress, and Coping, 13, 87–109.
Richards, J. M., & Gross, J. J. (2006). Personality and emotional memory: How regulating emotion impairs memory for emotional events. Journal of Research in Personality, 40, 631–651.
Rozenkrants, B., & Polich, J. (2008). Affective ERP processing in a visual oddball task: Arousal, valence, and gender. Clinical Neurophysiology, 119, 2260–2265.
Salters-Pedneault, K., Roemer, L., Tull, M. T., Rucker, L., & Mennin, D. S. (2006). Evidence of broad deficits in emotion regulation associated with chronic worry and generalized anxiety disorder. Cognitive Therapy and Research, 30, 469–480.
Schupp, H. T., Flaisch, T., Stockburger, J., & Junghöfer, M. (2006). Emotion and attention: Event-related brain potential studies. In S. Anders, G. Ende, M. Junghöfer, J. Kissler, & D. Wildgruber (Eds.), Progress in brain research: Understanding emotions (Vol. 156, pp. 31–51). Amsterdam: Elsevier.
Schupp, H. T., Junghöfer, M., Weike, A. I., & Hamm, A. O. (2003). Emotional facilitation of sensory processing in the visual cortex. Psychological Science, 14, 7–13.
Schupp, H. T., Junghöfer, M., Weike, A. I., & Hamm, A. O. (2004). The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology, 41, 441–449.
Schupp, H. T., Stockburger, J., Codispoti, M., Junghöfer, M., Weike, A. I., & Hamm, A. O. (2007). Selective visual attention to emotion. Journal of Neuroscience, 27, 1082–1089.
Shackman, A. J., Sarinopoulos, I., Maxwell, J. S., Pizzagalli, D. A., Lavric, A., & Davidson, R. J. (2006). Anxiety selectively disrupts visuospatial working memory. Emotion, 6, 40–61.
Sheppes, G., & Meiran, N. (2008). Divergent cognitive costs for online forms of reappraisal and distraction. Emotion, 8, 870–874.
Spielberger, C. D. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press.
Van Dillen, L. F., Heslenfeld, D. J., & Koole, S. L. (2009). Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage, 45, 1212–1219.
Van Dillen, L. F., & Koole, S. L. (2007). Clearing the mind: A working memory model of distraction from negative mood. Emotion, 7, 715–723.
van Reekum, C. M., Johnstone, T., Urry, H. L., Thurow, M. E., Schaefer, H. S., Alexander, A. L., et al. (2007). Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. NeuroImage, 36, 1041–1055.
Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. J. (2001). Effects of attention and emotion on face processing in the human brain an event-related fMRI study. Neuron, 30, 829–841.
Wager, T. D., Davidson, M. L., Hughes, B. L., Lindquist, M. A., & Ochsner, K. N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050.
Wangelin, B. C., Löw, A., McTeague, L. M., Bradley, M. M., & Lang, P. J. (2011). Aversive picture processing: Effects of a concurrent task on sustained defensive system engagement. Psychophysiology, 48, 112–116.
Weinberg, A., & Hajcak, G. (2010). Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion, 10, 767–782.
Weinberg, A., & Hajcak, G. (in press). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 1–14.
Zanto, T. P., & Gazzaley, A. (2009). Neural suppression of irrelevant information underlies optimal working memory performance. Journal of Neuroscience, 29, 3059–3066.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacNamara, A., Ferri, J. & Hajcak, G. Working memory load reduces the late positive potential and this effect is attenuated with increasing anxiety. Cogn Affect Behav Neurosci 11, 321–331 (2011). https://doi.org/10.3758/s13415-011-0036-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-011-0036-z