Abstract
The programming of rapid eye movements or “saccades” involves a large collection of neural substrates. The subcortical oculomotor center – the superior colliculus (SC) – contains a topographical motor map that encodes saccade vectors. Using a visual distractor task, the present study examined a classic model of the SC motor map, which assumes a symmetrical representation of the upper visual field (UVF) and lower visual field (LVF). Visual distractors are known to attract or repel the saccade trajectory, depending on their angular distance from the target. In the present study, the distractor (if presented) was placed at a location that mirrored the target in the opposite visual field (upper or lower). The symmetrical SC model predicts equivalent directional deviations for saccades into the UVF and LVF. The results, however, showed that the directional deviations evoked by visual distractors were much stronger for saccades directed to the LVF. We argue that this observation is consistent with the recent neurophysiological finding that the LVF is relatively under-represented, as compared to the UVF, in the SC and possibly in other oculomotor centers. We conclude the paper with a suggested revision to the SC model.
Similar content being viewed by others
Introduction
Humans make two to three rapid eye movements or “saccades” while awake (Rayner, 1998). Saccades are either voluntarily controlled or triggered by salient external events (i.e., oculomotor capture; Theeuwes et al., 1998). Saccades quickly shift gaze from one place to another so the brain can effectively sample information from the environment despite a tiny fovea on the retina. The control of saccades involves a collection of neural substrates (for an overview, see Munoz et al., 2007; Schall, 1997), including but not limited to the frontal eye fields (FEFs; for a review, see Schall, 1997), the lateral intraparietal cortices (LIP; for a review, see Glimcher, 2001), the basal ganglia (for a review, see Hikosaka et al., 2006), and the superior colliculus (SC; e.g., Robinson, 1972; for a review, see White & Munoz, 2011).
The SC has a layered structure. The superficial layers process external visual input, the deep layers process multisensory and higher cortical inputs, whereas the intermediate layers integrate all sources of inputs to trigger a saccade (White & Munoz, 2011). Visuomotor neurons in the intermediate layers of the SC encode the direction and amplitude of saccades; these neurons are topographically organized, much like the early visual cortices (e.g., Daniel & Whitteridge, 1961; Schwartz, 1977). The mapping between the visual space and the SC motor map is illustrated in Fig. 1. Hypothetically, delivering micro-stimulation to a population of neurons in the SC (T in Fig. 1b) will evoke a saccade towards a corresponding coordinate in the visual space (T’ in Fig. 1a). A similar but much coarser topographical map also exists in the FEFs, where neurons show selectivity to saccade amplitude and direction (e.g., Sommer & Wurtz, 2000).
a The visual space in polar coordinates. For saccades, the origin represents the current fixation, and the radial and angular coordinates represent saccade amplitude and direction, respectively. b The superior colliculus (SC) motor map; this map was drawn based on the mapping functions proposed by Ottes et al. (1986). In the SC, saccade amplitudes are represented along the rostral-caudal direction, whereas saccade directions are represented along the medial-lateral direction. HM horizontal meridian
Our current knowledge about the SC motor map is largely based on the early work by Robinson (1972), in which micro-stimulation was delivered to various SC sites to evoke saccades of varying directions and amplitudes. Based on 50 SC stimulation sites from two monkeys, Robinson (1972) constructed a contour map representing equal amplitudes and directions. This map assumed symmetrical mapping of the UVF and LVF in the SC. However, it is important to note that this map is constructed by first dividing the stimulation sites into strips of constant amplitude and direction “by eye” (see Robinson, 1972, pp. 1800-1801); it was never intended to be regarded as a precise description of the SC motor map, but rather a coarse approximation. In a recent neurophysiological study, Hafed and Chen (2016) showed that the response fields of SC neurons are larger for the LVF than for the UVF, suggesting the SC has less neural tissue representing the LVF. This anatomical asymmetry may have functional consequences, such that saccades to the LVF are less accurate (e.g., Hafed & Chen, 2016) and take longer to program (e.g., Abegg et al., 2015; Hafed & Chen, 2016; Heywood & Churcher, 1980).
