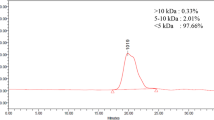
Abstract
Data have demonstrated that whey protein (WP) enhances the immune system. The aim of this study was to investigate and compare the effects of WP from three camel breeds on oxidative stress, blood lipid profile and the cytokine levels. Seventy five male mice were randomly split into five groups. The first served as a control group. The second, the third and the fourth groups were orally administrated the WP from Majaheim, Maghateer and Soffer camel breeds, respectively, at a dose of 100 mg/kg mouse body weight. The fifth group was supplemented with bovine serum albumin (BSA). Results showed similar electrophoretic patterns of the three whey proteins. WP was found to significantly inhibit the hydroperoxide and the Reactive Oxygen Species (ROS) in leukocytes, liver and skin as well as the blood cholesterol level in a time dependent manner. A significant enhancement of glutathione was revealed in WP groups. Furthermore, WP was found to significantly elevate the IL-2 with a significant time dependent enhance of IL-8. On contrast, a significant lowering effect of whey proteins on the pro-inflammatory cytokines, IL-1α, IL-1β, IL-6 and IL-10 was detected. Moreover, a mitogenic activity of WP was observed on the lymphocytes. Non-significant changes were observed in AST, ALT, creatinine and glucose level. These findings suggest that WP significantly improved the levels of the oxidative markers and the immune functions without any difference in the bioactivities of the three studied whey proteins.
Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine Aminotransferase
- AST:
-
Aspartate Aminotransferase
- IL:
-
Interleukin
- PBMCs:
-
Peripheral Blood Mononuclear Cells
- BSA:
-
Bovine serum albumin
- ROS:
-
Reactive Oxygen Species
- SDS-PAGE:
-
Sodium dodecyl sulfatepolyacrylamide gel electrophoresis
- WP:
-
Whey proteins
References
Akhavan T., Luhovyy B.L., Brown P.H., Cho C.E. & Anderson G.H. 2010. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am. J. Clin. Nutr. 91(4): 966–975. DOI:10.3945/ajcn.2009.28406
Akira S., Hira Akira S., Hirano T., Taga T. & Kishimoto T. 1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 4(11): 2860–2867. PMID: 2199284
Allain C.C., Poon L.S., Chan C.S., Richmond W. & Fu P.C. 1974. Enzymatic determination of total serum cholesterol. Clin. Chem. 20(4): 470–475.
Artym J. & Zimecki M. 2005. The role of lactoferrin in the proper development of newborns. Postepy Hig. Med. Dosw. 59: 421–432. PMID: 16106243
Badr G., Alwasel S., Ebaid H., Mohany M. & Alhazza I. 2011a. Perinatal supplementation with thymoquinone improves diabetic complications and T cell immune responses in rat off-spring. Cell Immunol. 267(2): 133–140. PMID: 21295287
Badr G., Bashandy S., Ebaid H., Mohany M. & Sayed D. 2011b. Vitamin C supplementation reconstitutes polyfunctional T cells in streptozotocin-induced diabetic rats. Eur. J. Nutr. DOI: 10.1007/s00394-011-0176-5
Ballard K.D., Bruno R.S., Seip R.L., Quann E.E., Volk B.M., Freidenreich D.J., Kawiecki D.M., Kupchak B.R., Chung M.Y., Kraemer W.J. & Volek J.S. 2009. Acute ingestion of a novel whey-derived peptide improves vascular endothelial responses in healthy individuals: a randomized, placebo controlled trial. Nut. J. 22: 8–34. DOI: 10.1186/1475-2891-8-34
Belokrylov G.A., Popova O.Y.A., Molchanova I.V., Sorochinskaya E.I. & Anokhina V.V. 1992. Peptides and their constituent amino acids influence the immune response and phagocytosis in different ways. Int. J. Immunopharmacol. 14(7): 1285–1292. http://dx.doi.org/10.1016/0192-0561(92)90065-S
