Abstract
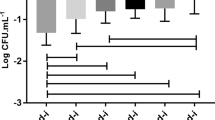
In the present study the biofilm-forming characteristics of 99 serotyped (DMC strains) and 41 genus level-identified (IS strains) Salmonella strains originating from Turkey were investigated. The strains were selected based on their ability to show the biofilm morphotype on Congo red agar plates. In addition, all strains were evaluated with regard to properties related to forming pellicle structures, physical differences of pellicles, any changes in the media associated with the formation of pellicles, and the presence of cellulose within the formed biofilm matrix as determined using 366 nm UV light. The Salmonella Typhimurium DMC4 strain was the best producer of biofilm grown on polystyrene microtiter plates (optical density at 595 nm: 3.418). In subsequent experiments industrial process conditions were used to investigate different morphotyped Salmonella strains’ biofilm-forming capability on stainless steel, a commonly preferred surface for the food industries, and on polystyrene surfaces. The effect of other important industrial conditions, such as temperature (5, 20, 37°C), pH (4.5, 5.5, 6.5, 7.4) and NaCl concentration (0.5, 1.5, 5.5, 10.5%) on the production of biofilm of the different morphotyped Salmonella strains (DMC4; red, dry and rough morphotyped S. Typhimurium, DMC12; brown, dry and rough morphotyped S. Infantis, DMC13; pink, dry and rough morphotyped S. subsp. Roughform) were also assessed. On the other hand, pH values exhibited variable effects on biofilm-forming features for different Salmonella strains on both polystyrene and stainless steel surfaces.
Similar content being viewed by others
Abbreviations
- te]bdar, brown:
-
dry and rough
- cfu:
-
colony forming unit
- CR:
-
Congo red
- LB:
-
Luria Bertani
- OD:
-
optical density
- pdar, pink:
-
dry and rough
- rdar, red:
-
dry and rough
- saw:
-
smooth and white
- sbam, smooth:
-
brown and mucoid
References
Agbaje M., Begum R.H., Oyekunle M.A., Ojo O. E. & Adenubi O.T. 2011. Evolution of Salmonella nomenclature a critical note. Folia Microbiol. 56: 497–503.
Anriany Y.A., Weiner R.M., Johnson J.A., De Rezende C.E. & Joseph S.W. 2001. Salmonella enterica serovar Typhimurium DT104 displays a rugose phenotype. Appl. Environ. Microbiol. 67: 4048–4056.
Avsaroglu M.D., Helmuth R., Junker E., Hertwig S., Schroter A., Akcelik M., Bozooglu F. & Guerra B. 2007. Plasmid-mediated quinolone resistance conferred by qnrS1 in Salmonella enterica serovar Virchow isolated from Turkish food avian origin. J. Antimicrob. Chemother. 60: 1146–1150.
Barnhart M.M. & Chapman M.R. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60: 131–147.
Bereksi N., Gavini F., Benezech T. & Faille C. 2007. Growth, morphology and surface properties of Listeria monocytogenes Scott A and LO28 under saline and acid environments. J. Appl. Microbiol. 92: 556–565.
Borucki M.K., Peppin J., White D., Loge D. & Call F.D.R. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69: 7336–7342.
Brown M.L., Aldrich H.C. & Gauthier J.J. 1995. Relationship between glycocalyx and povidone-iodine resistance in Pseudomonas aeruginosa (ATCC 27853) biofilms. Appl. Environ. Microbiol. 61: 187–193.
Cegelski L., Pinkner J.S., Hammer N.D., Cusumano C.K., Hung C.S., Chorel E., Aberg V., Walker J.N., Seed P.C., Almqvist F., Chapman M.R. & Hultgren S.J. 2009. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 5: 913–919.
Chavant P., Martinie B., Meylheuc T., Marie-Noëlle B.F. & Hebraud M. 2001. Listeria monocytogenes LO28: surface physicochemical properties and ability to form biofilms at different temperatures and growth phases. Appl. Environ. Microbiol. 68: 728–737.