As illustrated in Fig. 1, saccade vectors (amplitude and direction) are topographically mapped onto the SC motor maps. Compared to latency and landing position, behavioral effects in saccade amplitude and direction are more telling when examining the anatomical asymmetry suggested by Hafed and Chen (2016). The present study examined visual field asymmetry on oculomotor maps with a visual distractor task, in which a saccade target was accompanied by a visual distractor (see Fig. 2a and b, for an illustration). Visual distractors either attract or repel the direction of saccades, depending on their angular distance from the saccade target (e.g., Doyle & Walker, 2001; Fielding et al., 2006; McSorley et al., 2009; Wang et al., 2012; see Wang & Theeuwes, 2014, for a graphical meta-analysis). To examine whether meridians in the UVF and LVF are symmetrically represented on oculomotor maps, in the present study, the target and distractor were presented at locations mirroring the horizontal meridian. That is, if the saccade target was presented 30° (polar angle) above the horizontal meridian, the distractor would then be presented 30° below. The saccade targets were presented in either the UVF or LVF with equal probability. The rationale was straightforward. As shown in Fig. 2d, the distance between the target and the distractor on the SC map is the same regardless of the target appearing in the UVF or LVF and, consequently, the directional deviation caused by the visual distractor should be comparable in magnitude on the SC map (marked by red arrows in Fig. 2d). Assuming a symmetrical representation of the UVF and LVF in the SC, the magnitude of the directional deviation evoked by the visual director should be comparable across the visual fields. If the LVF is represented with less tissue in the SC and other oculomotor maps, as suggested by Hafed and Chen (2016), the same amount of directional deviation on the SC map will translate into a much larger directional deviation for LVF targets (see Fig. 2d). We acknowledge that behavioral experiments do not measure neuronal responses directly; nevertheless, carefully designed behavioral experiments can still provide supportive evidence for neurophysiological findings.
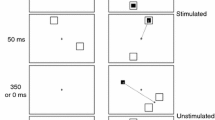
a An illustration of a distractor-absent trial in the visual distractor task. The initial direction of a saccade was estimated with the starting position and a sample at which the saccade had traveled 30% of its duration (see the main text for details). b An illustration of a distractor-present trial in the visual distractor task. c The directional deviation was quantified with the difference in initial saccade direction between distractor-present and -absent trials. d With the target and distractor mirroring the horizontal meridian, the distractor would evoke the same amount of directional deviation on the superior colliculus (SC) map (red arrows) regardless of the target appearing in the upper (UVF) or lower (LVF) visual field. If the representation of the LVF is relatively compressed compared to the UVF (as illustrated in panel D), the same amount of directional deviation on the SC map would translate into a much stronger directional deviation in the LVF. HM horizontal meridian
The experiment reported here was a close replication of a previous study that was briefly communicated at the 2014 Vision Sciences Society meeting (Wang, 2014). The previous study had only 30 trials per condition; to examine the impact of saccade latencies on directional deviations, the present study increased the number of trials by threefold to allow for a latency binning analysis. The most critical findings related to distractor-evoked directional directions were consistent in those two studies. For brevity, the results of the 2014 study are presented as Online Supplemental Materials (OSM).
Method
The research protocols reported in this paper were approved by a local ethics committee at Zhejiang University. All participants gave written informed consent.
Participants
Nineteen volunteers participated in the present study (seven females; mean age: 23.7 years (SD = 2.7); age range: 20–29 years), and no participant was excluded from the analysis. All participants had normal or corrected-to-normal visual acuity and reported no history of psychological or neurological disorders. They were paid 50 yuan per hour for their participation.
Apparatus
The experiment was carried out in a dimly lit laboratory. All stimuli were presented against a black background (2.45 cd/m2) on a 23.8-in. IPS monitor (DELL P2417H). The visible area of the monitor measured 32° × 20° at a view distance of about 90 cm (maintained with a chinrest). Gaze direction was monitored and recorded with an EyeLink® Portable Duo eye-tracker (SR Research Ltd., Oakville, Canada), sampling at 1,000 Hz. The tracking accuracy of the eye-tracker was typically in the 0.25–0.5° range (SR Research Ltd., 2022).
Task procedure and design
A trial started with the presentation of a white fixation cross that measured 1° × 1° (Weber contrast = 88.4). After a random delay of 500–700 ms, a saccade target (white circle; diameter = 1°, Weber contrast = 88.4) appeared at an eccentricity of 7.7°. A visual distractor (filled white square; 1° × 1°, Weber contrast = 88.4) appeared at the same time in 50% of the trials (see Fig. 2b). To discourage anticipatory response, the target was not presented in 1/16 of the trials, regardless of the presence of the distractor. The participant had 1,000 ms to initiate a saccade to the target if it appeared on the display. After a random inter-trial interval (750–1,500 ms), the next trial began.
