Abstract
There are thought to be two forms of inhibition of return (IOR) depending on whether the oculomotor system is activated or suppressed. When saccades are allowed, output-based IOR is generated, whereas input-based IOR arises when saccades are prohibited. In a series of 4 experiments, we mixed or blocked compatible and incompatible trials with saccadic or manual responses to investigate whether cueing effects would follow the same pattern as those observed with more traditional peripheral onsets and central arrows. In all experiments, an uninformative cue was displayed, followed by a cue-back stimulus that was either red or green, indicating whether a compatible or incompatible response was required. The results showed that IOR was indeed observed for compatible responses in all tasks, whereas IOR was eliminated for incompatible trials—but only with saccadic responses. These findings indicate that the dissociation between input- and output-based forms of IOR depends on more than just oculomotor activation, providing further support for the existence of an inhibitory cueing effect that is distinct to the manual response modality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inhibition of return (IOR) was first observed by Posner and Cohen (1984) in a study using the spatial cueing paradigm (Posner, 1980). In this paradigm, an uninformative peripheral cue is presented at various intervals before a target appears at either the same or opposite location as the cue. They found that participants responded faster on trials when the cue and target appeared at the same location (cued) than on trials when the cue and target appeared at different locations (uncued), but only when the interval between cue and target appearance (cue–target onset asynchrony; CTOA) was short—a form of behavioral facilitation. When the CTOA was longer than 300 ms, participants showed a reverse pattern of slower responses on cued trials—behavioral inhibition. These delayed responses were referred to as inhibition of return (Posner, Rafal, Choate, & Vaughan, 1985) and have since been observed under numerous conditions when individuals respond to a location they have previously attended (see Klein, 2000, for a review).
After decades of research since the first study of Posner and Cohen (1984), there is still little consensus regarding the neural mechanisms underlying IOR. This is largely owing to researchers using the umbrella term “IOR” to describe slowed responses to previously attended objects or locations despite wildly different tasks and stimuli that potentially engage different inhibitory mechanisms (Dukewich & Klein, 2015). The nomenclature used is particularly important because, without a clear definition, investigations into the causes and effects of IOR can become muddled. To address this issue, we will thus use the more general term inhibitory cueing effects (ICEs) when referring to cueing mechanisms that do not explicitly line up with Posner’s original definition of IOR (i.e., long lasting, in spatiotopic coordinates, with an activated oculomotor system)—a term suggested by Hilchey, Klein, and Satel (2014) in light of recent discoveries of multiple ICEs with different time courses and underlying mechanisms.
A great deal of research has suggested that there are at least two forms of ICE: (1) a sensory/input form of IOR when eye movements are actively suppressed and there is repeated peripheral stimulation, and (2) a motor/output form of IOR when the oculomotor system is activated by requiring eye movements to either the cue or target (Chica, Taylor, Lupiáñez, & Klein, 2010; Hilchey et al., 2014; Satel, Hilchey, Wang, Story, & Klein, 2013; Smith, Schenk, & Rorden, 2012; Sumner, Nachev, Vora, Husain, & Kennard, 2004; Taylor & Klein, 2000; Wang, Satel, & Klein, 2012). It is unclear, however, whether these two forms of ICE are completely independent and mutually exclusive or partially overlapping mechanisms.
Pro- and antilocalization
The notion that an activated oculomotor system automatically generates an output-based form of ICE has been challenged by several studies that used antisaccade tasks (Fecteau, Au, Armstrong, & Munoz, 2004; Khatoon, Briand, & Sereno, 2002; Rafal, Egly, & Rhodes, 1994). These tasks require participants to look away from the target to a laterally opposite location (antisaccade) rather than moving to the target (prosaccade). Unlike prosaccades that are primarily reflexive responses to stimuli, antisaccades are programmed volitionally. Making an antisaccade entails the suppression of reflexive eye movements to a peripheral stimulus followed by a voluntary eye movement toward the opposite location (Hallett & Adams, 1980). Compared to prosaccades, antisaccade latencies are generally slower and more error prone (see Hutton & Ettinger, 2006, for a review) as a consequence of the competition between reflexive and intentional responses that needs to be resolved (Massen, 2004).
With respect to ICEs, the antisaccade task dissociates the location of the target with that of the motor response (spatially incompatible stimulus-response mappings), thereby allowing examination of whether an observed ICE is closer to an input or output form of inhibition, or a conjunction of both. In other words, if an ICE primarily affects input/sensory processes, reaction times (RTs) should be delayed when the cue and the target appear at the same location, regardless of whether the task is to make a pro- or antisaccade. In contrast, if the ICE primarily affects output/motoric processes, RTs should be delayed in the antisaccade task when the target appears at an uncued location, because responses are made to the previously cued location.
Although many studies have found ICEs with prosaccades, only a few studies have observed ICEs with an antisaccade task (Fecteau et al., 2004; Khatoon et al., 2002; Rafal et al., 1994). Rafal et al. (1994, Exp. 2) tested, in separate blocks, pro- and antisaccade responses by investigating IOR when the reflexive oculomotor system was inhibited (by prohibiting eye movements during peripheral cueing) and when endogenously generated saccades activated the oculomotor system (by requiring eye movements during central cueing). Similarly, Khatoon et al. (2002, Exp. 2) tested pro- and antisaccades, in separate blocks, with an uninformative cue. Fecteau et al. (2004, Exp. 3) implemented a target–target design with the color of the fixation point indicating whether a pro- or antisaccade should be initiated in response to the target. All three of these experiments observed ICEs at the target stimulus location in anti- as well as prosaccade conditions. The available evidence is thus in favor of the ICE generated in antisaccade tasks being closer to the input form of IOR, because responses tend to be slowest when the cue and the target appear at the same location.
