Abstract
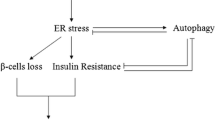
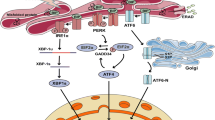
Endoplasmic reticulum (ER) plays a central role in the synthesis of proteins and their post-translational modification by folding newly synthesized proteins through the formation of disulfide bonds, which is necessary for their final stable conformational state. ER homeostasis is stressed when the influx of newly synthesized unfolded or misfolded polypeptide chains exceeds the ER capacity for repair and refolding. ER stress in diabetes can be caused by various factors that inhibit protein folding, such as glucose, nonesterified cholesterol, oxidized phospholipids, saturated fatty acids, and ROS. Chronic ER stress leads to the death of pancreatic β-cells, increases hyperglycemia, and is the main etiology of diabetes. Atherosclerosis (AS) is a chronic inflammatory disease that underlies the pathology of ischemic cardiovascular and cerebrovascular diseases. It has been documented that both endoplasmic reticulum (ER) stress and NLRP3 inflammasomes influence the progression of AS. The ER stress response in endothelial cells leads to inflammation and cell death in diabetes-related vascular diseases. ER stress also plays a key role in the onset of atherosclerosis in diabetes, which is a major consequence of endothelial dysfunction. Several independent risk factors for cardiovascular diseases, namely hyperhomocysteinemia, obesity, and dyslipidemia, as well as hyperglycemia, are also associated with ER stress, which indicates its integrating function in atherogenesis. The etiological role of low-level tissue inflammation in the formation of insulin resistance and β-cell dysfunction in type 2 diabetes is commonly recognized. Among innate immune receptors, NLRP3 plays a critical role in tissue inflammation associated with lipid overload or obesity. The research showed that ER stress is involved in inflammation and that ER plays a key role in the activation of NLRP3-inflammasomes, which trigger secretion of proinflammatory cytokines, such as IL-1β and IL-18. Metformin, an AMPK activator, inhibits ER stress and restores endothelial cell function in diabetes. Metformin inhibits NLRP3 inflammasome activation under ER stress through suppression of IL-6 and MCP-1 production induced by high glucose levels, lower TXNIP expression, and activation of autophagy via AMPK.

Similar content being viewed by others
REFERENCES
Agouni, A., Tual-Chalot, S., Chalopin, M., et al., Hepatic protein tyrosine phosphatase 1B (PTP1B) deficiency protects against obesity-induced endothelial dysfunction, Biochem. Pharmacol., 2014, vol. 92, pp. 607–617. https://doi.org/10.1016/j.bcp.2014.10.008
Bronner, D.N., Abuaita, B.H., Chen, X., et al., Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage, Immunity, 2015, vol 43, pp. 451–462. https://doi.org/10.1016/j.immuni.2015.08.008
Chai, T.F., Hong, S.Y., He, H., et al., A potential mechanism of metformin-mediated regulation of glucose homeostasis: inhibition of thioredoxin-interacting protein (TXNIP) gene expression, Cell Signal., 2012, vol. 24, pp. 1700–1705.https://doi.org/10.1016/j.cellsig.2012.04.017
Cheang, W.S., Tian, X.Y., Wong, W.T., et al., Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5' adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway, Arterioscler. Thromb. Vase Biol., 2014, vol. 34, no. 4, pp. 830–836. https://doi.org/10.1161/ATVBAHA.113.301938
Chen, X., Guo, X., Ge, Q., et al., ER stress activates the NLRP3 inflammasome: a novel mechanism of atherosclerosis, Oxid. Med. Cell Longev., 2019a, p. 3462530. https://doi.org/10.1155/2019/3462530
Chen, C., Kassan, A., Castaceda, D., et al., Metformin prevents vascular damage in hypertension through the AMPK/ER stress pathway, Hypertens. Res., 2019b, vol. 42, no. 7, pp. 960–969. https://doi.org/10.1038/s41440-019-0212-z
Chen, Y., Wang, J.J., Li, J., et al., Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes, Diabetologia, 2012, vol. 55, no. 9, pp. 2533–2545. https://doi.org/10.1007/sOO125-012-2594-1
