Abstract
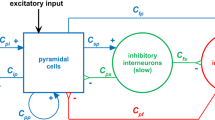
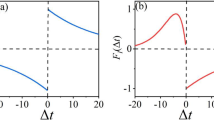
An asynchronous discrete model of nonsynaptic chemical interactions between neurons is proposed. The model significantly extends the previous work [1, 2] by novel concepts that make it more biologically plausible. In the model, neurons interact by emitting neurotransmitters to the shared extracellular space (ECS). We introduce the dynamics of membrane potentials that comprises two factors: the endogenous rates of change depending on the neuron’s firing type and the exogenous rates of change depending on the concentrations of neurotransmitters that the neuron is sensitive to. The neuron’s firing type is determined by the individual composition of endogenous rates. We consider three basic firing types: oscillatory, tonic, and reactive. Each firing type is essential for modeling central pattern generators—neural ensembles that generate rhythmic activity in the absence of external stimuli. Differences in endogenous rates of different neurons lead to asynchronous neural interactions and significant variability of phase durations in the activity patterns present in simple neural systems. The algorithm computing the behavior of the proposed model is provided.
Similar content being viewed by others
References
Bazenkov, N., Vorontsov, D., Dyakonova, V., Zhilyakova, L., Zakharov, I., Kuznetsov, O., Kulivets, S., and Sakharov, D., Discrete modeling of neuronal interactions in multi-neurotransmitter networks, Iskusstv. Intell. Prinyatie Reshenii, 2017, no. 2, pp. 55–73.
Bazenkov, N., Dyakonova, V., Kuznetsov, O., Sakharov, D., Vorontsov, D., and Zhilyakova, L., Discrete modeling of the multi-transmitter neural networks with neuronal competition, Biologically Inspired Cognitive Architectures (BICA) for Young Scientists; Adv. Intell. Syst. Comput., 2018, vol. 636, pp. 10–16.
McCulloch, W.S. and Pitts, W., A logical calculus of the ideas, Bull. Math. Biophys., 1943, vol. 5, pp. 115–133.
Hopfield, J.J., Neural networks and physical systems with emergent collective computational abilities, Proc. Natl. Acad. Sci., 1982, vol. 79, no. 8.
Haykin, S., Neural Networks and Learning Machines, Prentice Hall, 2009, 3rd ed.
LeCun, Y., Bengio, Y., and Hinton, G., Deep learning, Nature, 2015, vol. 521, no. 7553, pp. 436–444.
Goodfellow, I., Bengio, Y., and Courville, A., Deep Learning, MIT Press, 2016.
Deng, L. and Yu, D., Deep learning: Methods and applications, Found. Trends Signal Process., 2014, vol. 7, nos. 3–4, pp. 1–199.
Bengio, Y., Lamblin, P., Popovici, P., and Larochelle, H., Greedy layer-wise training of deep networks, Adv. Neural Inf. Process. Syst., 2007, vol. 19.
Hinton, G.E., Osindero, S., and Teh, Y.W., A fast learning algorithm for deep belief nets, Neural Comput., 2006, vol. 18, pp. 1527–1554.
Shumsky, S.A., Deep learning: Ten years later, XIX mezhdunarodnaya nauchno-tekhnicheskaya konferencia “Neiroinformatika-2017": Lekcii po neiroinformatike (XIX International Scientific and Technical Conference Neuroinformatics-2017: Lectures on Neuroinformatics), Moscow, 2017, pp. 98–131.
Abbott, L.F., Lapique’s introduction of the integrateand- fire model neuron, Brain Res. Bull., 1907, vol. 50, nos. 5–6, pp. 303–304.
Hodgkin, A.L. and Huxley, A.F., A quantitative description of the membrane current and its applications to conduction and excitation in nerve, J. Physiol. (London), 1952, vol. 116, pp. 500–544.
FitzHugh, R., Mathematical models of excitation and propagation in nerve, in Biological Engineering, Schwan, H.P., Ed., New York: McGraw-Hill Book Co., 1969, ch. 1, pp. 1–85.
Nagumo, J., Arimoto, S., and Yoshizawa, S., An active pulse transmission line simulating nerve axon, Proc. IRE, 1962, vol. 50, pp. 2061–2070.
Morris, C. and Lecar, H., Voltage Oscillations in the barnacle giant muscle fiber, Biophys. J., 1981, vol. 35, no. 1, pp. 193–213.
Vavoulis, D., Straub, V., Kemenes, I., Kemenes, G., Feng, J., and Benjamin, P., Dynamic control of a central pattern generator circuit: A computational model of the snail feeding network, Eur. J. Neurosci., 2007, vol. 25, pp. 2805–2818.
Izhikevich, E., Int. J. Bifurcation Chaos, 2000, vol. 10, no. 6, pp. 1171–1266.
Izhikevich, E., Which model to use for cortical spiking neurons?, IEEE Trans. Neural Networks, 2004, vol. 15, no. 5.