Manual and saccadic responses
One hypothesis put forth by Klein and Hilchey (2011) posited that the form of IOR generated is contingent upon the type of eye movement (pro- or antisaccade). They proposed that it is not simply the inactivation of reflexive eye movements but rather the active suppression of the oculomotor system that contributes to the generation of the input form of IOR. The antisaccade task, while requiring eye movements, also necessitates the suppression of mechanisms underlying the generation of reflexive saccades for the task to be performed correctly.
In addition to pro- and antisaccade responses, manual localization key presses (Briand, Larrison, & Sereno, 2000; Fischer, Pratt, & Neggers, 2003; Hunt & Kingstone, 2003; Pratt & Neggers, 2008; Reuter-Lorenz, Jha, & Rosenquist, 1996; Satel & Wang, 2012; Taylor & Klein, 2000) have also been used to investigate the dissociation between sensory/input and motor/output ICEs. The rationale behind this approach is that underlying spatial cueing (and spatial attention more generally) is a spatial map that provides a representation of visual space. For each response modality, the location of the target must be encoded (i.e., localization must occur) before a correct manual or saccadic response can be made. If IOR is primarily a sensory-based input process, it should not be affected by response modality—whether saccadic or manual responses are required—as long as both require spatial localization of the target. On the other hand, if IOR is primarily a motor-based output process, it should be affected by response modality, because the maps used to code for saccadic and manual responses are likely functionally distinct.
One early study of IOR that compared manual and saccadic responses observed no difference between them (Reuter-Lorenz et al., 1996). Briand et al. (2000) also found equivalent IOR in manual and saccadic responses in terms of magnitude, but, interestingly, the development from facilitation to inhibition differed across the two response modalities. Unlike Reuter-Lorenz et al. (1996), who used only relatively long CTOAs of 1,000 and 1,300 ms, Briand et al. (2000) used a range of CTOAs from 67 to 1,000 ms to examine the time course of IOR. This allowed them to observe a faster rate of decline for saccadic responses, such that IOR developed sooner for saccadic responses than for manual responses. The dissociation between saccadic and manual responses, particularly with inhibition overpowering facilitation at earlier CTOAs for saccadic responses, provides evidence for IOR as an oculomotor-based output process.
The present study
The goal of the present study was to tease apart further differential effects of pro- and antilocalizations with manual and saccadic responses to investigate how sensory (input) and oculomotor (output) processes contribute to ICEs. In our experiments, participants made either manual or saccadic responses to targets in cued and uncued trials of a pro- or antilocalization task. However, since it is unclear whether manual “localization” responses are equivalent in nature to saccadic localization responses, we will from here on use the terms compatible and incompatible to refer to prolocalization and antilocalization, respectively (as in Wascher, Schneider, & Hoffmann, 2015). Participants in all experiments completed a spatial cueing task (peripheral cue: maintain fixation, peripheral target: manual or saccadic response) in which they had to respond to targets with either a compatible or incompatible action, depending on instructions presented during each trial. The incompatible stimulus-response mapping task is thus able to dissociate sensory and motor processes, by separating the locations of the stimulus and the response. By removing repeated peripheral stimulation at the target location in the incompatible tasks, any ICE observed can be better attributed to oculomotor processing rather than to sensory processing. Furthermore, since manual responses require an actively inhibited oculomotor system—whereas eye movements are required on every trial in the saccadic response tasks—we can test the two-forms theory of IOR as well as examining the effects of response modality to further clarify discrepancies found in previous studies.
In line with previous work, saccadic responses should overall be faster than manual responses (Briand et al., 2000), as should compatible compared to incompatible tasks (Massen, 2004; Olk & Kingstone, 2003). We also expect to find ICEs in all compatible tasks, regardless of the response modality (Briand et al., 2000; Hunt & Kingstone, 2003). How ICEs interact with incompatible tasks and response modality, however, remains uncertain. If IOR is largely input based, we would expect to find equivalent ICEs in both compatible and incompatible tasks with manual responses. Similarly, with saccadic responses, we would expect to reproduce previous findings that ICEs affect the target stimulus location, not the unstimulated location to which a response is made.
Method
Participants
Fifty-eight undergraduate students from University of Nottingham–Malaysia Campus participated in this study in exchange for course credit or nonmonetary compensation. Data from three participants who completed less than 75% of the experiment were excluded from the analyses. A further four participants were removed because of error rates exceeding 10%, and two others were removed because their mean RTs were 3 standard deviations beyond the overall mean. Mean age of the remaining 49 participants (29 females, 45 right-handed) was 21.0 years. All reported normal or corrected-to-normal vision and no known neurological or psychiatric illness.
Stimuli and apparatus
Participants were seated in a dimly lit room during testing, with their head positioned on a chin-rest approximately 57 cm away from the display monitor. A 64-bit Windows 7 computer with a 3.4 GHz CPU and 8 GB of RAM running Python scripts was used for stimulus presentation and recording of behavioral data. Stimuli were presented on a 24-inch BenQ gaming monitor, and participants made manual responses on a standard QWERTY keyboard. A desktop-mounted eye-tracking system (EyeLink 1000 Plus) from SR Research was used to monitor participants’ eye movements throughout the experiment at a sampling rate of 1000 Hz. Saccadic response times (SRTs) to targets were recorded using the eye tracker, whereas manual response times (MRTs) were recorded via the keyboard.