Cnop, M., Toivonen, S., Igoillo-Esteve, M., and Salpea, P., Endoplasmic reticulum stress and eIF2a phosphorylation: the Achilles heel of pancreatic β cells, Mol. Metab., 2017, vol. 6, no. 9, pp. 1024–1039. https://doi.org/10.1016/j.molmet.2017.06.001
Davies, P.F., Civelek, M., Fang, Y., and Fleming, I., The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo, Cardiovasc. Res., 2013, vol. 99, no. 2, pp. 315–327. https://doi.org/10.1093/cvr/cvtl01
de la Roche, M., Hamilton, C., Mortensen, R., et al., Trafficking of cholesterol to the ER is required for NLRP3 inflammasome activation, J. Cell Biol., 2018, vol. 217, pp. 3560–3576. https://doi.org/10.1083/jcb.201709057
Dong, Y., Zhang, M., Wang, S., et al., Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo, Diabetes, 2010, vol. 59, no. 6, pp. 1386–1396. https://doi.org/10.2337/db09-1637
Flamment, M., Hajduch, E., Ferre, P., and Foufelle, F., New insights into ER stress-induced insulin resistance, Trends Endocrinol. Metab., 2012, vol. 23, pp. 381–390.https://doi.org/10.1016/j.tem.2012.06.003
Fonseca, S.G., Gromada, J., and Urano, F., Endoplasmic reticulum stress and pancreatic beta-cell death, Trends Endocrinol. Metab., 2011, vol. 22, no. 7, pp. 266–274.https://doi.org/10.1016/j.tem.2011.02.008
Galan, M., Kassan, M., Choi, S.K., et al., A novel role for epidermal growth factor receptor tyrosine kinase and its downstream endoplasmic reticulum stress in cardiac damage and microvascular dysfunction in type 1 diabetes mellitus, Hypertension, 2012, vol. 60, pp. 71–80. https://doi.org/10.1161/HYPERTENSIONAHA.11.192500
Galan, M., Kassan, M., Kadowitz, P.J., et al., Mechanism of endoplasmic reticulum stress-induced vascular endothelial dysfunction, Biochim. Biophys. Acta, 2014, vol.1843, pp. 1063–1075.https://doi.org/10.1016/j.bbamcr.2014.02.009
Gardner, B.M., Pincus, D., Gotthardt, K., et al., Endoplasmic reticulum stress sensing in the unfolded protein response, Cold Spring Harb. Perspect. Biol., 2013, vol. 5, art. a013169. https://doi.org/10.1101/cshperspect.a013169
Ghemrawi, R., Battaglia-Hsu, S.F., and Arnold, C., Endoplasmic reticulum stress in metabolic disorders, Cells, 2018, vol. 7, no. 6, p. 63. https://doi.org/10.3390/cells7060063
He, Y., Hara, H., and Nunez, G., Mechanism and regulation of NLRP3 inflammasome activation, Trends Biochem. Sci., 2016, vol. 41, pp. 1012–1021. doi . 09.002https://doi.org/10.1016/j.tibs.2016
Hossain, G.S., Lynn, E.G., Maclean, K.N., et al., Deficiency of TDAG51 protects against atheroclerosis by modulating apoptosis, cholesterol efflux, and peroxiredoxin-1 expression, J. Am. Heart Assoc., 2013, vol. 2, no. 3, e000134. https://doi.org/10.1161/JAHA.113.000134
Hu, M., Phan, F., Bourron, O., et al., Steatosis and NASH in type 2 diabetes, Biochimie, 2017, vol. 143, pp. 37–41. https://doi.org/10.1016/j.biochi.2017.10.019
Hur, K.Y. and Lee, M.S., New mechanisms of metformin action: focusing on mitochondria and the gut, J. Diabetes Invest., 2015, vol. 6, no. 6, pp. 600–609. https://doi.org/10.1111/jdi.12328
Inagi, R., Ishimoto, Y., and Nangaku, M., Proteostasis in endoplasmic reticulum—new mechanisms in kidney disease, Nat. Rev. Nephrol., 2014, vol. 10, no. 7, pp. 369–378. https://doi.org/10.1038/nrneph.2014.67
Incalza, M.A., D’Oria, R., Natalicchio, A., et al., Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases, Vasc. Pharmacol., 2018, vol. 100, pp. 1–19. https://doi.org/10.1016/j.vph.2017.05.005
Jamwal, S. and Sharma, S., Vascular endothelium dysfunction: a conservative target in metabolic disorders, Inflamm. Res., 2018, vol. 67, pp. 391–405. https://doi.org/10.1007/s00011-018-1129-8
Kim, S., Joe, Y., Jeong, S.O., et al., Endoplasmic reticulum stress is sufficient for the induction of IL-1 beta production via activation of the NF-kappa B and inflammasome pathways, Innate Immun., 2014, vol. 20, pp. 799–815. https://doi.org/10.1177/1753425913508593
Kornfeld, O.S., Hwang, S., Disatnik, M.H., et al., Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases, Circ. Res., 2015, vol. 116, pp. 1783–1799. https://doi.org/10.1161/CIRCRESAHA.116.305432
Lebeaupin, C., Proics, E., de Bieville, C.H., et al., ER stress induces NLRP3 inflammasome activation and hepatocyte death, Cell Death Dis., 2015, vol. 6, e1879. https://doi.org/10.1038/cddis.2015.248
