Abstract
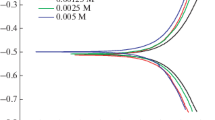
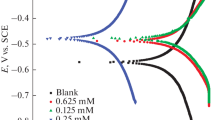
The research includes synthesis of bis thiourea phthalato nickel (II) complex (PTUNi) by reaction of NiCl2⋅6H2O with 2 mol thiourea and 1 mol phthalic anhydride. The (PTUNi) complex was identified by Fourier-transform infrared spectroscopy, UV-visible spectrophotometry, mass spectrophotometry, thermogravimetric differential thermogravimetry analyses, XRD techniques and magnetic susceptibility measurements. The complex was evaluated as corrosion inhibitor for carbon steel alloy (C1010) against a corrosive medium of 0.1 M hydrochloric acid at298 K and showed the maximal efficiency of 95.23% at a concentration of 3 ppm. The effect of temperature on the inhibition behavior was studied at 308, 318 and 328 K and the inhibitor revealed reducing efficiency as temperature raised. The inhibitor behaved as mixed inhibitor. The adsorption of the inhibitor on the surface of the alloy was studied by the Timken, Frumkin, Florry-Hugin and Langmuir adsorption isotherms. The best fitted isotherm was found to be the Langmuir isotherm. The thermodynamic functions like \(\Delta G_{{{\text{ads}}}}^{^\circ }\)and \(\Delta H_{{{\text{ads}}}}^{^\circ }\)were calculated and revealed that spontaneous adsorption, was exothermic where, the inhibitor was physiochemically adsorbed.

















Similar content being viewed by others
REFERENCES
El-Lateef, H.M.A., Adam, M.S.S., and Khalaf, M.M., Synthesis of polar unique 3D metal-imine complexes of salicylidene anthranilate sodium salt. Homogeneous catalytic and corrosion inhibition performance, J. Taiwan Inst. Chem. Eng., 2018, vol. 88, p. 286.
Kadhima, Z.N., Mahadia, M.A., and Al-Sawaada, H.Z., Synthesis, characterization and corrosion inhibitors evaluation of some Schiff base complexes of copper(II), and molybdenum(VI), Int. J. Acad. Stud., 2016, vol. 2, no. 11, p. 446.
Goyal, M., Kumar, S., Bahadur, I., Verma, C., et al., Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review, J. Mol. Liq., 2018, vol. 256, p. 565.
Liu, X., Okafor, P.C., Jiang, B., Hu, H., et al., Electrochemical study on the inhibition effect of phenanthroline and its cobalt complex as corrosion inhibitors for mild steel, J. Mater. Eng. Perform., 2015, vol. 24, no. 9, p. 3599.
Alwan, W.M., Synthesis, characterization and the corrosion inhibition study of two Schiff base ligands derived from urea and thiourea and their complexes with Cu(II) and Hg(II) ions, J. Phys.: Conf. Ser., 2018, vol. 1003, no. 1, art. ID 012017. https://doi.org/10.1088/1742-6596/1003/1/012017
Fawzy, A., Zaafarany, I.A., Ali, H.M., and Abdallah, M., New synthesized amino acids-based surfactants as efficient inhibitors for corrosion of mild steel in hydrochloric acid medium: Kinetics and thermodynamic approach, Int. J. Electrochem. Sci., 2018, vol. 13, no. 5, p. 4575.
Fawzy, A., Abdallah, M., Zaafarany, I.A., Ahmed, S.A., et al., Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media, J. Mol. Liq., 2018, vol. 265, p. 276.
Abdallah, M., Hazazi, O.A., Fawzy, A., El-Shafei, S., et al., Influence of N-thiazolyl-2-cyanoacetamide derivatives on the corrosion of aluminum in 0.01 M sodium hydroxide, Prot. Met. Phys. Chem. Surf., 2014, vol. 50, no. 5, p. 659.
Awad, M., Saad, A.F., Shaaban, M.R., AL Jahdaly, B.A., et al., New insight into the mechanism of the inhibition of corrosion of mild steel by some amino acids, Int. J. Electrochem. Sci., 2017, vol. 12, p. 1657.
Hazazi, O.A., Fawzy, A., Shaaban, M.R., and Awad, I.M., Enhanced 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol inhibition of corrosion of mild steel in 0.5 M H2SO4 by Cu(II), Int. J. Electrochem. Sci., 2014, vol. 9, p. 1378.
Takroni, K.M., El-Ghamry, H.A., and Fawzy, A., Evaluation of the catalytic activities of some synthesized divalent and trivalent metal complexes and their inhibition efficiencies for the corrosion of mild steel in sulfuric acid medium, J. Inorg. Organomet. Polym. Mater., 2019, vol. 29, no. 6, p. 1927.