Except for the “cue-back” fixation crosses, all stimuli were presented in white against a black background, with two peripheral boxes (4.5° × 4.5°, visual angle) presented as placeholders 8.7° to the left and right of the center along the horizontal meridian and a fixation cross (0.8° × 0.8°) that appeared at the center of the screen. Cues were presented as a highlighting (increased thickness) of one of the peripheral boxes, whereas cue backs appeared as either a green or red fixation cross (0.8° × 0.8°), with the color indicating whether the current trial was a compatible or incompatible trial, respectively. Participants responded to targets that were filled circles (2.4° in diameter) inside one of the peripheral boxes. A five-point calibration and validation procedure was performed on every participant prior to the experiment to ensure that the eye-tracking precision was within one degree of visual angle.
Design and procedure
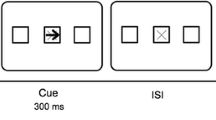
Figure 1 illustrates the sequence of events of a trial. For all four experiments, each trial began with a drift correction in which participants fixated on a small circle at the center of the screen, followed by a fixation cross that appeared on the screen for 500 ms. An uninformative cue came on immediately after and stayed on the screen for 300 ms. After a 400 ms delay, a green or red cue-back fixation cross flashed for 300 ms, indicating whether a compatible or incompatible response should be made to the upcoming target. There was another 500 ms delay prior to displaying the target, which participants were instructed to respond as quickly and accurately as possible. Trials ended as soon as a response was made or after 3,000 ms had elapsed, whichever came first.
Experimental paradigm used. Cues were uninformative of the subsequent location at which targets appeared. A green cue back called for (a) a compatible response to the target, whereas a red cue back called for (b) an incompatible response. Trials were considered (c) cued if the cue and target appeared at the same location and (d) uncued if the cue and target appeared at opposite locations (Color figure online)
Experiments E1 and E3 used a mixed design, with 600 intermixed compatible and incompatible trials per participant, separated into four blocks of 150 trials, with short breaks between the blocks and a five-point calibration preceding each block. Seventy-five compatible trials were cued to the left and 75 more cued to the right, making up 150 cued compatible trials. Similarly, 75 compatible trials were uncued with left targets and 75 more were uncued with right targets, making up 150 uncued compatible trials. The same ratios applied to incompatible trials. Trials were considered cued trials when cues and targets appeared at the same location and uncued when they appeared on opposite sides, regardless of the localization task required.
Experiments E2 and E4 (blocked) were identical to E1 and E3 (mixed) with the following exceptions: (1) the number of trials was reduced to a total of 320 trials that were split into four blocks of 80 trials to facilitate data collection, and (2) compatible and incompatible trials were blocked separately and counterbalanced, such that half of the participants completed two blocks of compatible trials followed by two blocks of incompatible trials, whereas the other half performed the blocks in the opposite order.
E1 and E2 (manual responses) required participants to stay fixated throughout each entire trial and make a manual response on the keyboard (“Z” for left, “/” for right) when the target appeared. E3 and E4 (saccadic responses) required participants to maintain central fixation until the target appeared and then make a saccade in response to the target. In all experiments, trials were abruptly terminated (with an error message presented on the screen) and randomly recycled if the participant’s gaze position deviated by more than 3° from the fixation stimulus at any point during a trial. In E3 and E4 (saccadic responses), however, saccades were allowed to valid response locations (target’s location or the opposite side, depending on localization task) within a 3° visual angle region centered in placeholders upon a target’s appearance, and any incorrect responses after target appearance were counted as errors.
Results
Trials with incorrect responses (3.65%), anticipatory responses (RTs faster than 2.5 median absolute deviation; MADFootnote 1 below the median of each subject for each factor level; 0.45%) and slow responses (RTs slower than 2.5 MAD above the median of each subject for each factor level; 6.74%) were removed prior to statistical analyses.
However, we further examined the incorrect responses to examine whether there was a speed–accuracy tradeoff. A 2 × 2 ANOVA on error rates revealed main effects of experimental design, F(1, 23) = 7.21, MSE = 16.21, p = .013, η2 = .24, where the mixed design showed a higher error rate (M = 4.03) than the blocked design (M = 2.29), and task condition, F(1, 23) = 16.96, MSE = 13.49, p < .001, η2 = .42, with a higher error rate in the incompatible conditions (M = 4.54) than the compatible conditions (M = 2.27). There was no significant interaction between the two factors.
The mean RTs for each level of cueing (cued vs. uncued), experimental design (mixed vs. blocked), response type (manual vs. saccade), and task condition (compatible vs. incompatible) can be found in Table 1. Cueing effects were calculated as the difference between RTs to cued and uncued targets (cued – uncued), with positive values representing inhibition, and are plotted in Fig. 2.
Manual responses omnibus
The results were first analyzed with a 2 × 2 × 2 repeated-measures omnibus ANOVA for the manual experiments (E1 and E2), with experimental design (2 levels: mixed vs. blocked) as a between-subjects factor and task condition (2 levels: compatible vs. incompatible) and cueing (2 levels: cued vs. uncued) as within-subjects factors. This ANOVA resulted in a main effect of task condition, F(1, 22) = 48.84, MSE = 1022.11, p < .001, η2 = 0.69, because compatible responses were faster than incompatible responses, and a main effect of cueing, F(1, 22) = 34.78, MSE = 213.65, p < .001, η2 = 0.61, because cued trials were overall slower than uncued trials, but no main effect of experimental design. There was also a three-way interaction between Experimental Design × Task Condition × Cueing, F(1, 22) = 5.01, MSE = 102.88, p = .036, η2 = 0.19, where ICEs were similar in the incompatible condition whether blocked or mixed, but ICEs were increased for blocked trials relative to mixed trials in the compatible condition.