The target and distractor could be 15°, 30°, 45°, 60°, or 75° (polar angle) away from the horizontal meridian (HM). When presented together, they always appeared at locations mirroring the horizontal meridian. Thus, the angular separation between them could be 30°, 60°, 90°, 120°, or 150°. By design, there were four blocks of 640 trials; so, a total of 2,560 trials were tested. As noted, the target was not presented on 1/16 of the trials; so, each block had 40 trials without the target. The remaining 600 trials were assigned with equal probability to the 20 experimental conditions [2 (visual field: UVF vs. LVF) × 5 (distance to HM: 15°, 30°, 45°, 60°, 75°) × 2 (distractor presence: present vs. absent)], with each condition having 30 trials.
Each participant came to the laboratory two to four times and completed one or two blocks of trials in each session.Footnote 1 In total, about 7–9 h were required to complete the task in the lab. Sixteen practice trials were provided at the beginning of each testing session. During testing, the participants could take a break after every 160 trials or whenever they felt they needed it. The eye-tracker was calibrated at the beginning of the testing session and re-calibrated following each break. Warning messages were displayed, and a trial was flagged as an “error” trial if the participants failed to maintain fixation before target onset, failed to respond within 1,000 ms, or the primary saccade missed the saccade target by more than 2°. All erroneous trials were later presented to the participants at the end of each block until all trials were successfully completed.
Dependent measures
The dependent measures of prime interest were saccade latencies, amplitudes, and directional deviations caused by the distractor.
Saccade latency
Saccade latency was the time interval between the onset of the stimulus (target, distractor, or both) and the onset of the primary saccade. Saccade onset was detected online with a velocity threshold of 30°/s and an acceleration threshold of 8,000°/s2.
Saccade amplitude
Saccade amplitude was the Euclidean distance between the start and landing positions.
Directional deviation
The initial direction of the primary saccade was determined by its starting position and the gaze position at which the saccade had traversed 30% of its duration (for similar measures, see Van der Stigchel et al., 2007; Wang & Theeuwes, 2014). The directional deviation was quantified as the difference in initial saccade direction between distractor-present and -absent trials (see Fig. 2a–c), with positive and negative values denoting deviation towards and away from the distractor, respectively.
Results
Only successfully completed trials were analyzed. Trials were excluded if they met one of the following criteria: (a) the latency of the primary saccade was below 80 ms or above 550 ms (3.85%), (b) the duration of the primary saccade was longer than 100 ms (1.10%), (c) the start position of the primary saccade deviated more than 2° from the fixation cross (0.86%), or (d) the initial direction of the primary saccade deviated from the target direction by more than 45° (1.86%). After data cleansing, 93.29% of the trials remained.
Saccade latency
Mean saccade latencies are presented in Table 1. A repeated-measures ANOVA (with Greenhouse-Geisser correction for sphericity) was performed on the saccade latencies, with variables visual field (target in UVF vs. LVF), target distance to HM (15°, 30°, 45°, 60°, 75°), and distractor presence (present vs. absent). The analysis revealed significant main effects for distractor presence, F(1, 18) = 45.91, p < 0.001, ηp2 = 0.72, visual field, F(1, 18) = 53.58, p < 0.001, ηp2 = 0.75, and distance to HM, F(4, 72) = 7.23, p < 0.001, ηp2 = 0.29. These effects occurred because saccade latencies were longer when the distractor was present, longer for LVF targets, and generally increased as the target moved away from the horizontal meridian (see Table 1). A significant two-way interaction occurred between visual field and target distance to HM, F(4, 72) = 16.81, p < 0.001, ηp2 = 0.48, as the effect of distance to HM was more prominent for LVF saccades. The three-way interaction was also significant, F(4, 72) = 2.76, p < 0.05, ηp2 = 0.13. Post hoc contrasts revealed that saccades to UVF targets had shorter latencies than those to LVF targets at all target to HM distances, all t > 3.20, all p < 0.01 (Bonferroni corrected). The latency increase due to the presence of a distractor is presented in Fig. 3a. An additional ANOVA on the distractor-evoked latency increase, with variables visual field and target distance to HM, revealed only a significant two-way interaction between distance to HM and visual field, F(4, 72) = 2.76, p = 0.034, ηp2 = 0.72. This interaction occurred because there was a trend for the distractor-evoked latency increase to increase with the target to HM distance in the UVF, whereas the opposite pattern of results was seen in the LVF. However, post hoc contrasts revealed no reliable difference between the UVF and LVF at all target to HM distances, all t < 1.53, all p > 0.14 (Bonferroni corrected).
