Abstract
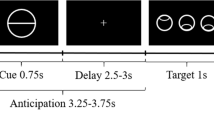
The goal of this research was to further our understanding of how the striatum responds to the delivery of affective feedback. Previously, we had found that the striatum showed a pattern of sustained activation after presentation of a monetary reward, in contrast to a decrease in the hemodynamic response after a punishment. In this study, we tested whether the activity of the striatum could be modulated by parametric variations in the amount of financial reward or punishment. We used an event-related fMRI design in which participants received large or small monetary rewards or punishments after performance in a gambling task. A parametric ordering of conditions was observed in the dorsal striatum according to both magnitude and valence. In addition, an early response to the presentation of feedback was observed and replicated in a second experiment with increased temporal resolution. This study further implicates the dorsal striatum as an integral component of a reward circuitry responsible for the control of motivated behavior, serving to code for such feedback properties as valence and magnitude.
Article PDF
Similar content being viewed by others
References
Aguirre, G. K., Zarahn, E., & D’Esposito, M. (1998). The variability of human, BOLD hemodynamic responses. NeuroImage, 8, 360–369.
Aharon, I., Etcoff, N., Ariely, D., Chabris, C. F., O’Connor, E., & Breiter, H. C. (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron, 32, 537–551.
Aosaki, T., Tsubokawa, H., Ishida, A., Watanabe, K., Graybiel, A.M., & Kimura, M. (1994). Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. Journal of Neuroscience, 14, 3969–3984.
Apicella, P., Ljungberg, T., Scarnati, E., & Schultz, W. (1991). Responses to reward in monkey dorsal and ventral striatum.Experimental Brain Research, 85, 491–500.
Apicella, P., Scarnati, E., Ljungberg, T., & Schultz, W. (1992). Neuronal activity in monkey striatum related to the expectation of predictable environmental events. Journal of Neurophysiology, 68, 945–960.
Bandettini, P. A. (1999). The temporal resolution of functional MRI. In C. Moonen, & P. A. Bandettini (Eds.), Functional MRI (pp. 205–220). New York: Springer-Verlag.
Bechara, A., Damasio, H., & Damasio, A. (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10, 295–307.
Berns, G. S., McClure, S. M., Pagnoni, G., & Montague, P. R. (2001). Predictability modulates human brain response to reward. Journal of Neuroscience, 21, 2793–2798.
Blamire, A. M., Ogawa, S., Ugurbil, K., Rothman, D., McCarthy,G., Ellermann, J. M., Hyder, F., Rattner, Z., & Shulman, R. G. (1992). Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proceedings of the National Academy of Sciences, 89, 11069–11073.
Breiter, H. C., Aharon, I., Kahneman, D., Dale, A., & Shizgal, P. (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron, 30, 619–639.
Breiter, H. C., & Rosen, B. R. (1999). Functional magnetic resonance imaging of brain reward circuitry in the human. In J. F. McGinty (Ed.), Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. In honor of Lennart Heimer (Annals of the New York Academy of Sciences, Vol. 877, pp. 523–547). New York: New York Academy of Sciences.
Brown, V. J., & Bowman, E. M. (1995). Discriminative cues indicating reward magnitude continue to determine reaction time of rats following lesions of the nucleus accumbens. European Journal of Neuroscience, 7, 2479–2485.
Buckner, R. L. (1998). Event-related fMRI and the hemodynamic response. Human Brain Mapping, 6, 373–377.
Buckner, R. L., Goodman, J., Burock, M., Rotte, M., Koutstaal, W., Schacter, D., Rosen, B., & Dale, A. M. (1998). Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron, 20, 285–296.
Buckner, R. L., & Logan, J. M. (2001). Functional neuroimaging methods: PET and fMRI. In R. Cabeza & A. Kingstone (Eds.), Handbook of functional neuroimaging of cognition (pp. 27–48). Cambridge, MA: MIT Press.
Cohen, J. D., Perlstein, W. M., Braver, T. S., Nystrom, L. E., Noll, D. C., Jonides, J., & Smith, E. E. (1997). Temporal dynamics of brain activation during a working memory task. Nature, 386, 604–608.
Courtney, S. M., Ungerleider, L. G., Keil, K., & Haxby, J. V. (1997). Transient and sustained activity in a distributed neural system for human working memory. Nature, 386, 608–611.
Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers & Biomedical Research, 29, 162–173.
Crespi, L. P. (1942). Quantitative variation of reinforcement and level of performance. American Journal of Psychology, 55, 467–517.
Delgado, M. R., Nystrom, L. E., Fissell, C., Noll, D. C., & Fiez, J.A. (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077.
Delgado, M. [R.], Sypher, H., Stenger, V., & Fiez, J. (2000). Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Society for Neuroscience Abstracts, 26, 1073.