Saccadic responses omnibus
A 2 ×2 × 2 repeated-measures omnibus ANOVA for the saccadic response experiments (E3 and E4) showed significant main effects of task condition, F(1, 23) = 64.15, MSE = 1263.14, p < .001, η2 = 0.74, because compatible responses were faster than incompatible responses, cueing, F(1, 23) = 21.64, MSE = 253.72, p < .001, η2 = 0.49, because cued trials were slower than uncued trials, and experimental design, F(1, 23) = 9.13, MSE = 8812.76, p = .006, η2 = 0.28, because overall SRTs for blocked trials were slower than SRTs for mixed trials. The two-way interaction between Task Condition × Cueing was significant, F(1, 23) = 70.21, MSE = 184.56, p < .001, η2 = 0.75, as cued trials were only responded to slower than uncued trials in the compatible condition. The two-way interaction between Experimental Design × Cueing was significant too, F(1, 23) = 7.60, MSE = 253.72, p = .011, η2 = 0.25, where responses to uncued trials were much faster than responses to cued trials in blocked conditions, but only slightly faster when trials were mixed. No other two- or three-way interactions were observed.
Manual responses
Next, 2 × 2 ANOVAs were performed on mean RTs separately for mixed and blocked experimental designs for each response type (manual and saccadic). In the manual mixed-design experiment (E1), there were significant main effects of task condition, F(1, 11) = 26.32, MSE = 799.81, p < .001, η2 = 0.71, and cueing, F(1, 11) = 16.08, MSE = 132.86, p = .002, η2 = 0.59, as well as a significant interaction between task condition and cueing, F(1, 11) = 9.48, MSE = 28.38, p = .010, η2 = 0.46, in which participants recorded overall faster responses in compatible tasks relative to incompatible tasks, and MRTs to uncued trials were faster than MRTs to cued trials. The interaction was significant, because the ICE was larger for incompatible than for compatible tasks. We further performed planned comparisons (two-tailed paired-samples t tests) which revealed that, for manual responses in the compatible task, there were significant differences between cued and uncued trials in the mixed-design experiment (9 ms), t(11) = 2.34, p = .040, d = 0.68, indicating a significant ICE. Results from a similar two-tailed paired-samples t test showed that this effect doubled in magnitude for the incompatible task (18 ms), t(11) = 4.95, p < .001, d = 1.43.
In the manual blocked-design experiment (E2), the ANOVA revealed significant main effects of task condition, F(1, 11) = 23.47, MSE = 1244.41, p < .001, η2 = 0.68, and cueing, F(1, 11) = 19.45, MSE = 294.44, p = .001, η2 = 0.64, where, again, compatible tasks were performed faster and an ICE was apparent. There was no interaction between task condition and cueing. Planned comparisons for the blocked-design experiment further revealed significant differences in manual responses to cued and uncued trials for compatible (26 ms), t(11) = 4.33, p = .001, d = 1.25, and incompatible tasks (17 ms), t(11) = 2.69, p = .021, d = 0.78, both showing robust ICEs.
Saccadic responses
In the saccadic mixed-design experiment (E3), the main effect of task condition was significant, F(1, 11) = 130.08, MSE = 215.30, p < .001, η2 = 0.92, but not that of cueing. The main effect of task condition was caused by the fact that compatible tasks had smaller SRTs than incompatible tasks. There was also a significant Task Condition × Cueing interaction, F(1, 11) = 90.18, MSE = 47.97, p < .001, η2 = 0.89, as an ICE was present in compatible tasks but absent in incompatible tasks, where responses to uncued trials were slower than responses to cued trials (i.e., facilitation). As before, planned comparisons were used to evaluate differences between cued and uncued trials for saccadic responses in compatible tasks (25 ms), t(11) = 5.82, p < .001, d = 1.68, and incompatible tasks (-13 ms), t(11) = -3.86, p = .003, d = 1.11, in the mixed-design experiment (E3), where an ICE was found in the former but facilitation was observed in the latter.
The ANOVA on its blocked-design counterpart (E4) revealed a main effect of both task condition, F(1, 12) = 24.62, MSE = 2223.66, p < .001, η2 = 0.67, and cueing, F(1, 12) = 19.21, MSE = 366.17, p < .001, η2 = 0.62, as well as a Task Condition × Cueing interaction, F(1, 12) = 28.93, MSE = 309.76, p < .001, η2 = 0.71, showing that mean SRTs for compatible tasks were smaller than those for incompatible tasks, mean SRTs for uncued trials were overall smaller than those for cued trials, and the difference between mean SRTs for uncued and cued trials was larger in the compatible than the incompatible tasks. Finally, in the blocked saccade experiment (E4), planned comparisons revealed that responses to uncued trials were significantly faster than cued trials when saccadic responses were made in compatible tasks (50 ms), t(12) = 5.19, p < .001, d = 1.44, showing an ICE that was not evident in incompatible tasks (-3 ms), t(12) = -0.83, p = .421, d = 0.23.
The results demonstrate an ICE regardless of response modality when the task was compatible. For incompatible tasks, an ICE also occurred for manual responses regardless of whether the task was mixed or blocked, but there were no ICEs when saccadic responses were made. Instead, for incompatible saccadic responses, we observed facilitation in the mixed-design experiment and no cueing effect whatsoever in the blocked-design experiment.
