Abstract
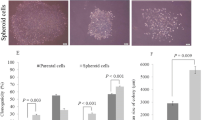
“Cancer stem cells” (CSCs) can initiate tumorigenesis and metastasis and show resistance against chemotherapy owing to the expression of prominent stem cell markers. CSCs are a subpopulation of highly heterogenic cancer spheroid cells. Pigment epithelium-derived factor (PEDF) is a neurotrophic, anti-tumorigenic, and anti-metastatic protein and its gene expression levels in A549 spheroids is still unknown. We aimed to compare clonogenicity and mRNA levels of PEDF, Oct4, and ALDH1A1 between A549 and spheroid cells. Spheroid and colony formation assays were performed with spheroid and A549 cells. We performed quantitative reverse transcription-polymerase chain reaction (qRT-PCR) for gene expression analysis. The clonogenic ratios for A549 and spheroids were ~60% and ~1% respectively. During spheroid formation, Oct4 mRNA level did not change but PEDF and ALDH1A1 levels decreased significantly. CSCs are characterized by elevated stem cell markers but spheroid cells consist of heterogeneous population including CSCs. In spheroid population, no increase in stem cell markers was observed. The reduced PEDF levels during spheroid transition can be a suppression mechanism of spheroid cells.


Similar content being viewed by others
REFERENCES
Adhikari, A.S., Agarwal, N., Wood, B.M., et al., CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance, Cancer Res., 2010, vol. 70, no. 11, pp. 4602–4612. https://doi.org/10.1158/0008-5472.CAN-09-3463
Allegra, A., Alonci, A., Penna, G., et al., The cancer stem cell hypothesis: a guide to potential molecular targets, Cancer Invest., 2014, vol. 32, pp. 470–495. https://doi.org/10.3109/07357907.2014.958231
Belkacemi, L. and Zhang, S.X., Anti-tumor effects of pigment epithelium-derived factor (PEDF): implication for cancer therapy. A mini-review, J. Exp. Clin. Cancer Res., 2016, vol. 35, art. ID 4. https://doi.org/10.1186/s13046-015-0278-7
Bonnet, D. and Dick, J.E., Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell, Nat. Med., 1997, vol. 3, pp. 730–737.
Bouck, N., PEDF: anti-angiogenic guardian of ocular function, Trends Mol. Med., 2002, vol. 8, pp. 330-334.
Chen, J., Ye, L., Zhang, L., et al., The molecular impact of pigment epithelium-derived factor, PEDF, on lung cancer cells and the clinical significance, Int. J. Oncol., 2009, vol. 35, pp. 159–166. https://doi.org/10.3892/ijo_00000324
Chen, Y.C., Hsu, H.S., Chen, Y.W., et al., Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells, PLoS One, 2008, vol. 3, art. ID e2637. https://doi.org/10.1371/journal.pone.0002637
Cheng, Y.H., Chen, Y.C., Brien, R., et al., Scaling and automation of a high-throughput single-cell-derived tumor sphere assay chip, Lab. Chip., 2016, vol. 16, pp. 3708–3717. https://doi.org/10.1039/c6lc00778c
Demestre, M., Terzi, M.Y., Mautner, V., et al., Effects of pigment epithelium derived factor (PEDF) on malignant peripheral nerve sheath tumors (MPNSTs), J. Neurooncol., 2013, vol. 115, pp. 391–399. https://doi.org/10.1007/s11060-013-1252-x
Dimou, A., Neumeister, V., Agarwal, S., et al., Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer, Am. J. Pathol., 2012, vol. 181, pp. 1436-1442. https://doi.org/10.1016/j.ajpath.2012.06.037
Duru, N., Candas, D., Jiang, G., et al., Breast cancer adaptive resistance: HER2 and cancer stem cell repopulation in a heterogeneous tumor society, J. Cancer Res. Clin. Oncol., 2014, vol. 140, pp. 1–14. https://doi.org/10.1007/s00432-013-1494-1
Eramo, A., Lotti, F., Sette, G., et al., Identification and expansion of the tumorigenic lung cancer stem cell population, Cell Death Differ., 2008, vol. 15, pp. 504-514. https://doi.org/10.1038/sj.cdd.4402283
Eyler, C.E. and Rich, J.N., Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis, J. Clin. Oncol., 2008, vol. 26, no. 17, pp. 2839–2845. https://doi.org/10.1200/JCO.2007.15.1829
Fernandez-Garcia, N.I., Volpert, O.V., and Jimenez, B., Pigment epithelium-derived factor as a multifunctional antitumor factor, J. Mol. Med., 2007, vol. 85, pp. 15–22. https://doi.org/10.1007/s00109-006-0111-z
