Abstract
We review the known information on the distribution and habitat affinities of the Micrargus herbigradus-species group in Poland. The analysis is based on a thorough literature survey, our own materials, and verification of some older collections. We give new diagnostic drawings and review the characters that are useful in identification of species within the group. Three species are present in Poland: M. herbigradus (Blackwall, 1854), M. apertus (O.-P. Cambridge, 1870) and M. georgescuae Millidge, 1976. The latter is recorded for the first time in the country, and we add numerous new localities for the two former species. Micrargus herbigradus is common and widespread in Poland, living in various habitats, with only a slight preference to forests. In contrast, M. apertus is widely distributed but rarely found, while its affinity to forests is the highest within the group. The records of this species are most numerous in lowland forests (up to c. 300 m a.s.l), but it can also be found at higher altitudes. M. georgescuae is found only in montane habitats, both in the Sudetes and the Carpathian Mountains, from above 650 m a.s.l. The adults of all three species occur the whole year round, but seem to be most abundant in May and June.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Micrargus herbigradus-group contains four Central European species: M. alpinus Rëlys and Weiss, 1997, M. apertus (O.-P. Cambridge, 1870), M. georgescuae Millidge, 1976 and M. herbigradus (Blackwall, 1854). Two species from this group, M. apertus and M. herbigradus, have been recorded in Poland. Here we add one more species to the list of the Polish fauna, i.e. M. georgescuae. Micrargus subaequalis (Westring, 1851), which is also present in Poland, belongs to a separate species group.
The species group discussed here was revised by Millidge (1976), and subsequently one further species was described from the Alps by Rëlys and Weiss (1997). Due to difficulties in the identification of Micragus species, their actual distribution in Poland was uncertain. Some authors suggested the presence of more than two herbigradus-group members (Staręga and Kupryjanowicz 1996), or the presence of some new species (Staręga 2003a), but they did not make final conclusions about the specimens’ identity. Since the species are difficult to identify we summarize all the features that are useful for their determination.
Although the biology of this species group is fairly well described in the literature, there is no thorough analysis of the data from Poland. Some of the published remarks on their ecology might have been simplifications. Thus, we summarize the features for accurate species identification, revise the data on the distribution of these species in the country, referring both to published information and our own findings, and analyze their characteristic habitats and phenology. Furthermore, we review the ambiguous data from previous publications.
Methods
We gathered information from different studies, both published and unpublished. The published data are listed in the results, for which some of the previous records were verified (Staręga and Kupryjanowicz 1996; Staręga 2003a). In total, 97 articles were used for summarizing information on the distribution, habitat preferences and phenology of the herbigradus-group in Poland. Additionally, we included unpublished records that emanate from our own research in the analysis, of which all the specimens were identified by us. The detailed list of new, unpublished records is attached as supplementary electronic material (Online Resource 1: Tables S1–S4). The analysis of the distribution is based on research utilizing diverse methods of sampling material, i.e. pitfall traps, sweep net, sieving or direct search. The intensity of research in different parts of the country was decisively uneven, thus some results might be biased.
For the analysis of the altitudinal distribution and habitat preferences, we used the single sampling plots as units and did not consider the numbers of recorded specimens. When different habitats were investigated in a particular study – even if they were situated very close to each other – we counted them as separate plots. Based on literature, we assigned each plot to an altitudinal interval at 100 m resolution, i.e. 0–100, 100–200 m a.s.l., etc. This resolution still allowed us to draw some meaningful conclusions, but it was the highest resolution that we could apply to data from the literature.
Furthermore, the properties of the habitats in each plot were studied. In the first step of the analysis we divided the habitats into open or forested. The second division was based on the humidity. We sorted habitats according to their humidity, with five successive levels. The attribution of each habitat to a humidity level does not refer to the exact place or time (which could not be verified), but to the typical features of a habitat type, e.g. all lowland alder forests or different mire types were recognized as ‘very moist’. The data in the literature were obviously of different quality or credibility, thus selected records were excluded from some counts. The exact number of records used in each analysis is given in the results.
For the analysis of phenology as a unit we have used a single record of a species in one plot, per year (i.e. records from the same plot and date coming from two different years were counted separately). We have divided the records into those coming from the first half of each month (1st–15th) and those from the second half (16th–end). For data from pitfall traps we have assigned the record to the respective period by taking the middle date of the trap exposure time.
Results
Distinguishing species
Males of the Micrargus herbigradus-group can be identified by comparing their embolus, size and form of the adjacent lamellae, and the shape of the process (apical spine) that accompanies the embolus. Micrargus georgescuae has a conspicuously wide basal part of the embolus (Figs. 4, 11), while that of M. apertus and M. herbigradus is thin (Figs. 17 and 19, respectively). The apical spine of the palp is slightly sinuous, with a truncated tip in M. herbigradus (Fig. 20), whereas M. apertus and M. georgescuae differ in having this process smooth apically (Figs. 18 and 12). This spine is shorter and straight in M. apertus (Fig. 18), but clearly longer and slightly bent in M. georgescuae (Fig. 12). The lamellae accompanying the process are much smaller in M. georgescuae (Fig. 15) compared to M. apertus, and have a distinctly different shape.
