Abstract
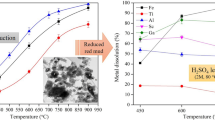
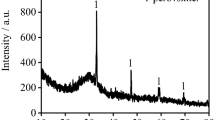
The extraction of Ti powder from SHS-produced Ti–MgO–Mg(–CaO) cakes by treatment in leaching solutions (HNO3, HCl, and NH4Cl) was explored and optimized in relation to such factors as concentration of leaching agent, leaching temperature, chemical resistance of target Ti powder, and extent of byproducts extraction. The type of leaching solution was found to affect the size, structure, and morphology of resultant Ti powder. Best results were obtained at 70°C with aqueous solutions of: (1) the nitric acid taken in a 6-fold excess to the Mg content of combustion product and (2) the ammonium chloride taken in a 20-fold excess to nominal Mg content.
Similar content being viewed by others
References
Kroll, W.J., The production of ductile titanium, Trans. Electrochem. Soc., 1940, vol. 78, no. 1, pp. 35–47. doi: 10.1149/1.3071290
Du, J., Research progress of titanium production technology, Rare Met. Mater. Eng., 2008, vol. 37, no. 10, pp. 1872–1875. doi 10.1007/s11771-012-1293-x
Ogasawara, T., Progress of the titanium production technology in Japan and future prospects of the field, Titanium Jpn., 2005, vol. 53, no. 2, pp. 103–108.
Zheng, H., Ito, H., and Okabe, T.H., Production of titanium powder by the calciothermic reduction of titanium concentrates or ore using the preform reduction process, Mater. Trans., 2007, vol. 48, no. 8, pp. 2244–2251 doi 10.2320/matertrans.MER2007115
Borovinskaya, I.P., Ignat’eva, T.I., Vershinnikov, V.I., Miloserdova, O.M., and Semenova, V.N., SHS of ultrafine and nanosized WCand TiC powders, Powder Metall. Met. Ceram., 2008, vol. 47, no. 9–10, pp. 505–511. doi 10.1007/s11106-008-9051-1
Ignat’eva, T.I., Miloserdova, O.M., Semenova, V.N., and Borovinskaya, I.P., Chemical dispersion as a method for segregation of ultrafine and nanosized TiC powders, Perspekt. Mater., 2009, no. 3, pp. 82–87.
Borovinskaya, I.P., Barinova, T.V., Vershinnikov, V.I., and Ignat’eva, T.I., SHS of ultrafine and nanosized refractory powders: An autoreview, Int. J. Self-Propag. High-Temp. Synth., 2010, vol. 19, no. 2, pp. 116–121 doi 10.3103/S1061386210020068
Sangwal, K., Dissolution kinetics of MgO crystals in aqueous acidic salt-solutions, J. Mater. Sci., 1982, vol. 17, no. 12, pp. 3598–3610 doi 10.1007/BF00752203
Sangwal, K. and Arora, S.K., Etching of MgO crystals in acids: Kinetics and mechanism of dissolution, J. Mater. Sci., 1978, vol. 13, no. 9, pp. 1977–1985 doi 10.1007/BF00552905
Ignat’eva, T.I., Vershinnikov, V.I., Semenova, V.N., and Miloserdova, O.M., Extraction of TiAl powder from SHS-produced TiAl–MgO semiproduct by treatment in different solutions, Int. J. Self-Propag. High-Temp. Synth., 2017, vol. 26, no. 2, pp. 115–118 doi 10.3103/S1061386217020066
Raschman, P., Leaching of calcined magnesite using ammonium chloride at constant pH, Hydrometallurgy, 2000, vol. 56, no. 1, pp. 109–123
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ignat’eva, T.I., Vershinnikov, V.I., Semenova, V.N. et al. Extraction of Ti Powder from Ti–MgO–Mg(–CaO) Cakes Produced by Magnesiothermic Reduction. Int. J Self-Propag. High-Temp. Synth. 27, 77–80 (2018). https://doi.org/10.3103/S1061386218020097
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1061386218020097