Discussion
Three primary findings emerge from this study. First, our study replicated previous findings: manual responses were consistently slower than saccadic responses (Briand et al., 2000), incompatible responses were slower than compatible responses whether they were manual or saccadic (Massen, 2004; Olk & Kingstone, 2003), and, in all of the compatible conditions, our results replicated past studies that have shown ICEs with both manual and saccadic responses (Briand et al., 2000; Hunt & Kingstone, 2003). These results serve to validate our experimental paradigm prior to assessing differences between manual and saccadic incompatible responses.
Second, the results in E2 replicate and expand upon those observed by Wascher et al. (2015) who found equivalent magnitudes of ICEs with manual responses for both compatible and incompatible trials when the two conditions were blocked. We found similar ICEs for manual incompatible responses, even when the trials were randomly intermixed in E1. Our study also used longer cue and target onsets than Wascher et al. did, a fixed CTOA of 1,500 ms versus their time course design (with CTOAs ranging from 80 to 1,240 ms), and a cue-back coloration to indicate response type—providing further validation that ICEs are observed with incompatible manual responses.
Third, the results in E4 failed to replicate those of previous studies where ICEs were observed with antisaccades (Fecteau et al., 2004; Khatoon et al., 2002; Rafal et al., 1994). However, these experiments were sufficiently different methodologically to suggest that different cueing effects could have been generated. Fecteau et al. (2004) used a target–target paradigm with a longer period of time between each target’s appearance (3,700 ms) where subjects made either pro- or antisaccades in response to targets depending on the color of the fixation point. Although they also observed an ICE for antisaccades, this paradigm is quite different from our own in these respects. In Rafal et al. (1994), where pro- and antisaccade trials were blocked, IOR was generated even with inhibition of the reflexive oculomotor system when no eye movements were allowed. Although there was an overall cueing effect where saccade latencies to cued targets were slower than those to uncued targets, Rafal et al. found no interaction between task condition and cueing. In other words, both pro- and antisaccades were slowest when the target appeared at cued locations, implying that IOR acted only by inhibiting detection of targets at tagged locations, thereby lending support to an input/sensory form of ICE being activated in this task. Our results contradict those because we found ICEs with prosaccades but not with antisaccades, which is consistent with an output/oculomotor form of ICE being generated (Hilchey et al., 2014) whereby a motor bias favors saccades directed away from the previously cued location. One potential explanation for these conflicting results lies in a difference in RT calculation across studies; whereas we analyzed mean RTs in a traditional manner, Rafal et al. did not report any RTs or the results of tests directly comparing cued and uncued conditions. They first determined the median for each participant and then analyzed the means of the median RTs. Khatoon et al. (2002) also only blocked their conditions, and furthermore included a third condition that used an indirect vertical eye-movement response. This was a time course study, wherein a very short onset cue (27 ms) was followed by a range of five CTOAs between 67 to 1,000 ms, resulting in only 12 trials per cell. Although an ICE was observed at the longest CTOA for antisaccades (16 ms), it was substantially smaller than that for prosaccades (31 ms).
The same experiment, when examined with mixed trials, adds an extra level of complexity to the task. Unlike prosaccades, subsequent programming of antisaccades did not suffer from inhibition of return to the cued targets. Instead, facilitation was observed when antisaccade responses were made with mixed trials (E3) and no cueing effect was observed when trials were blocked (E4). The element of uncertainty in mixed trials conceivably places greater demands on attentional resources as participants had to randomly switch back and forth from pro- and antisaccades. On top of the already difficult task, there is an additional memory component involved as participants in the mixed-design experiments also had to remember that the green cue back indicated compatible, whereas the red cue back indicated incompatible trials.
We know that stronger attentional control settings result in a delayed onset of inhibition for more difficult tasks from the works of Lupiáñez and colleagues (Lupiáñez, Milán, Tornay, Madrid, & Tudela, 1997; Lupiáñez & Milliken, 1999; Lupiáñez, Milliken, Solano, Weaver, & Tipper, 2001). For example, in a color discrimination task where one responded depending on the color of the target, Lupiáñez et al. (1997) showed that inhibition for the more difficult discrimination task begins at a later CTOA (of around 700 ms) than does inhibition for simpler detection tasks. Similarly, Lupiáñez et al. (2001) found that inhibition emerged at a 700 ms CTOA when discriminating between X and O, which was further delayed until 1,000 ms postcue in a more challenging discrimination task between M and N. Furthermore, when comparing regular and perceptually degraded targets that are more difficult to ascertain, Castel, Pratt, Chasteen, and Scialfa (2005) found that the onset of inhibition occurred later for harder detection tasks as well. Given the complexity of our task, inhibition could be incredibly delayed when pro- and antisaccade trials are intermixed, but only moderately so for the blocked trials.
It is worth mentioning that we observed a paradoxical improvement in terms of saccade latencies when pro- and antisaccades were mixed compared to blocked. Most current models of task switching describe a drop in performance, also known as time cost from switching between tasks (e.g., Meiran, Chorev, & Sapir, 2000; Rogers & Monsell, 1995; Wylie & Allport, 2000), and a similar mixing cost was expected in our experiments due to greater uncertainty when faced with randomly intermixed trials (Los, 1996, 1999a, 1999b). Cherkasova, Manoach, Intriligator, and Barton (2002) examined residual switch cost by comparing antisaccade latencies and error rates between switch and repeated trials in a randomized mixed block, but instead of a performance cost, antisaccade latencies were reduced when tasks were mixed. They reported a correlation between this improvement and indices of vigilance. This finding is in line with the notion of goal neglect, where certain tasks may not be adequately performed despite participants having understood instructions of the task (De Jong, Berendsen, & Cools, 1999; Nieuwenhuis, Broerse, Nielen, & De Jong, 2004). In the context of our results, the paradoxical decrease in saccade latency could thus be a result of allocating more attentional resources to the task requirements when pro- and antisaccade trials are intermixed and randomly presented compared to blocked trials that could require more internal effort to consistently focus on the task at hand. Alternatively, this effect could simply be the result of a speed–accuracy tradeoff. In the mixed design, with both saccadic and manual responses, accuracy is much worse than it is in the blocked design. That is, when pro- and antiresponses are mixed, responses are both faster and less accurate than when blocked.