a The distractor-evoked increases in saccade latency. b Directional deviations (differences in the initial direction between distractor-present and -absent trials) in upper (UVF) and lower (LVF) visual fields; negative values on the y-axis denote deviations away from the distractor. c The effect of saccade latency on directional deviation. In this figure, trials were binned according to the saccade latencies. HM horizontal meridian. Error bars denote ±1 SEM
Saccade amplitude
The mean saccade amplitudes are presented in Table 1. An ANOVA on the saccade amplitudes revealed a significant main effect for target distance to HM, F(4, 72) = 22.22, p < 0.001, ηp2 = 0.55, as saccades directed closer to the vertical meridian had a stronger tendency to undershoot. There was a significant two-way interaction between distractor presence and distance to HM, F(4, 72) = 3.16, p = 0.045, ηp2 = 0.15; however, the difference between distractor-present and -absent trials reached marginal significance only when the target to HM distance was 60°, t = 1.29, p = 0.070 (Bonferroni corrected). No other main effect or interaction reached significance, all F < 2.52, all p > 0.45.
Directional deviation
The directional deviation caused by the visual distractor was quantified with the difference in the initial saccade direction between distractor-present and -absent trials. The initial directions are presented in Table 1, whereas the directional deviations are presented in Fig. 3b. Note that, in Fig. 3b and c, positive and negative values on the y-axis denote directional deviations toward and away from the distractor, respectively.
An ANOVA on the directional deviations, with variables visual field and target distance to HM, revealed significant main effects for visual field, F(1, 18) = 15.96, p < 0.01, ηp2 = 0.47, and target distance to HM, F(4, 72) = 20.39, p < 0.001, ηp2 = 0.53. The directional deviation increased as the saccade target moved further away from the horizontal meridian;Footnote 2 most importantly, the directional deviation was much stronger for LVF targets (see Fig. 3a), suggesting the representation of the LVF is relatively compressed compared to the UVF (see Fig. 2d). The two-way interaction was also significant, F(4, 72) = 7.24, p < 0.001, ηp2 = 0.29. Post hoc contrasts revealed stronger directional deviations for saccades to LVF targets when the target was 45° and further away from the horizontal meridian, all t > 2.87, all p < 0.01 (Bonferroni corrected). No difference in directional deviation was observed between LVF and UVF targets when they were 15° or 30° away from the horizontal meridian, all t < 1.50, all p > 0.15. A similar pattern of results was seen in our 2014 study (see OSM).
One may wonder if the much stronger directional deviations of LVF saccades were due to a systematic visual field difference in saccade accuracy. To rule out this possibility, we analyzed the landing errors, i.e., the Euclidean distance between the target and the landing position of the primary saccade. The landing errors are presented in Fig. S1 in the OSM. Note that this analysis considered only distractor-absent trials. An ANOVA on the landing errors, with variables visual field and target distance to HM, revealed a significant main effect of target distance to HM, F(4, 72) = 4.63, p = 0.02, ηp2 = 0.21; saccades were overall less accurate when directed to regions close to the vertical meridian. However, the main effect of visual field did not reach significance, nor did the two-way interaction, all F < 1.7, p > 0.21, ηp2 < 0.09, suggesting that saccades directed to the LVF were as precise as, if not more precise than, those directed to the UVF (see Fig. S1, OSM).