Lee, H.M., Kim, J.J., Kim, H.J., et al., Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes, Diabetes, 2013, vol. 62, pp. 194–204. https://doi.org/10.2337/db12-0420
Lenna, S., Han, R., and Trojanowska, M., Endoplasmic reticulum stress and endothelial dysfunction, IUBMB Life, 2014, vol. 66, no. 8, pp. 530–537. https://doi.org/10.1002/iub.1292
Lerner, A.G., Upton, J.P., Praveen, P.V., et al., IRE1 a induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress, Cell Metab., 2012, vol. 16, pp. 250–264. https://doi.org/10.1016/j.cmet.2012.07.007
Li, A., Zhang, S., Li, J., et al., Metformin and resveratrol inhibit drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice, Mol. Cell Endocrinol., 2016, vol. 434, pp. 36–47. https://doi.org/10.1016/j.mce.2016.06.008
Liang, B., Wang, S., Wang, Q., et al., Aberrant endoplasmic reticulum stress in vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase-α 2 in vivo, Arterioscler. Thromb. Vasc. Biol., 2013, vol. 33, pp. 595–604. https://doi.org/10.1161/ATVBAHA.112.300606
Lisa, S., Domingo, B., Martinez, J., et al., Failure of prion protein oxidative folding guides the formation of toxic transmembrane forms, J. Biol. Chem., 2012, vol. 287, pp. 36693–36701. https://doi.org/10.1074/jbc.M112.398776
Liu, Q., Zhang, D., Hu, D., et al., The role of mitochondria in NLRP3 infiammasome activation, Mol. Immunol., 2018, vol. 103, pp. 115–124. https://doi.org/10.1016/j.molimm.2018.09.010
Lytrivi, M., Castell, A.L., Poitout, V., and Cnop, M., Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes, J. Mol. Biol., 2020, vol. 432, no. 5, pp. 1514–1534. https://doi.org/10.1016/j.jmb.2019.09.016
Maamoun, H., Zachariah, M., McVey, J.H., et al., Heme oxygenase (HO)-1 induction prevents endoplasmic reticulum stress-mediated endothelial cell death and impaired angiogenic capacity, Biochem. Pharmacol., 2017, vol. 127, pp. 46–59. https://doi.org/10.1016/j.bcp.2016.12.009
Maamoun, H., Abdelsalam, S.S., Zeidan, A., et al., Endoplasmic reticulum stress: a critical molecular driver of endothelial dysfunction and cardiovascular disturbances associated with diabetes, Int. J. Mol. Sci., 2019a, vol. 20, no. 7, p. 1658. https://doi.org/10.3390/ijms20071658
Maamoun, H., Benameur, T., Pintus, G., et al., Crosstalk between oxidative stress and endoplasmic reticulum (ER) stress in endothelial dysfunction and aberrant angiogenesis associated with diabetes: a focus on the protective roles of heme oxygenase (HO)-1, Front. Physiol., 2019b, vol. 10, p. 70. https://doi.org/10.3389/fphys.2019.00070
Misawa, T., Takahama, M., Kozaki, T., et al., Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome, Nat. Immunol., 2013, vol. 14, pp. 454–460. https://doi.org/10.1038/ni.2550
Mohan, S., Rani, P.R.M., Brown, L., et al., Endoplasmic reticulum stress: a master regulator of metabolic syndrome, Eur. J. Pharmacol., 2019, vol. 860, p. 172553. https://doi.org/10.1016/j.ejphar.2019.172553
Muller, C., Salvayre, R., Negre-Salvayre, A., and Vindis, C., HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs, Cell Death Differ., 2011, vol. 18, no. 5, pp. 817–828. https://doi.org/10.1038/cdd.2010.149
Oslowski, C.M., Hara, T., O’Sullivan-Murphy, B., et al., Thioredoxin-interacting protein mediates ER stress-induced B cell death through initiation of the inflammasome, Cell Metab., 2012, vol. 16, pp. 265–273. https://doi.org/10.1016/j.cmet.2012.07.005
Owen, C., Lees, E.K., Grant, L., et al., Inducible liver-specific knockdown of protein tyrosine phosphatase 1B improves glucose and lipid homeostasis in adult mice, Diabetologia, 2013, vol. 56, pp. 2286–2296. https://doi.org/10.1007/s00125-013-2992-z
Ozcan, L. and Tabas, I., Role of endoplasmic reticulum stress in metabolic disease and other disorders, Ann. Rev. Med., 2012, vol. 63, pp. 317–328.
Pushkarev, V.M., Sokolova, L.K., Pushkarev, V.V., and Tronko, M.D., The role of AMPK and mTOR in the development of insulin resistance and type 2 diabetes. The mechanism of metformin action, Probl. Endocrin. Pathol., 2016, vol. 3, pp. 77–90.
