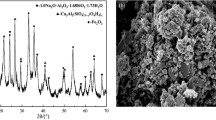
Abstract
The current study investigates hydrogen reduction of red mud followed by acid leaching to recover the valuable elements. The key objective is to reduce the hematite to metallic iron and consequently distort the interlocked structure of red mud. Reduction at 450 °C results in formation of magnetite with ~ 10% Fe metallization in 30 min, and increment in the temperature to 900 ºC results in 97% Fe metallization in 120 min. The metallic Fe particles possess characteristic spherical morphology. Gibbsite is dehydroxylated to alumina, and anatase (TiO2) is partially reduced to Ti2O3 above 750 °C. The H2SO4 leaching yielded higher dissolution of elements compared to HCl leaching of hydrogen-reduced product. Reduction at higher temperatures (750–900 °C) improves the dissolution of Fe and Ga but deteriorates for Sc, Al, and Ti. Sc is hosted by the anatase phase confirmed by the SEM mapping of feed/reduced products and a strong correlation between the dissolution values of Sc and Ti. Hydrogen reduction at 900 °C for 30 min with 0.5 L/min H2 flowrate followed by H2SO4 leaching (2 M, 2 h, 80 °C) results in 90% Fe, 95% Ga, and 54% Sc dissolution. The final residue contains TiO2, Al2O3, and FeTiO3 phases with 42 wt% TiO2 having yield of 35%. The leach solution contains 7.1 ppm Sc, 15.9 ppm Ga, 1.74 g/L Al, and 11.4 g/L Fe, which can be used for Sc and Ga recovery after Fe removal. The hydrogen reduction is attractive compared to carbothermal reduction because of low temperature (600–900 °C), less reductant use (107 g H2/kg red mud) and restricting fayalite and hercynite formation.
Graphical Abstract







Similar content being viewed by others
References
Agrawal S, Dhawan N (2021) Evaluation of red mud as a polymetallic source—a review. Miner Eng 171:107084
Evans K (2016) The history, challenges, and new developments in the management and use of bauxite residue. J Sustain Metall 2(4):316–331
Wang L, Sun N, Tang H, Sun W (2019) A review on comprehensive utilization of red mud and prospect analysis. Miner 9(6):362
Rai S, Bahadure S, Chaddha MJ, Agnihotri A (2020) Disposal practices and utilization of red mud (bauxite residue): a review in Indian context and abroad. J Sustain Metall 6(1):1–8
Liu Y, Naidu R (2014) Hidden values in bauxite residue (red mud): recovery of metals. Wast Manag 34(12):2662–2673
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Recovery of rare earths and other valuable metals from bauxite residue (red mud): a review. J Sustain Metall 2(4):365–386
Pasechnik LA, Skachkov VM, Bogdanova EA, Chufarov AY, Kellerman DG, Medyankina IS, Yatsenko SP (2020) A promising process for transformation of hematite to magnetite with simultaneous dissolution of alumina from red mud in alkaline medium. Hydromet 196:105438
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2(1):28–37
Alkan G, Yagmurlu B, Gronen L, Dittrich C, Ma Y, Stopic S, Friedrich B (2019) Selective silica gel-free scandium extraction from Iron-depleted red mud slags by dry digestion. Hydrometallurgy 185:266–272
Anawati J, Azimi G (2022) Integrated carbothermic smelting–acid baking–Water leaching process for extraction of scandium, aluminum, and iron from bauxite residue. J Clean Prod 330:129905
Agrawal S, Rayapudi V, Dhawan N (2019) Comparison of microwave and conventional carbothermal reduction of red mud for recovery of iron values. Miner Eng 132:202–210
Agrawal S, Dhawan N (2021) Microwave acid baking of red mud for extraction of titanium and scandium values. Hydrometallurgy 204:105704
Reid S, Tam J, Yang M, Azimi G (2017) Technospheric mining of rare earth elements from bauxite residue (red mud): process optimization, kinetic investigation, and microwave pretreatment. Sci Rep 7(1):1–9
BCavaliere P (2019) Clean ironmaking and steelmaking processes: efficient technologies for greenhouse emissions abatement. Clean Ironmak Steelmak 1–37
Rukini A, Rhamdhani MA, Brooks GA, Van den Bulck A (2022) Metals production and metal oxides reduction using hydrogen: a review. J Sustain Metall 8(1):1–24
Samouhos M, Taxiarchou M, Pilatos G, Tsakiridis PE, Devlin E, Pissas M (2017) Controlled reduction of red mud by H2 followed by magnetic separation. Miner Eng 105:36–43
Teplov OA, Lainer YA (2013) Rate of the reduction of the iron oxides in red mud by hydrogen and converted gas. Russian Metall (Metally) 1:25–32
Kapelari S, Gamaletsos PN, Van Der Donck T, Pontikes Y, Blanpain B (2021) H2-Based Processes for Fe and Al Recovery from Bauxite Residue (Red Mud): Comparing the Options. Mater Proceed 5(1):45
Pilla G, Hertel T, Kapelari S, Blanpain B, Pontikes Y (2022) Reactions and phase transformations during the low-temperature reduction of bauxite residue by H2 in the presence of NaOH, and recovery rates downstream. In: Proceedings of 40th International ICSOBA Conference, Athens, 10–14 October 2022
Skibelid OB, Velle SO, Vollan F, Van der Eijk C, Hoseinpur-Kermani A, Safarian J (2022) Isothermal hydrogen reduction of a lime-added bauxite residue agglomerate at elevated temperatures for iron and alumina recovery. Materials 15(17):6012
Spreitzer D, Schenk J (2019) Reduction of iron oxides with hydrogen—a review. Steel Res Int 90(10):1900108
Pepper RA, Couperthwaite SJ, Millar GJ (2016) Comprehensive examination of acid leaching behaviour of mineral phases from red mud: Recovery of Fe, Al, Ti, and Si. Miner Eng 99:8–18
Gelencsér A, Kováts N, Turóczi B, Rostási Á, Hoffer A, Imre K, Pósfai M (2011) The red mud accident in Ajka (Hungary): characterization and potential health effects of fugitive dust. Environ Sci Technol 45(4):1608–1615
Liu K, Chen Q, Yin Z, Hu H, Ding Z (2012) Kinetics of leaching of a Chinese laterite containing maghemite and magnetite in sulfuric acid solutions. Hydromet 125:125–136
Liu X, Gao P, Yuan S, Lv Y, Han Y (2020) Clean utilization of high-iron red mud by suspension magnetization roasting. Miner Eng 157:106553
Gostu S, Mishra B, Martins GP (2017) Low temperature reduction of hematite in red-mud to magnetite. Light metals 2017, Springer, Cham, 67–73.
Wang W, Cheng CY (2011) Separation and purification of scandium by solvent extraction and related technologies: a review. J Chem Technol Biotechnol 86(10):1237–1246
Oni AO, Anaya K, Giwa T, Di Lullo G, Kumar A (2022) Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Convers Manag 254:115245
Rivera RM, Xakalashe B, Ounoughene G, Binnemans K, Friedrich B, Van Gerven T (2019) Selective rare earth element extraction using high-pressure acid leaching of slags arising from the smelting of bauxite residue. Hydromet 184:162–174
Acknowledgements
This work was supported by the Faculty Initiation grant (FIG-100714) by the Indian Institute of Technology, Roorkee, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Dimitrios Panias.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agrawal, S., Dhawan, N. Hydrogen Reduction of Red Mud for Extraction of Metallic Values. J. Sustain. Metall. 9, 386–397 (2023). https://doi.org/10.1007/s40831-023-00655-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00655-8