Previous studies have found that the directional deviation caused by visual distractors increases with saccade latency. It is possible that, with longer saccade latencies, the brain has more time to suppress the distractor (e.g., McSorley et al., 2006; Theeuwes & Godijn, 2004) or the distractor has more time to compete with the target. Following this observation, one may speculate that the stronger directional deviation observed for LVF saccades (see Fig. 3b) may as well be the result of the overall longer latencies observed in the LVF (see Table 1). To rule out this possibility, we binned the saccade latencies into four quarters to examine how directional deviations varied with saccade latencies (see also McSorley et al., 2006, 2009; Theeuwes & Godijn, 2004). An additional ANOVA, with the latency bins as a factor, revealed significant main effects for visual field, F(1, 18) = 16.06, p < 0.01, ηp2 = 0.47, target distance to HM, F(1, 18) = 20.36, p < 0.001, ηp2 = 0.53, and latency bin, F(1, 18) = 9.10, p < 0.001, ηp2 = 0.34. The two-way interaction between visual field and target distance to HM was significant, F (3,54) = 7.27, p < 0.01, ηp2 = 0.28; the two-way interaction between latency bin and target distance to HM was marginal, F (12, 216) = 1.99, p = 0.069, ηp2 = 0.1. The latency bin effect replicated previous findings that the directional deviation generally increases with saccade latency. However, the two-way interaction between visual field and latency bin was not significant, F(3, 54) = 1.59, p = 0.20, ηp2 = 0.08, nor was the three-way interaction, F(12, 216) = 1.17, p = 0.30, ηp2 = 0.06. As shown in Fig. 3c, the visual field difference in directional deviations persisted even when the saccade latencies were matched between the UVF and LVF.
Discussion
In the present study, the target and distractor always appeared at locations mirroring the horizontal meridian. If the UVF and LVF were symmetrically represented on the SC map, the distance between the target and distractor on the SC map should be the same regardless of the target appearing in the UVF or LVF. Consequently, the directional deviations caused by the distractor should be comparable for UVF and LVF targets. The present study, however, observed a much stronger directional deviation for targets in the LVF (see Fig. 3b). The anatomical asymmetry in the SC maps observed by Hafed and Chen (2016) offers a straightforward interpretation of the present findings.Footnote 3 As schematically illustrated in Fig. 2d, if the SC has less neural tissue representing the LVF, a shift in neuronal activation along the medial-lateral direction would translate into a more pronounced change in direction for saccades into the LVF. As shown in Fig. 3b, the directional deviation evoked by visual distractors was much stronger for LVF targets that appeared 45° or further away from the horizontal meridian. This observation suggests that the compression of the LVF (relative to the UVF) in the SC is more prominent for regions close to the vertical meridian. This observation largely agrees with Drager and Hubel’s (1976) finding that the magnification factor in the SC was higher for regions 30° above the horizontal meridian.
Previous studies have found that stronger saccade trajectory deviations are linked to longer saccade latencies (e.g., McSorley et al., 2006). One may suspect that the present observation of stronger distractor-evoked directional deviation was the result of the overall longer saccade latencies in the LVF. We ruled out this possibility with a latency binning analysis, showing that the distractor-evoked directional deviation was stronger in the LVF even when the latency was matched for UVF and LVF saccades (see Fig. 3c). It is also worth noting that although the saccade latency was overall longer in the LVF, the distractor-evoked latency increase was comparable between the LVF and UVF at all targets to HM distances (see the statistical results in the Saccade latency section), whereas the distractor-evoked directional deviation was stronger in the LVF only when the target to HM distance was 45° or further. The stronger directional deviations observed in the LVF were unlikely confounded by saccade latency.
Previous studies have revealed various processing advantages in the LVF (for reviews, see Jóhannesson et al., 2018; Karim & Kojima, 2010; Skrandies, 1987). For instance, attention has been shown to have a finer resolution in the LVF (He et al., 1996), and visual performance is better below fixation along the vertical meridian (e.g., Carrasco et al., 2001). It is important to note that the present findings cannot be explained in terms of perceptual advantages in the LVF (e.g., Carrasco et al., 2001; Talgar & Carrasco, 2002). First, the perceptual advantage observed in LVF is largely restricted to the vertical meridian (e.g., Abrams et al., 2012; Cameron et al., 2002; Talgar & Carrasco, 2002), whereas in the present experiment, the target and distractor were never presented on the vertical meridian. Second, the LVF perceptual advantage on the vertical meridian is most noticeable for medium and high-frequency stimuli (Cameron et al., 2002; Skrandies, 1987); however, the visual target and distractor in the present study both had low spatial frequency. Finally, and most importantly, none of the LVF perceptual advantage findings could explain why latencies were longer for LVF saccades.
