Abstract
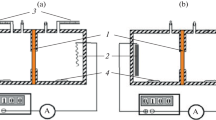
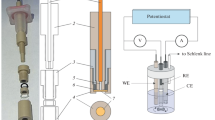
The experimental technique and equipment used to study the kinetics of ion exchange in weak electric fields in the ion–exchange resins using a computer resistometric measurement method are described. In the course of ion exchange in the NaCl + NaOH model solution–ion exchanger system the chemical composition dynamics of a solution measuring the solution resistance with the help of a resistometer is measured with a flowing sensor; its signal is computer processed. The pattern of interaction between electrical and diffusion ion fields in the solid phase of the ion exchanger is discussed. It is shown that when a weak directional electric field is applied on the spherical ion exchanger particle, the spherical symmetry of the ion displacement rate is distorted, and the balance of the ion flows is changed. Thus, the exchange process is accelerated in the direction of the electric field vector. The test unit for the study of the ion exchange dynamics, which includes a convective reactor, a built-in column with a portion of the exchanger, a temperature control system, a flowing sensor of the electrical resistance of the solution, an electronic resistometer, and a potentiostat–galvanostat, is described. Three experimental methods are described: a nonstationary method with the ion exchange suspension in the enclosed volume, the nonstationary method with filtration through the ion exchanger in the column, and the method of open filtration through the column. It is shown that the speed of the ion flux in the solid phase is constant and does not depend on the saturation value of the exchanger in the suspension mode of exchange in the basic interval time of the process. This effect is caused by the operation of the electroneutrality law on two ion fluxes with the charges of the same sign but with the opposite concentration gradients.










Similar content being viewed by others
REFERENCES
Koshel’, N.D., Magdych, E.A., and Akimov, A.M., et al., Vopr. Khim. Khim. Tekhnol., 2010, vol. 5, pp. 137–139.
Newman, J.S., Electrochemical System, New Jersey: Prentice-Hall, 1973.
Zoski, C.G., Handbook of Electrochemistry, Amsterdam: Elsevier, 2007.
Demirbas, A., Pehlivan, E., Gode, F., Altun, T., et al., J. Colloid Interface Sci., 2005, vol. 282, no. 1, pp. 20–25.
Pehlivan, E. and Altun, T., J. Hazard. Mater., 2006, vol. 134, nos. 1–3, pp. 149–156.
Koshel’, N.D. and Kostyrya, M.V., Surf. Eng. Appl. Electrochem., 2018, vol. 54, no. 1, pp. 103–110.
Spravochnik khimika. Tom 3. Khimicheskoe ravnovesie i kinetika svoistva rastvorov. Elektrodnye protsessy (Handbook of Coke Chemist, Vol. 3: Chemical Balance and Kinetics of Solution Properties. Electrode Processes), Moscow: Khimiya, 1965, 2nd ed.
Washburn, E.W., International Critical Tables of Numerical Data, Physics, Chemistry and Technology, New York: National Research Council, 2003, vol. 6.
McCleskey, R.B., et al., J. Chem. Eng. Data, 2011, vol. 56, no. 2, pp. 317–327.
Hunt, R.C., How To Increase the Accuracy of Solution Conductivity Measurements, Santa Ana, CA: Sensor Development, 1995. http://www.aitsouthwest.com.
Koshel, N.D., Smyrnova, O.V., and Kostyrya, M.V., The study of ion exchange kinetics on the anionite in weak electric fields, Proc. 8th International Conf. “Materials Science & Condensed Matter Physics,” September 12–16, 2016, Abstracts of Papers, Chisinau, 2016, p. 340.
Shaposhnik, V.A., Reshetnikova, A.K., Zolotareva, R.I., and Isaev, N.I., Zh. Prikl. Khim., 1973, vol. 46, no. 12, pp. 2659–2663.
Özgür, A., Ümran, Y., Nalan, K., and Mithat, Y., Desalination, 2014, vol. 342, pp. 16–22.
Smara, A., Delimi, R., Chai-Net, E., and Sandeaux, J., Sep. Purif. Technol., 2007, vol. 57, no. 1, pp. 103–110.
Akrama, M. and Andrew, F.A.H., Water Res., 2012, vol. 46, no. 10, pp. 3364–3376.
Alvarado, L. and Chen, A., Electrochim. Acta, 2014, vol. 132, pp. 583–597.
Dzyazko, Yu.S. and Belyakov, V.N., Desalination, 2004, vol. 162, pp. 179–189.
Dzyazko, Yu.S., Ponomaryova, L.N., Rozhdestvenskaya, L.M., Vasilyuk, S.L., et al., Desalination, 2014, vol. 342, pp. 43–51.
Spoor, P.B., Grabovska, L., Koene, L., and Janssen, L. J. Chem. Eng., 2002, vol. 89, nos. 1–3, pp. 193–202.
Gel’perin, N.I., Ainshtein, V.G., and Kvasha, V.B., Osnovy tekhniki psevdoozhizhenya (Fundamentals of Pseudofluidizing), Moscow: Khimiya, 1967.
Shvab, N.A., Stefanjak, N.V., Kazdobin, K.A., and Wragg, A.A., J. Appl. Electrochem., 2000, vol. 30, no. 11, pp. 285–1292.
Shvab, N.A., Stefanjak, N.V., Kazdobin, K.A., and Wragg, A.A., J. Appl. Electrochem., 2000, vol. 30, no. 11, pp. 1293–1298.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Myshkina
About this article
Cite this article
Koshel, N.D., Smirnova, E.V. & Koshel, S.A. Study of Ion Exchangers in Electric Fields Using Resistometric Measurement. Part 2. Methods and Equipment. Surf. Engin. Appl.Electrochem. 55, 576–586 (2019). https://doi.org/10.3103/S1068375519050065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375519050065