Biswas, A., Das, D., Lgaz, H., Pal, S., et al., Biopolymer dextrin and poly (vinyl acetate) based graft copolymer as an efficient corrosion inhibitor for mild steel in hydrochloric acid: electrochemical, surface morphological and theoretical studies, J. Mol. Liq., 2019, vol. 275, p. 867.
Abdallah, M., Fouda, A.S., Zaafarany, I., Fawzy, A., et al., Corrosion inhibition of iron in sulphuric acid solution by antibacterial cephalosporin, J. Am. Sci., 2013, vol. 9, no. 3, p. 209.
Bentiss, F., Lagrenee, M., Traisnel, M., and Hornez, J.C., The corrosion inhibition of mild steel in acidic media by a new triazole derivative, Corros. Sci., 1999, vol. 41, no. 4, p. 789.
Hazazi, O., Fawzy, A., and Awad, M., Synergistic effect of halides on the corrosion inhibition of mild steel in H2SO4 by a triazole derivative: Kinetics and thermodynamic studies, Int. J. Electrochem. Sci., 2014, vol. 9, p. 4086.
Bentiss, F., Lagrenée, M., and Traisnel, M., 2,5-Bis(n-pyridyl)-1,3,4-oxadiazoles as corrosion inhibitors for mild steel in acidic media, Corrosion, 2000, vol. 56, no. 7, p. 733.
Abdallah, M., AL Jahdaly, B.A., Salem, M.M., Fawzy, A., et al., Electrochemical behavior of nickel alloys and stainless steel in HNO3 using cyclic voltammetry technique, J. Mater. Environ. Sci., 2017, vol. 8, p. 1320.
Fuchs-Godec, R. and Pavlović, M.G., Synergistic effect between non-ionic surfactant and halide ions in the forms of inorganic or organic salts for the corrosion inhibition of stainless-steel X4Cr13 in sulphuric acid, Corros. Sci., 2012, vol. 58, p. 192.
Abdallah, M., Zaafarany, I., Fawzy, A., Radwan, M.A., et al., Inhibition of aluminum corrosion in hydrochloric acid by cellulose and chitosan, J. Am. Sci., 2013, vol. 9, p. 580.
Hazazi, O.A., Fawzy, A., and Awad, M.I., Sulfachloropyridazine as an eco-friendly inhibitor for corrosion of mild steel in H2SO4 solution, Chem. Sci. Rev. Lett., 2015, vol. 4, p. 67.
ELouadi, Y., Abrigach, F., Bouyanzer, A., and Touzani, R., Corrosion inhibition of mild steel by new N-heterocyclic compound in 1 M HCl: Experimental and computational study, Pharma Chem., 2015, vol. 7, no. 8, p. 265.
Cherrak, K., Dafali, A., Elyoussfi, A., El Ouadi, Y., et al., Two new benzothiazine derivatives as corrosion inhibitors for mild steel in hydrochloric acid medium, J. Mater. Environ. Sci., 2017, vol. 8, no. 2, p. 636.
El Ouadi, Y., Elmsellem, H., El Fal, M., Sebbar, N.K., et al., Effect of 1,5-di(prop-2-ynyl)-1H-pyrazolo[3,4-d]pyrimidine-4(5H)-thione on inhibition of mild steel corrosion in 1 M HCl, Pharma Chem., 2016, vol. 8, no. 1, p. 365.
Bader, A., Shaheen, U., Aborehab, M.A.S., El Ouadi, Y., et al., Inhibitory effect of Acacia hamulosa methanolic extract on the corrosion of mild steel, in 1 M hydrochloric acid, Bull. Chem. Soc. Ethiop., 2018, vol. 32, no. 2, p. 323.
Merimi, I., El Ouadi, Y., Ansari, K.R., Oudda, H., et al., Adsorption and corrosion inhibition of mild steel by ((Z)-4-((2,4-dihydroxybenzylidene)amino)-5-methy-2,4 dihydro-3H-1,2,4-triazole-3-thione) in 1 M HCl: Experimental and computational study, Anal. Bioanal. Electrochem., 2017, vol. 9, no. 5, p. 640.
Merimi, I., El Ouadi, Y., Benkaddour, R., Lgaz, H., et al., Improving corrosion inhibition potentials using two triazole derivatives for mild steel in acidic medium: experimental and theoretical studies, Mater. Today: Proc., 2019, vol. 13, p. 920.
Shen, C., Wang, S.G., Yang, H.Y., Long, K., et al., Corrosion and corrosion inhibition by thiourea of bulk nanocrystallized industrial pure iron in dilute HCl solution, Corros. Sci., 2006, vol. 48, no. 7, p. 1655.
Huong, D.Q., Duong, T., and Nam, P.C., Effect of the structure and temperature on corrosion inhibition of thiourea derivatives in 1.0 M HCl solution, ACS Omega, 2019, vol. 4, no. 11, p. 14478.