Previc (1990) has argued that the near (peripersonal) and far (extrapersonal) spaces roughly correspond to the LVF and UVF, respectively. The visual system is biased toward processing information in the LVF (near space). This functional bias may be the shaping force of a neural system favoring the LVF. In an early study, Van Essen et al. (1984) showed that the striate area devoted to the peripheral part of the LVF was, on average, about 1.25 times that devoted to the UVF. A ratio of 1.22 for the central visual field was later reported by Tootell et al. (1988). An anatomical asymmetry favoring the LVF also exists in the lateral geniculate nucleus (LGN), with a ratio of 1.13 (Connolly & Van Essen, 1984). The oculomotor system, which plays a critical role in visual search and orientation, is biased toward processing information in the UVF (far space). This functional bias may have shaped the asymmetrical organization of the UVF and LVF on oculomotor maps, as revealed in Hafed and Chen (2016) and the present study.
The mapping between the visual field and the striate cortex is described with a complex logarithmic model (Schwartz, 1977). Mathematically equivalent mapping functions have been suggested by Ottes et al. (1986) to describe the mapping of the visual field in the SC. These functions essentially transform the polar coordinates of the visual field into the Cartesian coordinates used to describe the anatomical structure of the SC. This model has two formulae (Eqs. 1 and 2).
In these formulae, u represents the anatomical distance (in mm) from the rostral pole in the SC along the axis representing the horizontal position (see Fig. 1b), v is the perpendicular distance; R and Φ are the retinal eccentricity and polar direction of the saccade target (in degrees), respectively. By approximating the SC motor map constructed by Robinson (1972), Ottes et al. (1986) estimated that Bu = 1.4 mm, Bv = 1.8 mm/rad, and A = 3°. Bu and Bv are scaling constants for the SC motor map along its u and v axes, respectively. As shown in the present study, the representation of polar directions in the LVF is compressed relative to that in the UVF. This LVF compression can be easily accommodated in the equations, by making Bv and/or Bu contingent on the polar direction of the saccade target. For the SC motor map illustrated in Fig. 2d, Bv was scaled by a sigmoidal transformation of the polar direction (Φ) (Eq. 3) and the transformation of the polar direction was largely linear (β = 0.2). This revised SC model is similar to that proposed by Hafed and Chen (2016), though both models did not consider the representation of saccade amplitudes. We speculate that the representation of saccade amplitudes may also be compressed in the LVF, i.e., neurons coding saccades of the same amplitudes are closer to the rostral pole of the SC (Wang et al., 2016; see OSM for an illustration). This supposition deserves further neurophysiological and neuroanatomical investigations.
Notes
Two participants came to the lab four times, six participants came to the lab three times, and 11 participants came to the lab twice to complete the task. The overall pattern of results on directional deviations was the same if we consider only the data collected from the 11 participants who came to the lab twice to complete the task (see Online Supplemental Materials).
Previous work had reported stronger distractor-evoked trajectory deviations for vertical than for horizontal saccades (e.g., van der Stigchel & Theeuwes, 2008), showing that vertical saccades are more susceptible to distractor influence. This vertical meridian effect may have contributed to the strong directional deviations in “near vertical” saccades, but it does not undermine the critical visual field difference observed in the present study.
Saccades into the UVF and LVF are likely modulated differently by gravity (e.g., Pierrot-Deseilligny, 2009); however, the stronger directional deviation observed for LVF saccades was unlikely the result of gravity. The directional deviation measures the impact of visual distractors and it is quantified with the difference in initial saccade direction between distractor-present and -absent trials. Saccades should have been similarly impacted by gravity across distractor-present and -absent trials.
References
Abegg, M., Pianezzi, D., & Barton, J. J. S. (2015). A vertical asymmetry in saccades. Journal of Eye Movement Research, 8(5), 3 1–10.
Abrams, J., Nizam, A., & Carrasco, M. (2012). Isoeccentric locations are not equivalent: The extent of the vertical meridian asymmetry. Vision Research, 52(1), 70–78. https://doi.org/10.1016/j.visres.2011.10.016
Cameron, E. L., Tai, J. C., & Carrasco, M. (2002). Covert attention affects the psychometric function of contrast sensitivity. Vision Research, 42(8), 949–967. https://doi.org/10.1016/S0042-6989(02)00039-1
Carrasco, M., Talgar, C. P., & Cameron, E. L. (2001). Characterizing visual performance fields: Effects of transient covert attention, spatial frequency, eccentricity, task and set size. Spatial Vision, 15(1), 61–75. https://doi.org/10.1163/15685680152692015
Connolly, M., & Van Essen, D. (1984). The representation of the visual field in parvicellular and magnocellular layers of the lateral geniculate nucleus in the macaque monkey. The Journal of Comparative Neurology, 226(4), 544–564. https://doi.org/10.1002/cne.902260408
Daniel, P. M., & Whitteridge, D. (1961). The representation of the visual field on the cerebral cortex in monkeys. The Journal of Physiology, 159(2), 203–221.