Shi, C.S., Shenderov, K., Huang, N.N., et al., Activation of autophagy by inflammatory signals limits IL-1b production by targeting ubiquitinated inflammasomes for destruction, Nat. Immunol., 2012, vol. 13, pp. 255–263. https://doi.org/10.1038/ni.2215
Sokolova, L.K., Pushkarev, V.M., Belchina, Y.B., et al., Effect of combined treatment with insulin and metformin on 5'AMP-activated protein kinase activity in lymphocytes of diabetic patients, Dopov. Nac. Akad. Nauk Ukr., 2018, vol. 5, pp. 100–104. https://doi.org/10.15407/dopovidi2018.05.100
Sokolova, L.K., Pushkarev, V.M., Pushkarev, V.V., et al., Diabetes mellitus and atherosclerosis. The role of inflammatory processes in pathogenesis, Mezhdunarod. Endokrinol. Zh., 2017, vol. 13, no. 7, pp. 486–498.
Son, S.M. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes, Diabetes Metab. J., 2012, vol. 36, pp. 190–198. https://doi.org/10.4093/dmj.2012.36.3.190
Tabas, I., The role of endoplasmic reticulum stress in the progression of atherosclerosis, Circ. Res., 2010, vol. 107, no. 7, pp. 839–850. doi . 224766https://doi.org/10.1161/CIRCRESAHA.110
Talty, A., Deegan, S., Ljujic, M., et al., Inhibition of IRE1alpha RNase activity reduces NLRP3 inflammasome assembly and processing of pro-IL1beta, Cell Death Dis., 2019, vol. 10, p. 622. https://doi.org/10.1038/s41419-019-1847-z
Thon, M., Hosoi, T., Yoshii, M., and Ozawa, K., Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells, Sci. Rep., 2014, vol. 4, p. 7096. https://doi.org/10.1038/srep07096
Tronko, N.D., Pushkarev, V.M., Sokolova, L.K., et al., Molecular Mechanisms of Pathogenesis of Diabetes and Its Complications, Kyiv: Medkniga, 2018.
Tufanli, O., Telkoparan Akillilar, P., Acosta-Alvear, D., et al., Targeting IRE1 with small molecules counteracts progression of atherosclerosis, Proc. Natl. Acad. Sci. U. S. A., 2017, vol. 114, pp. E1395–E1404. https://doi.org/10.1073/pnas.1621188114
Vandanmagsar, B., Youm, Y.H., Ravussin, A., et al., The NLRP3 inflammasome instigate obesity-induced inflammation and insulin resistance, Nat. Med., 2011, vol. 15, pp. 179–188. https://doi.org/10.1038/nm.2279
Walter, P. and Ron, D., The unfolded protein response: from stress pathway to homeostatic regulation, Science, 2011, vol. 334, pp. 1081–1086. https://doi.org/10.1126/science.1209038
Wang, Y.I., Bettaieb, A., Sun, C., et al., Triglyceride-rich lipoprotein modulates endothelial vascular cell adhesion molecule (VCAM)-1 expression via differential regulation of endoplasmic reticulum stress, PLoS One, 2013, vol. 8, no. 10, e78322. https://doi.org/10.1371/journal.pone.0078322
Ye, J., Mechanisms of insulin resistance in obesity, Front. Med., 2013, vol. 7, no. 1, pp. 14–24. https://doi.org/10.1007/s11684-013-0262-6
Zhou, J., Massey, S., Story, D., and Li, L., Metformin: an old drug with new applications, Int. J. Mol. Sci., 2018, vol. 19, no. 10, p. 2863. https://doi.org/10.3390/ijms19102863
Zhou, R., Tardivel, A., Thorens, B., et al., Thioredoxin-interacting protein links oxidative stress to inflammasome activation, Nat. Immunol., 2010, vol 11, pp. 136–140. https://doi.org/10.1038/ni.1831
Zhou, Y., Tong, Z., Jiang, S., et al., The roles of endoplasmic reticulum in NLRP3 inflammasome activation, Cell, 2020, vol. 9, no. 5, p. 1219. https://doi.org/10.3390/cells9051219
Zoungas, S., Chalmers, J., Ninomiya, T., et al., Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds, Diabetologia, 2012, vol. 55, no. 3, pp. 636–643. https://doi.org/10.1007/s00125-011-2404-1
Funding
The article was prepared within the budget funding of the National Academy of Medical Sciences of Ukraine according to the plan of research work of the Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Sciences of Ukraine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by K. Lazarev
About this article
Cite this article
Pushkarev, V.V., Sokolova, L.K., Kovzun, O.I. et al. The Role of Endoplasmic Reticulum Stress and NLRP3 Inflammasomes in the Development of Atherosclerosis. Cytol. Genet. 55, 331–339 (2021). https://doi.org/10.3103/S0095452721040113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0095452721040113