Costerton J.W. 1995. Overwiev of microbial biofilms. J. Ind. Microbiol. 15: 137–140.
Costerton J.W., Stewart P.S. & Greenberg E.P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322.
Crump J.A., Griffin P.M. & Angulo F.J. 2002. Bacterial contamination of animal feed and its relationship to human foodborne illness. J. Food Safety 35: 859–865.
Dhaliwal D.S., Cordier J.L. & Cox L.J. 1992. Impedimetric evaluation of the efficiency of disinfectants against biofilms. Lett. Appl. Microbiol. 15: 217–221.
Dhir V.K. & Dodd C.E.R. 1995. Susceptibility of suspended and surface-sttached Salmonella enteritidis to biocides and elevated temperatures. Appl. Environ. Microbiol. 61: 1731–1738.
Di Bonaventura G., Piccolomini R., Paludi D., D’Orio V., Vergara A., Conter M. & Ianieri A. 2007. Influence of temperature on biofilm formation by Listeria monocytogenes on various foodcontact surfaces: relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 104: 1552–1564.
Doyle M.E. & Glass K.A. 2010. Sodium reduction and ıts effect on food safety, food quality, and human health. Comp. Rev. Food Sci. Food Safety 9: 44–56.
Flint S.H., Bremer P.J & Brooks J.D. 1996. Biofilms in dairy manufacturing plant description, current concerns and methods of control. Biofouling 11: 81–97.
Giaouris E., Chorianoupoulos N. & Nychas G.J.E. 2005. Effect of temperature, pH, and water activity on biofilm formation by Salmonella enterica Enteritidis PT4 on stainless steel surfaces as ındicated by the bead vortexing method and conductance measurements. J. Food Prot. 68: 2149–2154.
Gilbert P., Allison D.G. & McBain A.J. 2002. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microbiol. 92: 98–110.
Gophna U., Barlev M., Seijffers R., Oelschlager T.A., Hacker J. & Ron E.Z. 2001. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect. Immun. 69: 2659–2665.
Gorski L., Palumbo J.D. & Mandrell R.E. 2002. Attachment of Listeria monocytogenes to radish tissue is dependent upon temperature and flagellar motility. Appl. Environ. Microbiol. 69: 258–266.
Herwald H., Mörgelin M., Olsen A., Rhen M., Dahlbäck B., Müller-Ester W. & Björck L. 1998. Activation of the contactphase system on bacterial surfaces — a clue to serious complications in infectious diseases. Nat. Med. 4: 298–302.
Holah J.T., Higgs C., Robinson S., Worthington D. & Spenceley H. 1990. A conductance-based surface disinfection test for food hygiene. Lett. Appl. Microbiol. 11: 255–259.
Hood S.K. & Zottola E.A. 1997. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 37: 145–153.
Humphrey T.J., Slater E., McAlpine K., Rowbury R.J. & Gilbert R.J. 1995. Salmonella enteritidis phage type 4 isolates more tolerant of heat, acid, or hydrogen peroxide also survive longer on surfaces. Appl. Environ. Microbiol. 61: 3161–3164.
Jones K., Bradshaw S.B. 1996. Biofilm formation by the Enterobacteriaceae: a comparison between Salmonella enteritidis, Escherichia coli and a nitrogen-fixing strain of Klebsiella pneumoniae. J. Appl. Microbiol. 80: 458–464.
Knight G.C. & Craven H.M. 2010. A model system for evaluating surface disinfection in dairy factory environments. Int. J. Food Microbiol. 137: 161–167.
Kumar C.G. & Anand S.K. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food Microbiol. 42: 9–27.
Lunden J.M., Miettinen M.K., Autio T.J. & Korkeala H.J. 2000. Persistent Listeria monocytogenes strains show enhanced adherence to food contact surface after short contact times. J. Food Prot. 63: 1204–1207.