Doyle, M., & Walker, R. (2001). Curved saccade trajectories: Voluntary and reflexive saccades curve away from irrelevant distractors. Experimental Brain Research, 139(3), 333–344. https://doi.org/10.1007/s002210100742
Drager, U. C., & Hubel, D. H. (1976). Topography of visual and somatosensory projections to mouse superior colliculus. Journal of Neurophysiology, 39(1), 91–101.
Fielding, J., Georgiou-Karistianis, N., Millist, L., Fahey, M., & White, O. (2006). Saccadic trajectory in Huntington’s disease. Journal of the International Neuropsychological Society, 12(04), 455–464. https://doi.org/10.1017/S1355617706060589
Glimcher, P. W. (2001). Making choices: The neurophysiology of visual-saccadic decision making. Trends in Neurosciences, 24(11), 654–659. https://doi.org/10.1016/s0166-2236(00)01932-9
Hafed, Z. M., & Chen, C.-Y. (2016). Sharper, stronger, faster upper visual field representation in primate superior colliculus. Current Biology, 26(13), 1647–1658. https://doi.org/10.1016/j.cub.2016.04.059
He, S., Cavanagh, P., & Intriligator, J. (1996). Attentional resolution and the locus of visual awareness. Nature, 383(6598), 334–337. https://doi.org/10.1038/383334a0
Heywood, S., & Churcher, J. (1980). Structure of the visual array and saccadic latency: Implications for oculomotor control. Quarterly Journal of Experimental Psychology, 32(2), 335–341. https://doi.org/10.1080/14640748008401169
Hikosaka, O., Nakamura, K., & Nakahara, H. (2006). Basal ganglia orient eyes to reward. Journal of Neurophysiology, 95(2), 567–584. American Physiological Society. https://doi.org/10.1152/jn.00458.2005
Jóhannesson, Ó. I., Tagu, J., & Kristjánsson, Á. (2018). Asymmetries of the visual system and their influence on visual performance and oculomotor dynamics. European Journal of Neuroscience, 48(11), 3426–3445. https://doi.org/10.1111/ejn.14225
Karim, A. K. M. R., & Kojima, H. (2010). The what and why of perceptual asymmetries in the visual domain. Advances in Cognitive Psychology, 6, 103–115. https://doi.org/10.2478/v10053-008-0080-6
McSorley, E., Haggard, P., & Walker, R. (2006). Time course of oculomotor inhibition revealed by saccade trajectory modulation. Journal of Neurophysiology, 96(3), 1420–1424. https://doi.org/10.1152/jn.00315.2006
McSorley, E., Cruickshank, A., & Inman, L. (2009). The development of the spatial extent of oculomotor inhibition. Brain Research, 1298, 92–98. https://doi.org/10.1016/j.brainres.2009.08.081
Munoz, D. P., Armstrong, I., & Coe, B. (2007). Using eye movement to probe development and dysfunction. In R. P. G. V. Gompel (Ed.), Eye movements: A window on mind and brain. Elsevier.
Ottes, F. P., Van Gisbergen, J. A., & Eggermont, J. J. (1986). Visuomotor fields of the superior colliculus: A quantitative model. Vision Research, 26(6), 857–873.
Pierrot-Deseilligny, C. (2009). Effect of Gravity on Vertical Eye Position. Annals of the New York Academy of Sciences, 1164(1), 155–165. https://doi.org/10.1111/j.1749-6632.2009.03864.x
Previc, F. (1990). Functional specialization in the lower and upper visual-fields in humans: Its ecological origins and neurophysiological implications. Behavioral and Brain Sciences, 13(3), 519–541.
Rayner, K. (1998). Eye movements in reading and information processing: 20 years of research. Psychological Bulletin, 124(3), 372–422.