The females can be identified by the arrangement of the seminal ducts, a structure seen on the inner side of the epigyne after dissecting it. We suggest not to try distinguishing species without dissecting this structure, although there might be some interspecific differences (Figs. 21, 25, 28). The seminal ducts of M. herbigradus form very distinctive long, wide and bent coils in the anterior part of the epigyne, relatively far from the spermathecae (Figs. 29, 30), whereas in M. georgescuae the ducts form a small narrow loop (Figs. 23, 24). The ducts of M. apertus have a much more complex structure: the first loop extends clearly in the anterior part of the epigyne, and subsequently forms a posterior coil (Figs. 26, 27). We summarize the features useful in species identification in Table 1.
The species differ also in the microsculpture of the stridulatory organs, which was shown by Rëlys and Weiss (1997). The other feature that distinguishes the herbigradus-group species is their size, M. apertus being the largest one, as mentioned by Millidge (1976). However, these characters are of little use in identifying spiders, unless one has all of the species available for comparison.
New localities
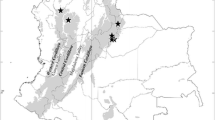
This study represents the first report of M. georgescuae in Poland. In total, 126 individuals (63 males and 63 females) were collected in several mountain ranges of the Carpathians – the Beskid Wyspowy, the Tatra Mountains, the Gorce (all in S-Central Poland); in the Orawa-Nowy Targ Basin, at high altitudes; and in the Sudetes – the Stołowe Mountains and the Giant Mountains (SW Poland; Fig. 31).
Micrargus georgescuae Millidge, 1976, male. 8. Cheliceral dentition; 9. Palpal organ, prolateral view; 10. Ditto, ventroprolateral view; 11. Ditto, ventral view; 12. Ditto, ventroretrolateral view; 13. Ditto, retrolateral view; 14. Ditto, dorsal view; 15. embolic part. Scales: 0.1 mm (9–14), 0.2 mm (8), eb - embolic base, as – apical spine, la – lamella
The specimens from the Gorce come from the study by Staręga and Kupryjanowicz (1996); most of this material belonged to M. georgescuae (Online Resource 1: Table S2). The species was present solely in the mountains, thus its range is restricted to the southern part of the country. It is not widespread, but in places where it lives, M. georgescuae may be quite numerous (Online Resource 1: Table S3). When one considers its broader European range, it was found in several major mountain ranges of Central Europe (see the Discussion).
Micrargus apertus is reported from five new localities in different parts of the country; it has already been recorded from 16 UTM (Universal Transverse Mercator) squares previously. The localities are scattered around the country, from the lowlands to the mountains (Fig. 32, Online Resource 1: Table S1).
Micrargus herbigradus is extremely common, and the relatively large number of published and newly added localities from Poland does not totally depict its true distribution, which is surely much broader. In addition to the 175 UTM squares that it has been reported from previously, we add 40 points to the grid. We confirm its presence in eight localities. In total, the species has now been recorded from 215 UTM grid squares (Fig. 33; Online Resource 1: Table S4).
Micrargus apertus (O.-P. Cambridge, 1870) and Micrargus herbigradus (Blackwall, 1854), females. 25. M. apertus, epigyne; 26. Ditto, spermathecae, ventral view; 27. Ditto, spermathecae, dorsal view; 28. Micrargus herbigradus, epigyne; 29. Ditto, spermathecae, ventral view; 30. Ditto, spermathecae, dorsal view. Scale: 0.1 mm
Distribution of Micrargus georgescuae in Poland. References: Staręga and Kupryjanowicz (1996): as a new species related to Micrargus georgescuae (only part of specimens). The red dots in all maps refer to our own findings or the material verified by us
Distribution of Micrargus apertus in Poland. References: Staręga (1996a), Staręga and Stankiewicz (1996), Chyży and Staręga (1997), Staręga (2003a), Rozwałka (2004), Kupryjanowicz (2005), Stańska (2005, 2007), Rozwałka (2009, 2010a), Hajdamowicz et al. (2016). The red dots refer to our own findings, the black dots to the data from the literature
Distribution of Micrargus herbigradus in Poland. References: Nowicki (1874), Kulczyński (1876, 1881), Dahl (1902), Schenkel (1929), Kajak (1960), Łuczak (1960), Pilawski (1962), Sanocka-Wołoszyn (1964), Pilawski (1965), Bednarz and Czajka (1966), Pilawski (1966a, b), Staręga (1966), Pilawski (1967), Pilawski (1970), Prószyński and Staręga (1971), Staręga (1971, 1972), Deltshev and Kajak (1974), Dziabaszewski (1974), Staręga (1974), Woźny (1975a, b), Czajka (1976), Czajka and Goos (1976), Staręga (1976), Woźny (1976), Staręga (1978), Jędryczkowski and Staręga (1980), Krzyżanowska et al. (1981), Puszkar (1981), Sanocka-Wołoszynowa (1981), Czajka and Kornalewicz (1982), Krzyżanowska (1982), Staręga (1984), Woźny (1985), Staręga (1988), Tomek (1988), Woźny et al. (1988), Dziabaszewski (1989), Dziabaszewski et al. (1989), Staręga (1989), Dziabaszewski (1991), Woźny (1992), Czajka and Domin (1993), Wojtaczka and Woźny (1993), Łęgowski (1995), Staręga (1995), Sielicki and Staręga (1996), Staręga (1996a, b), Staręga and Stankiewicz (1996), Woźny (1996), Baldy and Woźny (1998), Łuczak and Woźny (1999), Szymkowiak et al. (1999), Rozwałka (2000), Staręga (2000), Woźny and Szymkowiak (2000), Łęgowski (2001), Staręga and Kupryjanowicz (2001), Baldy (2002), Wolak (2002), Stańska et al. (2002), Kupryjanowicz (2003), Staręga (2003a, b), Kajak and Oleszczuk (2004), Rozwałka (2004), Szymkowiak and Górski (2004), Wolak (2004), Kupryjanowicz (2005), Łęgowski (2006), Rozwałka (2006a, b, c), Rozwałka (2007a, b), Rozwałka 2008, Stańska and Łydkowska (2008), Rozwałka and Juszczyński (2009), Rozwałka (2009), Oleszczuk (2010), Rozwałka (2010a, b, c), Rozwałka (2012), Cichocki and Rozwałka (2013), Rozwałka (2014a, b), Rozwałka et al. (2014). Colours of the dots as in the Fig. 32. The red-black dots show the regions, where presence of the species was confirmed
Number of forested or open plots with representatives of the Micrargus herbigradus-group in Poland and affinity of these species towards habitats of different moisture. Number of plots: M. herbigradus – 362, M. apertus – 28, M. georgescuae – 28 (habitat type); M. herbigradus – 373, M. apertus – 29, M. georgescuae – 28 (moisture)
Altitudinal distribution, habitats and phenology
The altitudinal distribution of the three species overlaps (Figs. 34–36). The only species with a clear preference to the mountains is M. georgescuae (Fig. 35), which in Poland occurs from about 670 m a.s.l., evenly distributed up to 1850 m a.s.l. (Online Resource 1: Tables S2, S3). Micrargus apertus and M. herbigradus were mostly found in Poland between 100 and 300 m a.s.l.. However, both of them appear in the mountains up to the subalpine level, and they sometimes cohabited with M. georgescuae.