In a variant of our study recently conducted by Hilchey, Dohmen, Crowder, and Klein (2016), ICEs were generated by pro- or anti-saccades to peripheral cues and measured with spatially congruent manual localization responses to central arrow targets (also see Redden, Hilchey, & Klein, 2016, for responses to peripheral targets). Indeed, with oculomotor activation during prosaccades, they found a robust ICE that was predominantly output based. Antisaccades, however, generated an ICE that was closer to the input form and could not be measured with manual responses to central targets. This finding is in accordance with Klein and Hilchey’s (2011) hypothesis of an input-based form of IOR that develops upon oculomotor suppression, but it does not explain the pattern of results presented here where an ICE was observed with incompatible responses when the oculomotor system was suppressed (in the manual incompatible condition), but not when activated (in the saccadic incompatible condition).
Since the generation of an antisaccade necessarily suppresses the oculomotor system (because a reflexive saccade must be suppressed to perform the task correctly), the absence of an observed ICE with antisaccadic responses suggests that an input form of IOR was combined with an output form of IOR in an additive manner. Such additivity of input-based and output-based ICEs was previously observed and suggested by Wang et al. (2012) and Satel and Wang (2012). However, the presence of an ICE with manual responses even though the oculomotor system was actively suppressed in that task as well (no eye movements were made) suggests that there is more to the generation of ICEs than simply sensory and oculomotor activation.
Alternatively, in light of recent studies that have brought unique evidence to the scholarship of IOR by challenging existing theories of attentional and oculomotor IOR, a “new” form of late ICE has been shown to emerge from reaching movements (Chang & Ro, 2005; Cowper-Smith, Eskes, & Westwood, 2013; Cowper-Smith & Westwood, 2013; Neyedli & Welsh, 2012). Cowper-Smith and Westwood (2013) conducted a test of purely motoric IOR by presenting targets on either the horizontal or vertical meridian in a target–target paradigm. Participants were required to use their index finger to point to the targets while maintaining fixation at a central location throughout each trial (to eliminate the contribution of an oculomotor ICE) and were given sufficiently long intervals to prepare for making a response (to remove the contribution of early sensory ICEs). Consistent with the idea of a separate motoric ICE, the authors observed longer RTs to cued targets than to uncued targets. This alternative motor form of ICE could potentially be contributing to the ICE observed in our incompatible task where manual responses were made by pressing spatially congruent and incongruent keys. This account would explain why an ICE was absent in the saccadic incompatible condition.
Although we do not yet know where in the brain this motor form of ICE is induced or which pathways it traverses, psychophysical studies have shown a dissociation between the effects of saccadic and manual responses on ICEs. For example, Sumner et al. (2004) investigated whether ICEs were mediated by the retinotectal pathway, which involves the superficial layer of the superior colliculus (SC; Munoz, Armstrong, & Coe, 2007) or by cortical structures in more endogenous pathways. This possibility was tested using stimuli that are only visible to short-wave sensitive cones (S-cones) that do not project to the SC (bypassed the SC), and typical stimuli that project to the SC (did not bypass the SC). Importantly, they asked the participants to make both saccadic and manual localization responses. They found an ICE in the manual localization condition regardless of whether the stimuli bypassed the SC, but only found an ICE in the saccadic condition when the stimuli did not bypass the SC. Based on this finding, Sumner and colleagues (2004) concluded that there are two dissociable generators of ICEs, namely a retinotectal generator in the SC, which causes saccadic ICEs, and a cortical generator in the cortical pathway, which contributes to both saccadic and manual behavioral ICEs (see Sumner, 2006, for supporting evidence).
This explanation is in accordance with the two-forms theory of IOR (Taylor & Klein, 2000), which suggests that there are two dissociable forms of IOR depending on whether the oculomotor system is actively suppressed (input/sensory) or engaged (output/motoric). That is, the retinotectal generator will primarily mediate the output/motoric based form of IOR, whereas the cortical generator will primarily mediate the input/sensory based form of IOR. This account could explain the overall slower responses in performing manual localization tasks as well as the presence of an ICE for compatible trials. This ICE was overshadowed by facilitation in incompatible trials, because inhibition generated from the cortical pathway starts later.
We have laid out a number of interpretations to our present findings that ICEs are produced in all tasks except incompatible responses with saccades. Neither solely an output-based nor input-based explanation can account for IOR occurring with manual incompatible but not saccadic incompatible responses, which suggests that differential mechanisms underlie the generation of ICEs that further interact with the response modality.
Notes
MAD is used instead of standard deviation because response time data is presumably skewed by outlying data points and thus the use of mean/standard deviation is unreliable as it is particularly sensitive to outliers. See Leys, Ley, Klein, Bernard, and Licata (2013) for evidence demonstrating the robustness of MAD compared to standard deviation for outlier removal.