Gradilone, A., Naso, G., Raimondi, C., et al., Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization, Ann. Oncol., 2011, vol. 22, no. 1, pp. 86–92. doihttps://doi.org/10.1093/annonc/mdq323
Herreros-Pomares, A., de-Maya-Girones, J.D., Calabuig-Farinas, S., et al., Lung tumorspheres reveal cancer stem cell-like properties and a score with prognostic impact in resected non-small-cell lung cancer, Cell Death Dis., 2019, vol. 10, art. ID 660. https://doi.org/10.1038/s41419-019-1898-1
Hong, H., Zhou, T., Fang, S., et al., Pigment epithelium-derived factor (PEDF) inhibits breast cancer metastasis by down-regulating fibronectin, Breast Cancer Res. Treat., 2014, vol. 148, pp. 61–72. https://doi.org/10.1007/s10549-014-3154-9
Jiang, F., Qiu, Q., Khanna, A., et al., Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer, Mol. Cancer Res., 2009, vol. 7, no. 3, pp. 330–338. https://doi.org/10.1158/1541-7786.MCR-08-0393
Jordan, C.T., Cancer stem cell biology: from leukemia to solid tumors, Curr. Opin. Cell Biol., 2004, vol. 16, pp. 708–712. https://doi.org/10.1016/j.ceb.2004.09.002
Kim, D., Choi, B.H., Ryoo, I.G., et al., High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling, Cell Death Dis., 2018, vol. 9, art. ID 896. https://doi.org/10.1038/s41419-018-0903-4
Lee, C.H., Yu, C.C., Wang, B.Y., et al., Tumorsphere as an effective in vitro platform for screening anti-cancer stem cell drugs, Oncotarget, 2016, vol. 7, pp. 1215–1226. https://doi.org/10.18632/oncotarget.6261
Li, D., Zhang, T., Gu, W., et al., The ALDH1(+) subpopulation of the human NMFH-1 cell line exhibits cancer stem-like characteristics, Oncol. Rep., 2015, vol. 33, pp. 2291–2298. https://doi.org/10.3892/or.2015.3842
Liu, J., Ma, L., Xu, J., et al., Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties, Int. J. Oncol., 2013, vol. 42, pp. 453–459. https://doi.org/10.3892/ijo.2012.1720
Massague, J. and Obenauf, A.C., Metastatic colonization by circulating tumour cells, Nature, 2016, vol. 529, pp. 298–306. https://doi.org/10.1038/nature17038
Minkevich, N.I., Lipkin, V.M., and Kostanyan I.A., PEDF—a noninhibitory serpin with neurotrophic activity, Acta Naturae, 2010, vol. 2, pp. 62–71.
Morrison, B.J., Steel, J.C., and Morris, J.C., Sphere culture of murine lung cancer cell lines are enriched with cancer initiating cells, PLoS One, 2012, vol. 7, art. ID e49752. https://doi.org/10.1371/journal.pone.0049752
Nwani, N.G., Deguiz, M.L., Jimenez, B., et al., Melanoma cells block PEDF production in fibroblasts to induce the tumor-promoting phenotype of cancer-associated fibroblasts, Cancer Res., 2016, vol. 76, pp. 2265–2276. https://doi.org/10.1158/0008-5472.CAN-15-2468
Pang, L., Ding, J., Ge, Y., et al., Single-cell-derived tumor-sphere formation and drug-resistance assay using an integrated microfluidics, Anal. Chem., 2019, vol. 91, pp. 8318–8325. https://doi.org/10.1021/acs.analchem.9b01084
Patel, M., Lu, L., Zander, D.S., et al., ALDH1A1 and ALDH3A1 expression in lung cancers: Correlation with histologic type and potential precursors, Lung Cancer, 2008, vol. 59, pp. 340–349. https://doi.org/10.1016/j.lungcan.2007.08.033
Qureshi-Baig, K., Ullmann, P., Rodriguez, F., et al., What do we learn from spheroid culture systems? Insights from tumorspheres derived from primary colon cancer tissue, PLoS One, 2016, vol. 11, art. ID e0146052. https://doi.org/10.1371/journal.pone.0146052
Ribaux, P., Britan, A., Thumann, G., et al., Malignant ascites: a source of therapeutic protein against ovarian cancer?, Oncotarget, 2019, vol. 10, pp. 5894–5905. https://doi.org/10.18632/oncotarget.27185
Roudi, R., Madjd, Z., Ebrahimi, M., et al., CD44 and CD24 cannot act as cancer stem cell markers in human lung adenocarcinoma cell line A549, Cell. Mol. Biol. Lett., 2014, vol. 19, pp. 23–36. https://doi.org/10.2478/s11658-013-0112-1
Seo, D.C., Sung, J.M., Cho, H.J., et al., Gene expression profiling of cancer stem cell in human lung adenocarcinoma A549 cells, Mol. Cancer, 2007, vol. 6, art. ID 75. https://doi.org/10.1186/1476-4598-6-75