All of the species show some preference towards forests compared to open habitats (Figs. 37–39), although they are not considered to be typical forest-dwellers. The percentage of the forested plots occupied by M. apertus was the highest of the three species, suggesting a stronger association with this habitat type (Fig. 37).
Micrargus apertus and M. georgescuae occur more frequently in humid habitats (Figs. 40, 41). The latter species is typical for the mountains, which – in Poland – have a cooler and more humid climate than in the other parts of the country. Micrargus herbigradus is present in all the habitat types (Fig. 42), from xerothermic, extremely dry stands, to periodically flooded forests and the mires. However, the majority of records come from stands of moderate moisture. The habitats of M. apertus are also diverse, including former sand quarries, crops, different forest types, caves and a subalpine bog. The diversity of M. georgescuae’s habitats is also conspicuous, i.e. montane spruce, alder or beech forests, dwarf pine shrubs, meadows, mountainous grasslands or different mire types.
The adults of the three species are present for most of year. Micrargus apertus was recorded from April to November. The data on its occurrence are scarce, but evenly distributed within the period (Fig. 43). The adults of M. georgescuae have two activity peaks, in late spring and early autumn (Fig. 44). The temporal distribution of M. herbigradus spans the whole year, with the highest peak in May and June (Fig. 45).
Discussion
Our data update information on the distribution of the M. herbigradus-species group in Central Europe. Micrargus georgescuae is newly recorded from Poland, where it occurs in the mountains and is locally abundant. It inhabits several European mountain ranges, as the Alps (Thaler 1978; Maurer and Walter 1980; Rëlys and Weiss 1997; Höfer et al. 2010), but also various lower massifs in Germany (Arachnologische Gesellschaft 2017), Czechia (Buchar and Růžička 2002; The Czech Society of Arachnology 2017) and Slovakia (Franc 2002; Svatoň and Kovalčík 2006). It is also present in some other mountain ranges of the Carpathians (Gajdoš et al. 2014), namely in the Ukrainian Chornohora (Hirna et al. 2016) and in Romania (Georgescu 1971; Millidge 1976; Urák and Samu 2008). The species has already been recorded in the mountains close to the study sites referred to in the text, such as the Ore Mountains (Růžička and Hajer 2000), the Hrubý Jeseník (Majkus 2006) or the Great Fatra (Franc 2002), but also in the same mountain ranges, i.e. the Giant Mountains (Kůrka and Vaněk 2001; Kůrka and Vaněk 2009; Materna et al. 2010), the Central Sudetes (Buchar and Růžička 2002) and the Tatra Mountains (Svatoň and Kovalčík 2006). Micrargus georgescuae was not found in the mountains of SE Poland (the Bieszczady and the Eastern Beskidy), even though these ranges have been very intensively surveyed recently. It is also absent in the Slovak part of this mountain massif (Svatoň et al. 2003 only list M. herbigradus). Similarly, M. georgescuae was not found in the Izera Mountains (the Western Sudetes) that are close to the other mountain ranges where the species occurs. These mountains have also been intensively surveyed during the last decade.
Micrargus apertus is a species that is rarely found. Considering the arrangement of its localities in other countries, e.g. in Germany (Arachnologische Gesellschaft 2017) or the Czech Republic (Czech Society of Arachnology 2017), and the uneven, apparently disordered distribution in Poland, it might be expected that M. apertus is much more widespread, although uncommon. On the British Islands it seems to be more common in the northern parts (British Arachnological Society 2017). The species might often have been misidentified. Micrargus herbigradus is widespread and common almost throughout Europe (Nentwig et al. 2017), and the presented distribution map of Poland is surely incomplete.