References
Briand, K. A., Larrison, A. L., & Sereno, A. B. (2000). Inhibition of return in manual and saccadic response systems. Perception & Psychophysics, 62, 1512–1524. doi:10.3758/BF03212152
Castel, A. D., Pratt, J., Chasteen, A. L., & Scialfa, C. T. (2005). Examining task difficulty and the time course of inhibition of return: Detecting perceptually degraded targets. Canadian Journal of Experimental Psychology, 59, 90–98. doi:10.1037/h0087464
Chang, E., & Ro, T. (2005). Inhibition of return in perception and action. Visual Cognition, 12, 443–472. doi:10.1080/13506280444000391
Cherkasova, M. V., Manoach, D. S., Intriligator, J. M., & Barton, J. J. (2002). Antisaccades and task-switching: Interactions in controlled processing. Experimental Brain Research, 144, 528–537. doi:10.1007/s00221-002-1075-z
Chica, A. B., Taylor, T. L., Lupiáñez, J., & Klein, R. M. (2010). Two mechanisms underlying inhibition of return. Experimental Brain Research, 201, 25–35. doi:10.1007/s00221-009-2004-1
Cowper-Smith, C. D., Eskes, G. A., & Westwood, D. A. (2013). Motor inhibition of return can affect prepared reaching movements. Neuroscience Letters, 541, 83–86. doi:10.1016/j.neulet.2013.02.033
Cowper-Smith, C. D., & Westwood, D. A. (2013). Motor IOR revealed for reaching. Attention, Perception, & Psychophysics, 75, 1914–1922. doi:10.3758/s13414-013-0528-8
De Jong, R., Berendsen, E., & Cools, R. (1999). Goal neglect and inhibitory limitations: Dissociable causes of interference effects in conflict situations. Acta Psychologica, 101, 379–394. doi:10.1016/S0001-6918(99)00012-8
Dukewich, K. R., & Klein, R. M. (2015). Inhibition of return: A phenomenon in search of a definition and a theoretical framework. Attention, Perception, & Psychophysics, 77, 1647–1658. doi:10.3758/s13414-015-0835-3
Fecteau, J. H., Au, C., Armstrong, I. T., & Munoz, D. P. (2004). Sensory biases produce alternation advantage found in sequential saccadic eye movement tasks. Experimental Brain Research, 159, 84–91. doi:10.1007/s00221-004-1935-9
Fischer, M. H., Pratt, J., & Neggers, S. F. (2003). Inhibition of return and manual pointing movements. Perception & Psychophysics, 65, 379–387. doi:10.3758/BF03194569
Hallett, P. E., & Adams, B. D. (1980). The predictability of saccadic latency in a novel voluntary oculomotor task. Vision Research, 20, 329–339. doi:10.1016/0042-6989(80)90019-X
Hilchey, M. D., Dohmen, D., Crowder, N. A., & Klein, R. M. (2016). When is inhibition of return input- or output-based? It depends on how you look at it. Canadian Journal of Experimental Psychology, 70, 325–334. doi:10.1037/cep0000075
Hilchey, M. D., Klein, R. M., & Satel, J. (2014). Returning to “inhibition of return” by dissociating long-term oculomotor IOR from short-term sensory adaptation and other nonoculomotor “inhibitory” cueing effects. Journal of Experimental Psychology: Human Perception and Performance, 40, 1603–1616. doi:10.1037/a0036859
Hunt, A. R., & Kingstone, A. (2003). Inhibition of return: Dissociating attentional and oculomotor components. Journal of Experimental Psychology: Human Perception and Performance, 29, 1068–1074. doi:10.1037/0096-1523.29.5.1068
Hutton, S. B., & Ettinger, U. (2006). The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology, 43, 302–313. doi:10.1111/j.1469-8986.2006.00403.x
Khatoon, S., Briand, K. A., & Sereno, A. B. (2002). The role of response in spatial attention: Direct versus indirect stimulus-response mappings. Vision Research, 42, 2693–2708. doi:10.1016/S0042-6989(02)00327-9
Klein, R. M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4, 138–147. doi:10.1016/S1364-6613(00)01452-2
Klein, R. M., & Hilchey, M. D. (2011). Oculomotor inhibition of return. In S. P. Liversedge, I. D. Gilchrist, & S. Everling (Eds.), The Oxford handbook of eye movements (pp. 471–492). Oxford, UK: Oxford University Press.
Leys, C., Ley, C., Klein, O., Bernard, P., & Licata, L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49, 764–766. doi:10.1016/j.jesp.2013.03.013
Los, S. A. (1996). On the origin of mixing costs: Exploring information processing in pure and mixed blocks of trials. Acta Psychologica, 94, 145–188. doi:10.1016/0001-6918(95)00050-x
Los, S. A. (1999a). Identifying stimuli of different perceptual categories in mixed blocks of trials: Evidence for cost in switching between computational processes. Journal of Experimental Psychology: Human Perception and Performance, 25, 3–23. doi:10.1037/0096-1523.25.1.3
Los, S. A. (1999b). Identifying stimuli of different perceptual categories in pure and mixed blocks of trials: Evidence for stimulus-driven switch costs. Acta Psychologica, 103, 173–205. doi:10.1016/S0001-6918(99)00031-1
Lupiáñez, J., Milán, E. G., Tornay, F. J., Madrid, E., & Tudela, P. (1997). Does IOR occur in discrimination tasks? Yes, it does, but later. Perception & Psychophysics, 59, 1241–1254. doi:10.3758/BF03214211
Lupiáñez, J., & Milliken, B. (1999). Inhibition of return and the attentional set for integrating versus differentiating information. Journal of General Psychology, 126, 392–418. doi:10.1080/00221309909595373
Lupiáñez, J., Milliken, B., Solano, C., Weaver, B., & Tipper, S. P. (2001). On the strategic modulation of the time course of facilitation and inhibition of return. The Quarterly Journal of Experimental Psychology. A, 54, 753–773. doi:10.1080/713755990
Massen, C. (2004). Parallel programming of exogenous and endogenous components in the antisaccade task. The Quarterly Journal of Experimental Psychology. A, 57, 475–498. doi:10.1080/02724980343000341
Meiran, N., Chorev, Z., & Sapir, A. (2000). Component processes in task switching. Cognitive Psychology, 41, 211–253. doi:10.1006/cogp.2000.0736
Munoz, D. P., Armstrong, I., & Coe, B. (2007). Using eye movements to probe development and dysfunction. In R. P. G. van Gompel, M. H. Fischer, W. S. Murray, & R. L. Hill (Eds.), Eye movements: A window on mind and brain (pp. 99–124). Amsterdam, Netherlands: Elsevier.