Di Chiara, G., Tanda, G., Bassareo, V., Pontieri, F., Acquas, E., Fenu, S., Cadoni, C., & Carboni, E. (1999). Drug addiction as a disorder of associative learning: Role of nucleus accumbens shell/ extended amygdala dopamine. In J. F. McGinty (Ed.), Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. In honor of Lennart Heimer (Annals of the New York Academy of Sciences, Vol. 877, pp. 461–485). New York: New York Academy of Sciences.
Elliott, R., Friston, K. J., & Dolan, R. J. (2000). Dissociable neural responses in human reward systems. Journal of Neuroscience, 20, 6159–6165.
Elliott, R., Sahakian, B. J., Michael, A., Paykel, E. S., & Dolan, R. J. (1998). Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychological Medicine, 28, 559–571.
Everitt, B. J., Parkinson, J. A., Olmstead, M. C., Arroyo, M., Robledo, P., & Robbins, T. W. (1999). Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. In J. F. McGinty (Ed.), Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. In honor of Lennart Heimer (Annals of the New York Academy of Sciences, Vol. 877, pp. 412-438). New York: New York Academy of Sciences.
Forman, S. D., Cohen, J. D., Fitzgerald, M., Eddy, W. F., Mintun, M. A., & Noll, D. C. (1995). Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance Medicine, 33, 636–647.
Grant, S., Contoreggi, C., & London, E. D. (2000). Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia, 38, 1180–1187.
Groenewegen, H. J., Wright, C. I., Beijer, A. V., & Voorn, P. (1999). Convergence and segregation of ventral striatal inputs and outputs. In J. F. McGinty (Ed.), Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. In honor of Lennart Heimer (Annals of the New York Academy of Sciences, Vol. 877, pp. 49-63). New York: New York Academy of Sciences.
Haber, S. N., Kunishio, K., Mizobuchi, M., & Lynd-Balta, E. (1995). The orbital and medial prefrontal circuit through the primate basal ganglia. Journal of Neuroscience, 15, 4851–4867.
Hassani, O. K., Cromwell, H. C., & Schultz, W. (2001). Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. Journal of Neurophysiology, 85, 2477–2489.
Hikosaka, O., Sakamoto, M., & Usui, S. (1989). Functional properties of monkey caudate neurons: III. Activities related to expectation of target and reward. Journal of Neurophysiology, 61, 814–832.
Hollerman, J. R., Tremblay, L., & Schultz, W. (1998). Influence of reward expectation on behavior-related neuronal activity in primate striatum. Journal of Neurophysiology, 80, 947–963.
Hollerman, J. R., Tremblay, L., & Schultz, W. (2000). Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Progress in Brain Research, 126, 193–215.
Ito, R., Dalley, J. W., Robbins, T. W., & Everitt, B. J. (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience, 22, 6247–6253.
Kahneman, D., & Tversky, A. (1979). Prospect theory: An analysis of decision under risk. Econometrica, 47, 263–291.
Kawagoe, R., Takikawa, Y., & Hikosaka, O. (1998). Expectation of reward modulates cognitive signals in the basal ganglia. Nature Neuroscience, 1, 411–416.
Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21, RC159.
Knutson, B., Fong, G. W., Adams, C. M., Varner, J. L., & Hommer, D. (2001). Dissociation of reward anticipation and outcome with eventrelated fMRI. NeuroReport, 12, 3683–3687.
Knutson, B., Westdorp, A., Kaiser, E., & Hommer, D. (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12, 20–27.
Koepp, M. J., Gunn, R. N., Lawrence, A. D., Cunningham, V. J., Dagher, A., Jones, T., Brooks, D. J., Bench, C. J., & Grasby, P. M. (1998). Evidence for striatal dopamine release during a video game. Nature, 393, 266–268.
Koob, G. F. (1999). The role of the striatopallidal and extended amygdala systems in drug addiction. In J. F. McGinty (Ed.), Advancing from the ventral striatum to the extended amygdala: Implications for neuropsychiatry and drug abuse. In honor of Lennart Heimer (Annals of the New York Academy of Sciences, Vol. 877, pp. 445–460). New York: New York Academy of Sciences.
Koob, G. F., & Nestler, E. J. (1997). The neurobiology of drug addiction. Journal of Neuropsychiatry & Clinical Neurosciences, 9, 482–497.
Kwong, K. K., Belliveau, J. W., Chesler, D. A., Goldberg, I. E., Weisskoff, R. M., Poncelet, B. P., Kennedy, D. N., Hoppel, B. E., Cohen, M. S., Turner, R., Cheng, H., Brady, T. J., & Rosen, B. R. (1992). Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proceedings of the National Academy of Sciences, 89, 5675–5679.