The results on the altitudinal preferences of the three species in Poland might be biased both by the geography of the country, with its area situated mostly at the altitudes between 0 and 300 m a.s.l., and by the way the arachnologists have chosen investigated regions. There are numerous gaps, i.e. regions that have received little or no interest historically (e.g. the Western Pomerania and some parts of Central Poland). There is a clear affinity of M. georgescuae towards mountainous regions, with the lowest localities situated approximately at 650 m a.s.l. A similar pattern might be observed in Germany, where the species clearly prefers mountains (Arachnologische Gesellschaft 2017). However, in the Czech Republic the spider was found at lower altitudes, from 350 m a.s.l., with a peak around 400–600 m (Buchar and Růžička 2002). It might be linked to abundance of montane areas in this country and the availability of specific habitats such as wetlands or stone gorges, with which the species might by partly associated there (Buchar and Růžička 2002). Altitudinal ranges of the species from the herbigradus-group overlap both in Poland, in the Czech Republic (Buchar and Růžička 2002) and – for example – the Alps (Muster and Leipold 2001). In the latter case the authors mentioned some separation of the habitats within the group (additionally with M. alpinus), although sometimes the species were observed together (Muster and Leipold 2001). We have also observed their cohabitation in some localities. Micrargus is therefore an example of the genera that undergo high diversification in Europe, with a few examples of altitudinal vicariants, such as M. alpinus or M. georgescuae. Care should be taken in investigating material from the mountains, because some other new forms might be expected in the other massifs.
Micrargus herbigradus was sometimes described as a silvicolous species (Kupryjanowicz 2008; Nentwig et al. 2017), only sporadically occurring in open habitats (Buchar and Růžička 2002). However, this might be a broad simplification, or its preferences are geographically changeable, according to – for instance – the availability of specific habitat types in the region. In Poland and some other European countries (Hänggi et al. 1995) M. herbigradus is a common, eurytopic species, with no specific habitat preference. Generally, it is commoner in humid habitats and has no preference towards open or shaded places (Entling et al. 2007). In Poland, M. apertus seems to have an affinity towards shaded habitats. However, the total number of its records is considerably low, which may have influenced the results. Micrargus georgescuae does not have special habitat preferences, apart from living in cool and humid climates of the mountains.
Micrargus herbigradus also inhabits some specific microhabitats (Online Resource 1: Table S4), as we have observed this species in the (slightly destroyed) nest of the ant Lasius fuliginosus in a tree. It is also a common cave-dweller (Sanocka-Wołoszynowa 1964, Sanocka-Wołoszynowa 1981). The species was observed in the diet of dunnock hatchlings (Prunella modularis) by Tomek (1988).
The results on the phenology of the M. herbigradus-species group are consistent with those from some other studies. Micrargus apertus is recorded in almost all of the seasons (British Arachnology Society 2017), with the highest abundance from May to October (Arachnologische Gesellschaft 2017; Czech Society of Arachnology 2017), while M. georgescuae was recorded mostly from April to October (Czech Society of Arachnology 2017), similar to our study. One of our records of M. georgescuae comes from pitfall traps left for the duration of winter, from October to April (Online Resource 1: Tables S2, S3). Some species with winter activity of adults may be detected by leaving the traps beneath the snow cover. The adults of M. herbigradus can be found all year round, with a peak between May and July (Arachnologische Gesellschaft 2017; British Arachnological Society 2017; Czech Society of Arachnology 2017). In selected studies – some of which we had to exclude from the analysis – the authors observed two abundance peaks in late spring and in autumn (Staręga 2000, 2003a), or recorded considerable winter activity of this Micrargus species (Woźny 1992).