Neyedli, H. F., & Welsh, T. N. (2012). The processes of facilitation and inhibition in a cue-target paradigm: Insight from movement trajectory deviations. Acta Psychologica, 139, 159–165. doi:10.1016/j.actpsy.2011.11.001
Nieuwenhuis, S., Broerse, A., Nielen, M. M. A., & De Jong, R. (2004). A goal activation approach to the study of executive function: An application to antisaccade tasks. Brain and Cognition, 56, 198–214. doi:10.1016/j.bandc.2003.12.002
Olk, B., & Kingstone, A. (2003). Why are antisaccades slower than prosaccades? A novel finding using a new paradigm. NeuroReport, 14, 151–155. doi:10.1097/00001756-200301200-00028
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25. doi:10.1080/00335558008248231
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. Bouwhuis (Eds.), Attention and performance X: Control of language processes (pp. 531–556). London, UK: Erlbaum.
Posner, M. I., Rafal, R. D., Choate, I. S., & Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2, 211–228. doi:10.1080/02643298508252866
Pratt, J., & Neggers, B. (2008). Inhibition of return in single and dual tasks: Examining saccadic, keypress, and pointing responses. Perception & Psychophysics, 70, 257–265. doi:10.3758/PP.70.2.257
Rafal, R., Egly, R., & Rhodes, D. (1994). Effects of inhibition of return on voluntary and visually guided saccades. Canadian Journal of Experimental Psychology, 48, 284–300. doi:10.1037/1196-1961.48.2.284
Redden, R. S., Hilchey, M. D., & Klein, R. M. (2016). Peripheral stimuli generate different forms of inhibition of return when participants make prosaccades versus antisaccades to them. Attention, Perception, & Psychophysics, 78, 2283–2291. doi:10.3758/s13414-016-1175-7
Reuter-Lorenz, P. A., Jha, A. P., & Rosenquist, J. N. (1996). What is inhibited in inhibition of return. Journal of Experimental Psychology: Human Perception and Performance, 22, 367–378. doi:10.1037/0096-1523.22.2.367
Rogers, R. D., & Monsell, S. (1995). The cost of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General, 124, 207–231. doi:10.1037/0096-3445.124.2.207
Satel, J., Hilchey, M. D., Wang, Z., Story, R., & Klein, R. M. (2013). The effects of ignored versus foveated cues upon inhibition of return: An event-related potential study. Attention, Perception, & Psychophysics, 75, 29–40. doi:10.3758/s13414-012-0381-1
Satel, J., & Wang, Z. (2012). Investigating a two causes theory of inhibition of return. Experimental Brain Research, 223, 469–478. doi:10.1007/s00221-012-3274-6
Smith, D. T., Schenk, T., & Rorden, C. (2012). Saccade preparation is required for exogenous attention but not endogenous attention or IOR. Journal of Experimental Psychology: Human Perception and Performance, 38, 1438–1447. doi:10.1037/a0027794
Sumner, P. (2006). Inhibition versus attentional momentum in cortical and collicular mechanisms of IOR. Cognitive Neuropsychology, 23, 1035–1048. doi:10.1080/02643290600588350
Sumner, P., Nachev, P., Vora, N., Husain, M., & Kennard, C. (2004). Distinct cortical and collicular mechanisms of inhibition of return revealed with S cone stimuli. Current Biology, 14, 2259–2263. doi:10.1016/j.cub.2004.12.021
Taylor, T. L., & Klein, R. M. (2000). Visual and motor effects in inhibition of return. Journal of Experimental Psychology: Human Perception and Performance, 26, 1639–1656. doi:10.1037/0096-1523.26.5.1639
Wang, Z., Satel, J., & Klein, R. M. (2012). Sensory and motor mechanisms of oculomotor inhibition of return. Experimental Brain Research, 218, 441–453. doi:10.1007/s00221-012-3033-8
Wascher, E., Schneider, D., & Hoffmann, S. (2015). Does response selection contribute to inhibition of return? Psychophysiology, 52, 942–950. doi:10.1111/psyp.12430
Wylie, G., & Allport, A. (2000). Task switching and the measurement of “switch costs”. Psychological Research, 63, 212–233. doi:10.1007/s004269900003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eng, V., Lim, A., Kwon, S. et al. Stimulus-response incompatibility eliminates inhibitory cueing effects with saccadic but not manual responses. Atten Percept Psychophys 79, 1097–1106 (2017). https://doi.org/10.3758/s13414-017-1295-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-017-1295-8