Lauwereyns, J., Takikawa, Y., Kawagoe, R., Kobayashi, S. Koizumi, M., Coe, B., Sakagami, M., & Hikosaka, O. (2002). Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron, 33, 463–473.
Lauwereyns, J., Watanabe, K., Coe, B., & Hikosaka, O. (2002). A neural correlate of response bias in monkey caudate nucleus. Nature, 418, 413–417.
LeDoux, J. E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184.
Leon, M. I., & Shadlen, M. N. (1999). Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron, 24, 415–425.
Lesieur, H. R., & Blume, S. B. (1987). The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. American Journal of Psychiatry, 144, 1184–1188.
Macwhinney, B., Cohen, J., & Provost, J. (1997). The PsyScope experiment-building system. Spatial Vision, 11, 99–101.
Middleton, F. A., & Strick, P. L. (2000). Basal ganglia output and cognition: Evidence from anatomical, behavioral, and clinical studies. Brain & Cognition, 42, 183–200.
Noll, D. C., Cohen, J. D., Meyer, C. H., & Schneider, W. (1995). Spiral K-space MR imaging of cortical activation. Journal of Magnetic Resonance Imaging, 5, 49–56.
O’Doherty, J., Kringelbach, M. L., Rolls, E. T., Hornak, J., & Andrews, C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience, 4, 95–102.
Pagnoni, G., Zink, C. F., Montague, P. R., & Berns, G. S. (2002). Activity in human ventral striatum locked to errors of reward prediction. Nature Neuroscience, 5, 97–98.
Robbins, T. W., & Everitt, B. J. (1992). Functions of dopamine in the dorsal and ventral striatum. Seminars in the Neurosciences, 4, 119–127.
Robbins, T. W., & Everitt, B. J. (1996). Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology, 6, 228–236.
Rogers, R. D., Owen, A. M., Middleton, H. C., Williams, E. J., Pickard, J. D., Sahakian, B. J., & Robbins, T. W. (1999). Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience, 19, 9029–9038.
Rolls, E. T. (1999). The brain and emotion. Oxford: Oxford University Press.
Rolls, E. T. (2000). The orbitofrontal cortex and reward. Cerebral Cortex, 10, 284–294.
Salinas, J. A., Packard, M. G., & McGaugh, J. L. (1993). Amygdala modulates memory for changes in reward magnitude: Reversible post-training inactivation with lidocaine attenuates the response to a reduction in reward. Behavioural Brain Research, 59, 153–159.
Salinas, J. A., & White, N. M. (1998). Contributions of the hippocampus, amygdala, and dorsal striatum to the response elicited by reward reduction. Behavioral Neurosciences, 112, 812–826.
Schultz, W. (2000). Multiple reward signals in the brain. Nature Reviews: Neurosciences, 1, 199–207.
Schultz, W., Apicella, P., Scarnati, E., & Ljungberg, T. (1992). Neuronal activity in monkey ventral striatum related to the expectation of reward. Journal of Neuroscience, 12, 4595–4610.
Schultz, W., Tremblay, L., & Hollerman, J. R. (1998). Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology, 37, 421–429.
Shidara, M., Aigner, T. G., & Richmond, B. J. (1998). Neuronal signals in the monkey ventral striatum related to progress through a predictable series of trials. Journal of Neuroscience, 18, 2613–2625.
Takahashi, N., & Kawamura, M. (2002). Pure topographical disorientation: The anatomical basis of landmark agnosia. Cortex, 38, 717–725.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: An approach to medical cerebral imaging. New York: Thieme Medical Publishers.
Tversky, A., & Kahneman, D. (1981). The framing of decisions and the psychology of choice. Science, 211, 453–458.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Jayne, M., Franceschi, D., Wong, C., Gatley, S. J., Gifford, A. N., Ding, Y. S., & Pappas, N. (2002). “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse, 44, 175–180.
Woods, R. P., Cherry, S. R., & Mazziotta, J. C. (1992). Rapid automated algorithm for aligning and reslicing PET images. Journal of Computer Assisted Tomography, 16, 620–633.
Woods, R. P., Mazziotta, J. C., & Cherry, S. R. (1993). MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography, 17, 536–546.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by NIH Grant ROIDA14103, NSF Grant LIS 9720350, and an NSF Graduate Research Fellowship.
Rights and permissions
About this article
Cite this article
Delgado, M.R., Locke, H.M., Stenger, V.A. et al. Dorsal striatum responses to reward and punishment: Effects of valence and magnitude manipulations. Cognitive, Affective, & Behavioral Neuroscience 3, 27–38 (2003). https://doi.org/10.3758/CABN.3.1.27
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.3758/CABN.3.1.27