Lundmark K., Westermark G.T., Olsen A. & Westermark P. 2004. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: Cross-seeding as a disease mechanism. Proc. Natl. Acad. Sci. USA 102: 6098–6102.
Moretro T., Midtgaard E.S., Nesse L.L. & Langsrud S. 2003. Susceptibility of Salmonella isolated from fish feed factories to disinfectants and air-drying at surfaces. Vet. Microbiol. 94: 207–217.
Morin P., Camper A., Jones W., Gatel D. & Goldman J.C. 1996. Colonization and disinfection of biofilms hosting coliformcolonized carbon fines. Appl. Environ. Microbiol. 62: 4428–4432.
Olsen A., Jonsson A. & Normark S. 1989. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 338: 652–655.
Rainey P.B. & Travisano M. 1998. Adaptive radiation in an heterogeneous environment. Nature 394: 69–72.
Römling U. & Rohde M. 1999. Flagella modulate the multicellular behavior of Salmonella typhimurium on the community level. FEMS Microbiol. Lett. 180: 91–102.
Römling U., Sierralta W.D., Eriksson K., Normark S 1998. Multicellular and aggregative behaviour of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28: 249–264.
Ryu J.H. & Beuchat L.R. 2005. Biofilm formation by Escherichia coli O157:H7 on stainless steel: effect of exopolysaccharide and curli production on its resistance to chlorine. Appl. Environ. Microbiol. 71: 247–254.
Sauer K., Rickard A.H. & Davies D.G. 2007. Biofilm and biocomplexity. Features 2: 347–353.
Scher K., Römling U. & Yaron S. 2004. Effect of heat, acidifi-cation, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 71: 1163–1168.
Shi X. & Zhu X. 2009. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 20: 407–413.
Solano C., Garcia B., Valle J., Berasain C., Ghigo J.M., Gamazo C. & Lasa I. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43: 793–808.
Somers E.B., Schoeni J.L. & Wong A.C.L. 2002. Effect of trisodium phosphate on biofilm and planktonic cells of Campylobacter jejuni, Escherichia coli O157: H7, Listeria monocytogenes and Salmonella typhimurium. Int. J. Food Microbiol. 22: 269–276.
Spiers A.J., Bohannon J., Gehrig S.M. & Rainey P.B. 2003. Biofilm formation at the air-liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol. Microbiol. 50: 15–27.
Spiers A.J., Kahn S.G., Bohannon J., Travisano M. & Rainey P.B. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161: 33–46.
Stepanovic S., Vukovic D., Dakic I., Savic B. & Svabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 40: 175–179.
Tresse O., Shannon K., Pinon A., Malle P., Vialette M. & Midelet-Bourdin G. 2007. Varaible adhesion of Listeria monocytogenes isolates from food-processing facilities and clinical cases to inert surfaces. J. Food Prot. 70: 1569–1578.
Vestby L.K., Moretro T., Langsrud S., Heir E. & Nesse L.L. 2009. Biofilm forming abilities of Salmonella are correlated with persistence in fish meal- and feed factories. BMC Vet. Res. 5: 20–26.
Woodward M.J., Sojka M., Sprigings K.A. & Humphrey T.J. 2000. The role of sef 14 and sef17 fimbriae in the adherence of Salmonella enterica serotype Enteritidis to inanimate surfaces. J. Med. Microbiol. 49: 481–487.
Xu H., Zou Y., Lee H. & Ahn J. 2010. Effect of NaCl on the biofilm formation by foodborne pathogens. J. Food Sci. 75: 80–85.
Yildiz F.H. & Schoolnik G.K. 1998. Vibrio cholerae O1 El Tor: Identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96: 4028–4033.
Zogaj X., Nimtz M., Rohde M., Bokranz W. & Römling U. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39: 1452–1463.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karaca, B., Akcelik, N. & Akcelik, M. Biofilm-producing abilities of Salmonella strains isolated from Turkey. Biologia 68, 1–10 (2013). https://doi.org/10.2478/s11756-012-0138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-012-0138-2