Ajibade, P.A. and Zulu, N.H., Metal complexes of diisopropylthiourea: synthesis, characterization and antibacterial studies, Int. J. Mol. Sci., 2011, vol. 12, no. 10, p. 7186.
Binzet, G., Kavak, G., Külcü, N., Özbey, S., Flörke, U., and Arslan, H., Synthesis and characterization of novel thiourea derivatives and their nickel and copper complexes, J. Chem., 2013, vol. 2013, art. ID 536562. https://doi.org/10.1155/2013/536562
Halim, N.I.M., Kassim, K., Fadzil, A.H., and Yamin, B.M., Sintesis, pencirian dan kajian aktiviti antibakteria kompleks Cu(II) tiourea, Malays. J. Anal. Sci., 2012, vol. 16, no. 1, p. 56.
Larouj, M., Ourrak, K., El M’Rabet, M., Zarrok, H., et al., Thermodynamic study of corrosion inhibition of carbon steel in acidic solution by new pyrimidothiazine derivative, J. Mater. Environ. Sci., 2017, vol. 8, no. 11, p. 3921.
Devika, B., Doreswamy, B., and Tandon, H., Corrosion behaviour of metal complexes of antipyrine based azo dye ligand for soft-cast steel in 1 M hydrochloric acid, J. King Saud Univ. Sci., 2020, vol. 32, no. 1, p. 881.
Fernandes, C.M., Alvarez, L.X., dos Santos, N.E., Barrios, A.C.M., et al., Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses, Corros. Sci., 2019, vol. 149, p. 185.
Manssouri, M., El Ouadi, Y., Znini, M., Costa, J., et al., Adsorption proprieties and inhibition of mild steel corrosion in HCl solution by the essential oil from fruit of Moroccan Ammodaucus leucotrichus, J. Mater. Environ. Sci., 2015, vol. 6, no. 3, p. 631.
Radey, H.H., Khalaf, M.N., and Al-Sawaad, H.Z., Novel corrosion inhibitors for carbon steel alloy in acidic medium of 1 N HCl synthesized from graphene oxide, Open J. Org. Polym. Mater., 2018, vol. 8, no. 04, p. 53.
AL-Sawaad, H.Z., Evaluation of the ceftriaxone as corrosion inhibitor for carbon steel alloy in 0.5 M of hydrochloric acid, Int. J. Electrochem. Sci., 2013, 8, p. 3105.
Fouda, A., El-morsi, M.A., Gaber, M., and Fakeeh, M., A comparative study of the corrosion inhibition of carbon steel in HCl solution by 1-[(5-mercapto-1H-1,2,4-triazole-3-yl) diazenyl] naphthalene-2-ol (HL) and its manganese complex, Chem. Data Collect., 2020, vol. 28, art. ID 100479.
Fateh, A., Aliofkhazraei, M., and Rezvanian, A., Review of corrosive environments for copper and its corrosion inhibitors, Arab. J. Chem., 2020, vol. 13, no. 1, p. 481.
El Aoufir, Y., Aslam, R., Lazrak, F., Marzouki, R., et al., The effect of the alkyl chain length on corrosion inhibition performances of 1,2,4-triazole-based compounds for mild steel in 1.0 M HCl: Insights from experimental and theoretical studies, J. Mol. Liq., 2020, vol. 303, art. ID 112631.
Hsissou, R., Benhiba, F., About, S., Dagdag, O., et al., Trifunctional epoxy polymer as corrosion inhibition material for carbon steel in 1.0 M HCl: MD simulations, DFT and complexation computations, Inorg. Chem. Commun., 2020, vol. 115, art. ID 107858. https://doi.org/10.1016/j.inoche.2020.107858
Ardakani, E.K., Kowsari, E., and Ehsani, A., Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: experimental and computational study, Colloids Surf., A, 2020, vol. 586, art. ID 124195.
Lebrini, M., Robert, F., and Roos, C., Adsorption properties and inhibition of C38 steel corrosion in hydrochloric solution by some indole derivates: temperature effect, activation energies, and thermodynamics of adsorption, Int. J. Corros., 2013, vol. 2013, art. ID 139798. https://doi.org/10.1155/2013/139798
AL-Jubanawi, I.M., AL-Sawaad, H.Z., and AL-Waaly, A.A., Bis thiourea phthalato cobalt(II) complex: synthesis and studying as corrosion inhibitors for carbon steel alloy (C1010) in 0.1 M HCl, J. Mater. Environ. Sci., 2020, vol. 11, p. 1386.
El-Lateef, H.M.A., El-Sayed, A.R., Mohran, H.S., and Shilkamy, H.A.S., Corrosion inhibition and adsorption behavior of phytic acid on Pb and Pb–In alloy surfaces in acidic chloride solution, Int. J. Ind. Chem., 2019, vol. 10, no. 1, p. 31.