Brunel, N., Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons, J. Comput. Neurosci., 2000, vol. 8, no. 3, pp. 183–208. https://doi.org/.10.1023/A:1008925309027
Ladenbauer, J., Augustin, M., Shiau, L., and Obermayer, K., Impact of adaptation currents on synchronization of coupled exponential integrate-and-fire neurons, PLoS Comput. Biol., 2012, vol. 8, no. 4, e1002478. https://doi.org/.10.1371/journal.pcbi.1002478
Delahunt, C.B., Riffell, J.A., and Kutz, J.N., Biological mechanisms for learning: A computational model of olfactory learning in the Manduca sexta moth, with applications to neural nets, ArXiv.org: 1802.02678. https://arxiv.org/abs/1802.02678. Accessed April 10, 2018.
Balaban, P.M., Vorontsov, D.D., D’yakonova, V.E., D’yakonova, T.L., Zakharov, I.S., Korshunova, T.A., Orlov, O.Yu., Pavlova, G.A., Panchin, Yu.V., Sakharov, D.A., and Falikman, M.V., Central pattern generators, Neurosci. Behav. Physiol., 2015, vol. 45, no. 1, pp. 42–57.
Mulloney, B. and Smarandache, C., Fifty years of CPGs: Two neuroethological papers that shaped the course of neuroscience, Front. Behav. Neurosci., 2010, vol. 4, no. 45, pp. 1–8.
Dynamic Biological Networks: The Stomatogastric Nervous System, Harris-Warrick, R.M., Marder, E., Selverston, A.I., and Moulins, M., Eds., Cambridge, MA: MIT Press, 1992.
Vizi, E.S., Kiss, J.P., and Lendvai, B., Nonsynaptic communication in the central nervous system, Rev. Neurochem. Int., 2004, vol. 45, pp. 443–451.
De-Miguel, F.F. and Trueta, C., Synaptic and extrasynaptic secretion of serotonin, Cell Mol. Neurobiol., 2005, vol. 25, pp. 297–312.
Sem’yanov, A.V., Diffusional extrasynaptic neurotransmission via glutamate and GABA, Neurosci. Behav. Physiol., 2005, vol. 35, pp. 253–266.
Dyakonova, T.L. and Dyakonova, V.E., Coordination of rhythm-generating units via NO and extrasynaptic neurotransmitter release, J. Comput. Physiol. A, 2010, vol. 196, no. 8, pp. 529–541.
Bargmann, C.I., Beyond the connectome: How neuromodulators shape neural circuits, BioEssays, 2012, vol. 34, no. 6, pp. 458–465.
Artemov, N.M., Sakharov, D.A., and Koshtoyants, K.S., Raboty po khimicheskim osnovam mekhanizmov nervnoy deyatelnosti (Works on Chemical Foundations of Nervous Activity Mechanisms), Moscow: Nauka, ch. 3, pp. 106–162.
Brezina, V., Beyond the wiring diagram: Signalling through complex neuromodulator networks, Philos. Trans. R. Soc. Lond. B. Biol. Sci., 2010, vol. 12, vol. 365, no. 1551, pp. 2363–2374.
Sakharov, D.A., The biological substrate for the generation of behavioral acts, Zh. Obshch. Biol., 2012, vol. 73, no. 5, pp. 334–348.
Agnati, L.F., Guidolin, D., Guescini, M., Genedani, S., and Fuxe, K., Understanding wiring and volume transmission, Brain Res. Rev., 2010, vol. 64, pp. 137–159.
Dyakonova, V.E., Neurotransmitter mechanisms of context-dependent behavior, Zh. Vyssh. Nerv. Deyat., 2012, vol. 62, no. 6, pp. 1–17.
Marder, E. and Bucher, D., Central pattern generators and the control of rhythmic movements, Curr. Biol., 2001, vol. 11, no. 23, pp. R986–996.
Amari, S.I., Learning patterns and pattern sequences by self-organizing nets of threshold elements, IEEE Trans. Comput., 1972, vol. C–21, no. 11, pp. 1197–1206.
Wang, R.-S. and Albert, R., Effects of community structure on the dynamics of random threshold networks, Phys. Rev. E, 2013, vol. 87.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.P. Kuznetsov, N.I. Bazenkov, B.A. Boldyshev, L.Yu. Zhilyakova, S.G. Kulivets, I.A. Chistopolsky, 2018, published in Iskusstvennyi Intellekt i Prinyatie Reshenii, 2018, No. 2, pp. 3–20.
About this article
Cite this article
Kuznetsov, O.P., Bazenkov, N.I., Boldyshev, B.A. et al. An Asynchronous Discrete Model of Chemical Interactions in Simple Neuronal Systems. Sci. Tech. Inf. Proc. 45, 375–389 (2018). https://doi.org/10.3103/S0147688218060072
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0147688218060072