Our review on the Micrargus herbigradus-species group is one of the first works of its kind that deals with the spider fauna of Poland. It shows that there is still a need to clarify data on spider distribution and ecology, and to revisit published works. The knowledge of spiders is dispersed in various articles, predominantly only of local importance, and reviewing their information will enable more thorough analysis and allow them to be considered within a broader European context.
References
Arachnologische Gesellschaft (2017) Atlas of the European Arachnids. http://atlas.arages.de. Accessed 30 June 2017
Baldy K (2002) Rola zgrupowań pająków w zooindykacji szczelin piaskowcowych Gór Stołowych. Parki nar Rez Przyr 21:451–469
Baldy K, Woźny M (1998) Stan zbadania araneofauny na terenie Parku Narodowego Gór Stołowych. Szczeliniec 2:89–96
Bednarz S, Czajka M (1966) Drugie stanowisko pająka Saloca diceros (Cambridge) (Micryphantidae) w Polsce. Przegl Zool 10:397–398
British Arachnological Society (2017) Spider and Harvestman Recording Scheme website. http://srs.britishspiders.org.uk/. Accessed 30 June 2017
Buchar J, Růžička V (2002) Catalogue of spiders of the Czech Republic. Peres Publishers, Praha
Chyży I, Staręga W (1997) Pająki (Araneae) rezerwatu Antoniuk. Parki nar Rez Przyr 16:27–39
Cichocki W, Rozwałka R (2013) Pająki rezerwatu torfowiskowego „Bór na Czerwonem”. Chrońmy Przyr Ojcz 69:41–54
Czajka M (1976) Nowe stanowiska rzadkich gatunków pająków (Aranei) w Polsce. Zesz Przyr Opol TPN 16:19–130
Czajka M, Domin L (1993) Pająki (Aranei) okolic Ruszowa w Borach Dolnośląskich. Zesz Przyr Opol TPN 29:7–30
Czajka M, Goos M (1976) Pająki (Aranei) pól buraków cukrowych w Pawłowicach Wielkich koło Wrocławia. Pol Pis Ent 46:179–185
Czajka M, Kornalewicz W (1982) Pająki (Aranei) kilku biotopów Doliny Odry. Zesz Przyr Opol TPN 21:133–140
Czech Society of Arachnology (2017) Online Atlas, Araneae. https://www.arachnology.cz/rad/araneae-1.html. Accessed 30 June 2017
Dahl F (1902) Über Stufenfänge echter Spinnen im Riesengebirge. Sitz-ber Ges naturf Freu Berlin 1902:185–203
Deltshev K, Kajak A (1974) Analysis of a sheep pasture ecosystem in the Pieniny Mountains (the Carpathians). XVI. Effect of pasture management on the number and biomass of spiders (Araneae) in two climatic regions (the Pieniny and the Średnia Góra mountains). Ekol pol 22:693–710
Dziabaszewski A (1974) Z badań nad pająkami (Aranei) Wielkopolski II. Bad Fizjogr Pol Zach, ser. C-Zool 27:53–67
Dziabaszewski A (1989) Uwagi faunistyczne o rzadszych gatunkach pająków (Aranei) z Poznania (z listą 302 stwierdzonych gatunków). Bad Fizjogr Pol Zach, ser. C-Zool 38:5–21
Dziabaszewski A (1991) Pająki grądowego rezerwatu „Jakubowo” ze szczegółowym uwzględnieniem fenologii oraz struktury zgrupowań Aranei. Prace Kom Biol Pozn TPN 73:35–55
Dziabaszewski A, Dziabaszewska J, Ciołek J, Jankowski A (1989) Pająki (Aranei) grądów Wielkopolski. Bad Fizjogr Pol Zach, ser. C-Zool 38:35–42
Entling W, Schmidt MH, Bacher S, Brandl R, Nentwig W (2007) Niche properties of Central European spiders: shading, moisture and the evolution of the habitat niche. Glob Ecol Biogeogr 16:440–448. https://doi.org/10.1111/j.1466-8238.2006.00305.x
Franc V (2002) Contribution to the knowledge on spiders (Araneae) of the Vel'ká Fatra Mts. Matthias Belivs Univ Proc, Suppl 2:155–163
Gajdoš P, Moscaliuc LA, Rozwałka R (2014) Red list of spiders (Araneae) of the Carpathian Mts. In: Kadlečík J (ed) Carpathian Red List of forest habitats and species. Carpathian list of invasive alien species (draft). The State Nature Conservancy of the Slovak Republic, Banská Bystrica, pp 118–135
Georgescu M (1971) Quelques considerations sur le genre Micrargus (Dahl) en Roumanie. Trav Institut Spéol "Émile Racovitza" 10:235–244
Hajdamowicz I, Stańska M, Król A, Hirler A, Nicewicz Ł (2016) Rzadkie i zagrożone gatunki pająków w zbożach w województwie lubelskim. Kulon 21:49–61
Hänggi A, Stöckli E, Nentwig W (1995) Lebensräume mitteleuropäischer Spinnen. Charakterisierung der Lebensräume der häufigsten Spinnenarten Mitteleuropas und der mit diesen vergesellschafteten Arten. Misc Faun Helv 4, Neuchâtel
Hirna A, Gnelitsa V, Zhukovets E (2016) A checklist of the spiders (Araneae) of the Chornohora Mountain massif (Ukrainian Carpathians). Arachnol Mitt / Arachnology Letters 51:16–38. https://doi.org/10.5431/aramit5104
Höfer H, Blick T, Muster C, Paulsch D (2010) Artenvielfalt und Diversität der Spinnen (Araneae) auf einem beweideten Allgäuer Grasberg (Alpe Einödsberg) und unbeweideten Vergleichsstandorten im Naturschutzgebiet Allgäuer Hochalpen. Andrias 18:53–78
Jędryczkowski W, Staręga W (1980) Bezkręgowce lądowe (Isopoda, Diplopoda, Aranei, Opiliones) rezerwatu kserotermicznego „Kulin”. Fragm faun 25:179–197