Shukla, S.K. and Ebenso, E.E., Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium, Int. J. Electrochem. Sci., 2011, vol. 6, no. 8, p. 3277.
El-Tabesh, R., Abdel-Gaber, A.M., Hammud, H., and Oweini, R., Effect of mixed-ligands copper complex on the corrosion inhibition of carbon steel in sulfuric acid solution, J. Bio- Tribo-Corros., 2020, vol. 6, no. 2, p. 29.
Al-Sawaad, H.Z., Faili, N.T., and Essa, A.H., Evaluation of vicine as a corrosion inhibitor for carbon steel alloy, Port. Electrochim. Acta, 2019, vol. 37, no. 4. p. 205.
El-Gammal, O.A., Fouda, A.E.-A.S., and Nabih, D.M., Novel Mn2+, Fe3+, Co2+, Ni2+ and Cu2+ complexes of potential OS donor thiosemicarbazide: design, structural elucidation, anticorrosion potential study and antibacterial activity, J. Mol. Struct., 2020, vol. 1204, art. ID 127495.
Rao, C., Venkataraghavan, R., and Kasturi, T., Contribution to the infrared spectra of organosulphur compounds, Can. J. Chem., 1964, vol. 42, no. 1, p. 36.
Zakaria, S.A., Muharam, S.H., Yusof, M.S.M., Khairul, W.M., et al., Spectroscopic and structural study of a series of pivaloylthiourea derivatives, Malays. J. Anal. Sci., 2011, vol. 15, no. 1, p. 37.
Akpomie, K.G., Fayomi, O.M., Ezeofor, C.C., Sha’Ato, R., et al., Insights into the use of metal complexes of thiourea derivatives as highly efficient adsorbents for ciprofloxacin from contaminated water, Trans. R. Soc. South Afr., 2019, vol. 74, no. 2, p. 180.
Sonmez, M., Synthesis and characterization of copper(II), nickel(II), cadmium(II), cobalt(II) and zinc(II) complexes with 2-benzoyl-3-hydroxy-1-naphthylamino-3-phenyl-2-propen-1-on, Turk. J. Chem., 2001, vol. 25, no. 2, p. 181.
Ibrahim, O.B., Complexes of urea with Mn(II), Fe(III), Co(II), and Cu(II) metal ions, Adv. Appl. Sci. Res., 2012, vol. 3, no. 6, p. 18.
Ghazal, K., Shoaib, S., Khan, M., Khan, S., et al., Synthesis, characterization, X-ray diffraction study, in-vitro cytotoxicity, antibacterial and antifungal activities of nickel(II) and copper(II) complexes with acyl thiourea ligand, J. Mol. Struct., 2019, vol. 1177, p. 124.
Refat, M.S., El-Deen, I.M., Zein, M.A., Adam, A.M., et al., Spectroscopic, structural and electrical conductivity studies of Co(II), Ni(II) and Cu(II) complexes derived from 4-acetylpyridine with thiosemicarbazide, Int. J. Electrochem. Sci., 2013, vol. 8, no. 7, p. 9894.
Tan, S.S., Al-Abbasi, A.A., Tahir, M.I.M., and Kassim, M.B., Synthesis, structure and spectroscopic properties of cobalt(III) complexes with 1-benzoyl-(3,3-disubstituted)thiourea, Polyhedron, 2014, vol. 68, p. 287.
Monshi, A., Foroughi, M.R., and Monshi, M.R., Modified Scherrer equation to estimate more accurately nano-crystallite size using XRD, World J. Nano Sci. Eng., 2012, vol. 2, no. 3, p. 154.
Breviglieri, S.T., Cavalheiro, E.T.G., and Chierice, G.O., Correlation between ionic radius and thermal decomposition of Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) diethanoldithiocarbamates, Thermochim. Acta, 2000, vol. 356, nos. 1–2, p. 79.
El-Sawaf, A.K., El-Essawy, F., Nassar, A.A., and El-Samanody, E.A., Synthesis, spectral, thermal and antimicrobial studies on cobalt(II), nickel(II), copper(II), zinc(II) and palladium(II) complexes containing thiosemicarbazone ligand, J. Mol. Struct., 2018, vol. 1157, p. 381.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
About this article
Cite this article
Israa M. Al-Jubanawi, Al-Sawaad, H.Z. & Alwaaly, A.A. Synthesis Characterization and Corrosion Inhibition of Thiourea and Phthalic Anhydride Complex with Ni(II) for Carbon Steel Alloy C1010 0.1 M Hydrochloric Acid. Surf. Engin. Appl.Electrochem. 57, 595–606 (2021). https://doi.org/10.3103/S1068375521050057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375521050057