Singh, A.K., Arya, R.K., Maheshwari. S., et al., Tumor heterogeneity and cancer stem cell paradigm: updates in concept, controversies and clinical relevance, Int. J. Cancer, 2015, vol. 136, pp. 1991–2000. https://doi.org/10.1002/ijc.28804
Sladek, N.E., Human aldehyde dehydrogenases: Potential pathological, pharmacological, and toxicological impact, J. Biochem. Mol. Toxicol., 2003, vol. 17, pp. 7–23. https://doi.org/10.1002/jbt.10057
Sourisseau, T., Hassan, K.A., Wistuba, I., et al., Lung cancer stem cell: fancy conceptual model of tumor biology or cornerstone of a forthcoming therapeutic breakthrough?, J. Thorac. Oncol., 2014, vol. 9, pp. 7–17. https://doi.org/10.1097/JTO.0000000000000028
Spyra, M., Kluwe, L., Hagel, C., et al., Cancer stem cell-like cells derived from malignant peripheral nerve sheath tumors, PLoS One, 2011, vol. 6, art. ID e21099. https://doi.org/10.1371/journal.pone.0021099
Sung, J.M., Cho, H.J., Yi, H., et al., Characterization of a stem cell population in lung cancer A549 cells, Biochem. Biophys. Res. Commun., 2008, vol. 371, no. 1, pp. 163–167. https://doi.org/10.1016/j.bbrc.2008.04.038
Teng, Y., Wang, X., Wang, Y., et al., Wnt/β-catenin signaling regulates cancer stem cells in lung cancer A549 cells, Biochem. Biophys. Res. Commun., 2010, vol. 392, no. 3, pp. 373–379. https://doi.org/10.1016/j.bbrc.2010.01.028
Terzi, M.Y., Casalis, P., Lang, V., et al., Effects of pigment epithelium-derived factor on traumatic brain injury, Restor. Neurol. Neurosci., 2015, vol. 33, pp. 81–93. https://doi.org/10.3233/RNN-140417
Ucar, D., Cogle, C.R., Zucali, J.R., et al., Aldehyde dehydrogenase activity as a functional marker for lung cancer, Chem. -Biol. Interact., 2009, vol. 178, nos. 1–3, pp. 48–55. https://doi.org/10.1016/j.cbi.2008.09.029
Wang, K., Wu, X., Wang, J., et al., Cancer stem cell theory: therapeutic implications for nanomedicine, Int. J. Nanomed., 2013, vol. 8, pp. 899–908. https://doi.org/10.2147/IJN.S38641
Wang, Q., Zhang, Z., Ding, T., et al., Mesenchymal stem cells overexpressing PEDF decrease the angiogenesis of gliomas, Biosci. Rep., 2013, vol. 33, art. ID e00019. https://doi.org/10.1042/BSR20110124
Xu, Y., Hu, J., Zhu, Q., et al., Co-detection of ALDH1A1, ABCG2, ALCAM and CD133 in three A549 subpopulations at the single cell level by one-step digital RT-PCR, Integr. Biol., 2018, vol. 10, no. 6, pp. 364–369. https://doi.org/10.1039/c8ib00042e
Zhang, L., Chen, J., Ke, Y., et al., Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome, Int. J. Mol. Med., 2006, vol. 17, no. 5, pp. 937–944.
Zhou, C., Chen, X., Zeng, W., et al., Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway, Oncotarget, 2016, vol. 7, pp. 68314–68327. https://doi.org/10.18632/oncotarget.11599
Zhou, D., Zhang, M., Xu, P., et al., Expression of pigment epithelium-derived factor is associated with a good prognosis and is correlated with epithelial-mesenchymal transition-related genes in infiltrating ductal breast carcinoma, Oncol. Lett., 2016, vol. 11, pp. 116–124. https://doi.org/10.3892/ol.2015.3880
ACKNOWLEDGMENTS
None.
Funding
This research was funded by the Scientific Research Project Funds of Hatay Mustafa Kemal University (Project no. 18.M. 102).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
AUTHOR CONTRIBUTION
MYT designed the study. MYT and HMO performed the experiments. M.Y.T., H.M.O., G.G.D., and M.U.K. analyzed the data statistically and M.Y.T. prepared all figures. M.Y.T. prepared tables. All authors wrote/drafted/edited/revised the manuscript and interpreted the results. All authors read, edited, and gave an approval for the final version of the present manuscript. All authors are aware of the order of authorship and that no further changes in authorship will be performed after submission.
About this article
Cite this article
Terzi, M.Y., Okuyan, H.M., Gülbol-Duran, G. et al. Reduced Expression of PEDF and ALDH1A1 during Spheroid Transition of Lung Cancer Cells: An In Vitro Study. Cytol. Genet. 56, 172–178 (2022). https://doi.org/10.3103/S0095452722020104
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452722020104