Kajak A (1960) Zmiany liczebności pająków na kilku łąkach. Ekol pol, ser B 8:199–228
Kajak A, Oleszczuk M (2004) Effect of shelterbelts on adjoining cultivated fields: patrolling intensity of carabid beetles (Carabidae) and spiders (Araneae). Pol J Ecol 52:155–172
Krzyżanowska E (1982) Pająki (Aranei) skarpy wiślanej w Warszawie. Fragm faun 27:59–66
Krzyżanowska E, Dziabaszewski A, Jackowska B, Staręga W (1981) Spiders (Arachnoidea, Aranei) of Warsaw and Mazovia. Memorabilia Zool 34:87–110
Kulczyński W (1876) Dodatek do fauny pajęczaków Galicyi. Spraw Kom Fiz 10:41–67
Kulczyński W (1881) Wykaz pająków Tatr, Babiéj Góry i Karpat szlązkich z uwzględnieniem pionowego rozsiedlenia pająków w Galicyi zachodniéj. Spraw Kom Fiz 15:248–322
Kupryjanowicz J (2003) Spiders (Araneae) of open habitats in the Biebrza National Park, Poland. Fragm faun 46:209–237
Kupryjanowicz J (2005) Pająki (Araneae) Biebrzańskiego Parku Narodowego. In: Dyrcz A, Werpachowski C (eds) Przyroda Biebrzańskiego Parku Narodowego. Biebrzański Park Narodowy, Osowiec-Twierdza, pp 275–299
Kupryjanowicz J (2008) Pająki, Araneae. In: Bogdanowicz W, Chudzicka E, Pilipiuk I, Skibińska E (eds) Fauna Polski. Charakterystyka i wykaz gatunków. Muzeum i Instytut Zoologii PAN, Warszawa, pp 223–239
Kůrka A, Vaněk J (2001) Spiders (Araneae) of shaded and non-shaded sites in the tundra of the western Giant Mountains (Czech Republic). Opera Corcontica 38:219–233
Kůrka A, Vaněk J (2009) Fauna pavouků (Araneae) jižního svahu Sněžky (Krkonoše). Opera Corcontica 46:149–158
Łęgowski D (1995) Antropogeniczne przeobrażenia zgrupowań pająków (Aranei) w ekosystemach borów sosnowych. In: Szujecki A (ed) Antropogeniczne przeobrażenia epigeicznej i glebowej entomofauny borów sosnowych. SGGW, Warszawa, pp 381–460
Łęgowski D (2001) Waloryzacja Puszczy Białowieskiej metodą zooindykacyjną na podstawie pająków (Aranei). In: Szujecki A (ed) Próba szacunkowej waloryzacji lasów w Puszczy Białowieskiej metodą zooindykacyjną. SGGW, Warszawa, pp 207–233
Łęgowski D (2006) Spiders (Aranei). In: Szujecki A (ed) Zooindication-based monitoring of anthropogenic transformations in Białowieża Primeval Forest. SGGW, Warszawa, pp 247–291
Łuczak J (1960) Rozmieszczenie piętrowe pająków w lesie. Ekol pol, ser B 6:39–50
Łuczak J, Woźny M (1999) Effect of spruce forest decline on spider communities of Karkonosze Mts. Pol J Ecol 41:429–447
Majkus Z (2006) Arachnofauna rašelinišť NPR Praděd. Čas Slez Zem Muz. Opava (A) 55:239–248
Materna J, Vaněk J, Kůrka A, Vonička P (2010) Epigeičtí pavouci (Araneae), sekáči (Opiliones) a střevlíci (Coleoptera: Carabidae) vybraných rostlinných společenstev krkonošské a skandinávské tundry. Opera Corcontica 47:187–210
Maurer R, Walter JE (1980) Für die Schweiz neue und bemerkenswerte Spinnen (Araneae). Mitt Schweiz entomol Ges 53:157–162
Millidge AF (1976) Re-examination of the erigonine spiders "Micrargus herbigradus" and "Pocadicnemis pumila" (Araneae: Linyphiidae). Bull Brit Arach Soc 3:145–155
Muster C, Leipold D (2001) Drei für Deutschland neue Zwergspinnen aus dem bayerischen Alpenraum (Araneae: Linyphiidae, Erigoninae). Arachnol Mitt 22:1–10. https://doi.org/10.5431/aramit2201
Nentwig W, Blick T, Gloor D, Hänggi A, Kropf C (2017) Spiders of Europe, version 01.10.15 www.araneae.unibe.ch. Accessed 30 June 2017
Nowicki M (1874) Dodatek do fauny pajęczaków Galicyi. Spraw Kom Fiz 8:2–11
Oleszczuk M (2010) Refugia śródpolne, jako siedliska rzadziej spotykanych i zagrożonych gatunków pająków (Araneae) w Polsce. Chrońmy Przyr Ojcz 66:361–375
Pilawski S (1962) Wstępne badania pająków w Karkonoskim Parku Narodowym. Acta Univ Wrat 3. Prace zool 1:181–188
Pilawski S (1965) O kilkunastu gatunkach pająków złowionych w Sudetach Śląskich nowych dla fauny Dolnego Śląska i Polski. Przegl Zool 9:254–265
Pilawski S (1966a) Wstępne badania pająków w Górach Świętokrzyskich. Acta Univ Wrat 51. Prace zool 2:1–70
Pilawski S (1966b) Wstępne badania pająków okolic Kudowy Zdroju (woj. wrocławskie). Przegl Zool 10:19–66
Pilawski S (1967) Materiały do znajomości pająków (Araneae) Wzgórz Trzebnickich. Przegl Zool 11:391–404
Pilawski S (1970) Przyczynek do ekologii niektórych gatunków pająków (Aranei) z Dolnego Śląska. Przegl Zool 14:47–61
Prószyński J, Staręga W (1971) Pająki – Aranei. Katalog Fauny Polski 33, PWN, Warszawa
Puszkar T (1981) Zmiany wybranych elementów zoocenoz w agroekosystemach poddawanych silnej presji emisji przemysłowych. IUNG Puławy 157:1–79
Rëlys V, Weiss I (1997) Micrargus alpinus sp. n., eine weitere Art der M. herbigradus-Gruppe aus Österreich (Arachnida: Araneae: Linyphiidae). Rev Suisse Zool 104:491–501. https://doi.org/10.5962/bhl.part.80007