Robinson, D. A. (1972). Eye movements evoked by collicular stimulation in the alert monkey. Vision Research, 12(11), 1795–1808. https://doi.org/10.1016/0042-6989(72)90070-3
Schall, J. D. (1997). Visuomotor Functions in the Frontal Lobe. Cerebral Cortex, 12, 527–638. https://doi.org/10.1146/annurev-vision-082114-035317
Schwartz, E. L. (1977). Spatial mapping in the primate sensory projection: Analytic structure and relevance to perception. Biological Cybernetics, 25(4), 181–194. https://doi.org/10.1007/BF01885636
Skrandies, W. (1987). The upper and lower visual field of man: Electrophysiological and functional differences. In P. D. H. Autrum, P. D. D. Ottoson, P. D. E. R. Perl, P. D. R. F. Schmidt, P. D. H. Shimazu, & P. D. W. D. Willis (Eds.), Progress in Sensory Physiology (pp. 1–93). Springer: Berlin Heidelberg.
Sommer, M. A., & Wurtz, R. H. (2000). Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. Journal of Neurophysiology, 83(4), 1979–2001.
SR Research Ltd. (2022). EyeLink® Portable Duo User Manual (version 1.0.9). SR Research Ltd..
Talgar, C. P., & Carrasco, M. (2002). Vertical meridian asymmetry in spatial resolution: Visual and attentional factors. Psychonomic Bulletin & Review, 9(4), 714–722. https://doi.org/10.3758/BF03196326
Theeuwes, J., & Godijn, R. (2004). Inhibition-of-return and oculomotor interference. Vision Research, 44(12), 1485–1492. https://doi.org/10.1016/j.visres.2003.09.035
Theeuwes, J., Kramer, A. F., Hahn, S., & Irwin, D. E. (1998). Our eyes do not always go where we want them to go: Capture of the eyes by new objects. Psychological Science, 9(5), 379–385. https://doi.org/10.1111/1467-9280.00071
Tootell, R. B., Switkes, E., Silverman, M. S., & Hamilton, S. L. (1988). Functional anatomy of macaque striate cortex. II. Retinotopic organization. The Journal of Neuroscience, 8(5), 1531–1568.
Van der Stigchel, S., & Theeuwes, J. (2008). Differences in distractor-induced deviation between horizontal and vertical saccade trajectories. Neuroreport, 19(2), 251–254. https://doi.org/10.1097/WNR.0b013e3282f49b3f
Van der Stigchel, S., Meeter, M., & Theeuwes, J. (2007). The spatial coding of the inhibition evoked by distractors. Vision Research, 47(2), 210–218. https://doi.org/10.1016/j.visres.2006.11.001
Van Essen, D. C., Newsome, W. T., & Maunsell, J. H. R. (1984). The visual field representation in striate cortex of the macaque monkey: Asymmetries, anisotropies, and individual variability. Vision Research, 24(5), 429–448. https://doi.org/10.1016/0042-6989(84)90041-5
Wang, Z. (2014). Deviation in saccade trajectories suggests asymmetric representation of the upper and lower visual fields in the superior colliculus. Journal of Vision, 14(10), 1219–1219. https://doi.org/10.1167/14.10.1219
Wang, Z., & Theeuwes, J. (2014). Distractor evoked deviations of saccade trajectory are modulated by fixation activity in the superior colliculus: Computational and behavioral evidence. PLoS One, 9(12), e116382. https://doi.org/10.1371/journal.pone.0116382
Wang, Z., Kruijne, W., & Theeuwes, J. (2012). Lateral interactions in the superior colliculus produce saccade deviation in a neural field model. Vision Research, 62(1), 66–74. https://doi.org/10.1016/j.visres.2012.03.024
Wang, Z., Wang, B., & Finkbeiner, M. (2016). Asymmetrical representation of the upper and lower visual fields in oculomotor maps (preprint). Neuroscience. https://doi.org/10.1101/086397
White, B. J., & Munoz, D. P. (2011). The superior colliculus. In S. Liversedge, I. Gilchrist, & S. Everling (Eds.), Oxford Handbook of Eye Movements (pp. 195–213). Oxford University Press.
Acknowledgements
We thank Haoyun Yang for his assistance in lab management and data collection.
Author note
This project was supported by grants from the National Natural Science Foundation of China (Grant No.: 31333137) and Zhejiang Provincial Natural Science Foundation (grant No.: LY13C090007) to Z. Wang.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practices statement
Data, experimental scripts, and analysis tools are available from the corresponding author upon reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 451 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Zhang, S. & Wang, Z. Distractor-evoked deviation in saccade direction suggests an asymmetric representation of the upper and lower visual fields on oculomotor maps. Atten Percept Psychophys 85, 1150–1158 (2023). https://doi.org/10.3758/s13414-023-02701-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-023-02701-9