Rozwałka R (2000) Pająki (Araneae) zespołu Brachypodio-Teucrietum rezerwatu „Stawska Góra”. In: Łętowski J (ed) Walory przyrodnicze Chełmskiego Parku Krajobrazowego i jego najbliższych okolic. Wydawnictwo UMCS, Lublin, pp 109–118
Rozwałka R (2004) Materiały do znajomości pająków (Araneae) Roztocza. Nowy Pam Fizjogr 3:101–116
Rozwałka R (2006a) Pająki (Araneae) Nadwieprzańskiego Parku Krajobrazowego. Nowy Pam Fizjogr 4:55–66
Rozwałka R (2006b) Materiały do poznania pająków (Araneae) Poleskiego Parku Narodowego. Nowy Pam Fizjogr 4:67–82
Rozwałka R (2006c) Pająki (Araneae) stanowiska roślinności kserotermicznej w Żmudzi koło Chełma. Parki nar Rez Przyr 25:51–68
Rozwałka R (2007a) Materiały do znajomości pająków (Araneae) Wyżyny Lubelskiej. Nowy Pam Fizjogr 5:145–173
Rozwałka R (2007b) Pająki (Araneae) Kazimierskiego Parku Krajobrazowego. Parki nar Rez Przyr 26:83–100
Rozwałka R (2008) Wykaz krytyczny pająków (Araneae) Ojcowskiego Parku Narodowego. Parki nar Rez Przyr 27:63–79
Rozwałka R (2009) Pająki (Araneae) Parku Krajobrazowego Lasy Janowskie. Nowy Pam Fizjogr 6:45–70
Rozwałka R (2010a) Uzupełnienia i sprostowania informacji o pająkach (Araneae) z Parku Narodowego Gór Stołowych. Przyr Sud 13:99–113
Rozwałka R (2010b) Zbiór pająków (Araneae) Muzeum Górnośląskiego w Bytomiu. Acta ent Sil 18:79–94
Rozwałka R (2010c) Materiały do znajomości pająków Araneae Bieszczadów i Bieszczadzkiego Parku Narodowego. Rocz Bieszcz 18:167–177
Rozwałka R (2012) Materiały do znajomości pająków Araneae Bieszczadzkiego Parku Narodowego. Rocz Bieszcz 20:156–195
Rozwałka R (2014a) Materiały do znajomości pająków Araneae Beskidu Wschodniego. Rocz Bieszcz 22:239–350
Rozwałka R (2014b) Pająki (Araneae) Magurskiego Parku Narodowego. Rocz Bieszcz 22:351–370
Rozwałka R, Juszczyński P (2009) Pająki (Araneae) dwu nalessowych stanowisk kserotermicznych w okolicach Lublina. Nowy Pam Fizjogr 6:87–106
Rozwałka R, Renn K, Sienkiewicz P (2014) Pająki (Araneae) i kosarze (Opiliones) Lednickiego Parku Krajobrazowego (I). Przegl Przyr 25:42–63
Růžička V, Hajer J (2000) Pavouci (Araneae) mokřadů Lučiny u Tisé (Boh. bor. occ.) Sbor Okres muz Most, řada přírodověd 22:13–18
Sanocka-Wołoszyn E (1964) Uwagi nad rozmieszczeniem i ekologią pająków (Araneae) z jaskiń Gór Świętokrzyskich. Seminarium speleologiczne I Ogólnopolskiego Zjazdu Badaczy Krasu, Kielce:73–86
Sanocka-Wołoszynowa E (1981) Badania pajęczaków (Aranei, Opiliones, Pseudoscorpionida) Wyżyny Krakowsko-Częstochowskiej. Acta Univ Wrat 548. Prace Zool 11:1–92
Schenkel E (1929) Spinnen vom Riesengebirge, gesammelt von E. Nielsen. Entomol Medd 16:335–338
Sielicki M, Staręga W (1996) Pająki (Araneae) ekotonu ols-łąka w okolicach Białegostoku. Fragm faun 39:169–177
Stańska M (2005) Pająki (Araneae) jako element monitoringu ekologicznego w wybranych środowisk leśnych Puszczy Białowieskiej. Leś Pr Bad 1:65–79
Stańska M (2007) Rare and threatened spider species (Araneae) in selected types of deciduous forests in the Białowieża Forest. Nat Conserv 64:13–29
Stańska M, Hajdamowicz I, Żabka M (2002) Epigeic spiders of alder swamp forests in Eastern Poland. In: Toft S, Scharff N (eds) Proceedings of the 19th European Colloquium of Arachnology. Aarhus University Press, Aarhus, pp 191–197
Stańska M, Łydkowska M (2008) Pająki (Araneae) dąbrowy świetlistej w rezerwacie Dębniak. Leś Pr Bad 69:309–320
Staręga W (1966) Przyczynek do poznania fauny pająków (Aranei) Polski. Fragm faun 13:175–186
Staręga W (1971) Pająki (Aranei) Bieszczadów. Fragm faun 17:53–126
Staręga W (1972) Nowe dla fauny Polski i rzadsze gatunki pająków (Aranei), z opisem Lepthyphantes milleri sp. n. Fragm faun 18:55–98
Staręga W (1974) Materiały do znajomości rozmieszczenia pająków (Aranei) w Polsce. Fragm faun 19:395–420
Staręga W (1976) Pająki (Aranei) Pienin. Fragm faun 21:233–330
Staręga W (1978) Materiały do znajomości rozmieszczenia pająków (Aranei) w Polsce, III–VII. Fragm faun 23:259–302
Staręga W (1984) Materiały do znajomości rozmieszczenia pająków (Aranei) w Polsce, VIII–X. Fragm faun 28:79–136
Staręga W (1988) Pająki (Aranei) Gór Świętokrzyskich. Fragm faun 31:185–359
Staręga W (1989) Spiders (Aranei) of moist meadows on the Mazovian Lowland. Memorabilia Zool 43:37–60
Staręga W (1995) Pająki Puszczy Knyszyńskiej. In: Czerwiński A (ed) Puszcza Knyszyńska, Monografia przyrodnicza. Zespół Parków Krajobrazowych w Supraślu, Supraśl, pp 279–298
Staręga W (1996a) Spinnen (Araneae) aus der Borkenheide und anderen Lokalitäten der Masurischen Seenplatte. Fragm faun 39:287–311
Staręga W (1996b) Spinnen (Araneae) von oberschlesischen Abraumhalden des Steinkohlebergbaus. Fragm faun 39:329–344
Staręga W (2000) Spinnen aus Roztocze und anliegenden Gebieten. Fragm faun 43:59–89
Staręga W (2003a) Pająki (Araneae) Puszczy Knyszyńskiej. Nowy Pam Fizjogr 1:95–206
Staręga W (2003b) Pająki z Nadbużańskiego Parku Krajobrazowego. Parki nar Rez Przyr 22:531–541
Staręga W, Kupryjanowicz J (1996) Beitrag zur Kenntnis der Spinnen (Araneae) des Gorce-Gebirges. Fragm faun 39:313–328
Staręga W, Kupryjanowicz J (2001) Subclassis (podgromada): Araneae – pająki. In: Gutowski JM, Jaroszewicz B (eds) Katalog fauny Puszczy Białowieskiej, IBL Warszawa, pp 55–64
Staręga W, Stankiewicz A (1996) Beitrag zur Spinnenfauna einiger Moore Nordostpolens. Fragm faun 39:345–361
Svatoň J, Kovalčík R (2006) Present state of knowledge of araneo-fauna in the Tatras National Park. Oecol Mont 15:1–14
Svatoň J, Thomka V, Gajdoš P (2003) Pavúky (Araneae). In: Mašán P, Svatoň J (eds) Pavúkovce Národného Parku Poloniny. Štátna ochrana prírody SR, Banská Bystrica a S–NP Poloniny, Snina, Humenné, pp 21–113
Szymkowiak P, Górski G (2004) Spider communities in the contact zone between open areas and spruce forest in the Karkonosze National Park. Opera Corcontica 41:309–315
Szymkowiak P, Woźny M, Błażejczyk M (1999) A comparison of the species composition of spider communities over sixty years in the vicinity of Krotoszyn. Fragm faun 42:29–40
Thaler K (1978) Über wenig bekannte Zwergspinnen aus den Alpen-V (Arachnida: Aranei, Erigonidae). Beitr Entomol 28:183–200
Tomek T (1988) The breeding biology of the Dunnock Prunella modularis modularis (Linnaeus, 1758) in the Ojców National Park (South Poland). Acta Zool Crac 31:115–166
Urák I, Samu F (2008) Contribution to the spider fauna of the Mohoş peat bog from Transylvania, with some new data for Romania. North-West J Zool 4:50–60
Wojtaczka M, Woźny M (1993) Pająki (Aranei) podłoża Gór Złotych. Acta Univ Wrat 977. Prace Zool 26:39–64
Wolak M (2002) The spider fauna of balks. In: Toft S, Scharff N (eds) Proceedings of the 19th European Colloquium of Arachnology. Aarhus University Press, Aarhus, pp 229–326
Wolak M (2004) The significance of unmanaged “island” habitats for epigeic spiders in a uniform agricultural landscape. In: Samu F, Szinetár C (eds) Proceedings of the 20th European Colloquium of Arachnology. Budapest – Szombathely, pp 327–336
Woźny M (1975a) Pająki (Aranei) południowej Opolszczyzny. Prace OTPN. Wydział III Nauk Przyrodniczych 50:1–100
Woźny M (1975b) Nowe dane do znajomości fauny pająków (Aranei) Gór Opawskich. Zesz Przyr Opol TPN 14–15:209–218
Woźny M (1976) Niektóre dane o kilku rzadkich pająkach (Aranei) dla fauny Polski. Zesz Przyr Opol TPN 16:131–136
Woźny M (1985) Pająki (Aranei) Wału Trzebnickiego. Fragm faun 29:39–76
Woźny M (1992) Wpływ wilgotności podłoża na zgrupowania pająków oraz dynamika liczebności gatunków dominujących borów sosnowych Wzgórz Ostrzeszowskich. Acta Univ Wrat 1124. Prace Zool 23:25–82
Woźny M (1996) Pająki (Aranei). In: Jahn A, Kozłowski S, Pulina M (eds) Masyw Śnieżnika, zmiany w środowisku przyrodniczym. Polska Agencja Ekologiczna S.A, Warszawa, pp 244–249
Woźny M, Czajka M, Pilawski S, Bednarz S (1988) Pająki (Araneae) Polskich Sudetów, Acta Univ Wrat 972. Prace Zool 19:53–128
Woźny M, Szymkowiak P (2000) Epigeic spiders of the pastures of northern Wielkopolska. Arachnol Mitt 20:1–25. https://doi.org/10.5431/aramit2001
Acknowledgments
We would like to thank the numerous institutions responsible for nature protection in Poland for giving us their permission and support for our research. We give thanks to all of the researchers that supplied us with material or who gave their assistance during field work. Special thanks to Marzena Stańska and Janusz Kupryjanowicz for providing the specimens for the analysis and to Charles Haddad for proofreading the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
Table S1 New localities of Micrargus apertus. Table S2 Verified localities of Micrargus georgescuae. Table S3 New localities of Micrargus georgescuae. Table S4 New localities of Micrargus herbigradus. (PDF 50 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wiśniewski, K., Rozwałka, R. & Wesołowska, W. Distribution, habitat affinities and phenology of the Micrargus herbigradus-species group (Araneae: Linyphiidae) in Poland. Biologia 73, 151–164 (2018). https://doi.org/10.2478/s11756-018-0026-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0026-5