Bounous G. 2000. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anti-Cancer Res. 20(6C): 4785–4792. PMID: 11205219
Bounous G., Batist G. & Gold P. 1989. Immunoenhancing property of dietary whey protein in mice: role of glutathione. Clin. Invest. Med. 12(3): 154–161. PMID: 2743633
Bounous G. & Molson J.H. 2003. The Antioxidant system. Anticancer Res. 23(2B): 1411–1405. PMID: 12820403
Castro G.A., Carvalho J.E., Tinti S.V., Possenti A. & Sgarbieri V.C. 2010. Anti-ulcerogenic effect of a whey protein isolate and collagen hydrolysates against ethanol ulcerative lesions on oral administration to rats. J. Med. Food 13(1): 83–90. DOI:10.1089/jmf.2008.0277
Chan P., Liu I.M., Tzeng T.F., Yang T.L. & Cheng J.T. 2007. Mechanism for blockade of angiotensin subtype 1 receptors to lower plasma glucose in streptozotocin-induced diabetic rats. Diabetes Obes. Metab. 9(1): 39–49. DOI: 10.1111/j.1463-1326.2005.00566.x
Clark K.D., Lu Z. & Strand M.R. 2010. Regulation of melanization by glutathione in the moth Pseudoplusia includens. Insect Biochem. Molec. Biol. 40(6): 460–467. DOI:10.1016/j.ibmb.2010.04.005
David O.L. 1999. Breakthrough technology produces concentrated whey protein with bioactive immunoglobulins. Clin. Nutr. Insights 6(21): 1–4.
Devaraj S. & Jialal I. 2000. Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: the effect of α-tocopherol supplementation. Circulation 102(2): 191–196. DOI:10.1161/01.CIR.102.2.191
Ebaid H., Hassnein K. & El-Feki M. 2005. The un-denatured whey protein enhanced wound healing in mice. J. Egypt. Germ. Soc Zool. 40: 2–27.
Ebaid H., Salem A., Sayde A. & Metwalli A. 2011. Whey protein enhances normal inflammatory responses during cutaneous wound healing in diabetic rats. Lipids in Health Disease. 10(1): 235. DOI:10.1186/1476-511X-10-235
Elagamy E.I., Ruppanne R., Ismail A., Champagne C.P. & Assaf R. 1996. Purification and characterization of lactoferrin, lactoperoxidase, lysozyme and immunoglobulins from camel’s milk. Int. Dairy J. 6(2): 129–145. DOI: 10.1016/0958-6946(94)00055-7
Elagamy E.I., Nawar M.A., Shamsia S.H. & Awad S. 2008. The convenience of camel milk proteins for the nutrition of cow milk allergic children. J. Saudi Soc. Food Nutr. 3: 42–55.
Farah Z. 1986. Effect of heat treatment on whey proteins of camel milk. Milchwissenschaft 41(12): 763–765.
Gauthier S.F., Pouliot Y. & Saint-Sauveur D. 2006. Imunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 16(11): 1315–1323. DOI:10.1016/j.idairyj.2006.06.014
Grellnera W., Georgb T. & Wilskea J. 2000. Quantitative analysis of proinflammatory cytokines (IL-1β, IL-6, TNF-α) in human skin wounds. Foren. Sci. Int. 113(1–3): 251–264. DOI: 10.1016/S0379-0738(00)00218-8
Han S.N., Leka L.S., Lichtenstein A.H., Ausman L.M. & Meydani S.N. 2003. Effect of a therapeutic lifestyle change diet on immune functions of moderately hypercholesterolemic humans. J. Lipid Res. 44(12): 2304–2310. DOI:10.1194/jlr.M300181-JLR200
Kawasaki H. 1993. Comparative studies on proteodermatan sulfate of bovine gastrointestinal tract. Tohoku J. Exp. Med. 171(3): 255–266. PMID: 8160181
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227(5259): 680–685. DOI: 10.1038/227680a0
Lands L.C., Grey V., Smountas A.A., Kramer V.G. & McKenna D. 1999. Lymphocyte glutathione levels in children with cystic fibrosis. Chest 116(1): 201–205. DOI:10.1378/chest.116.1.201
Marchetti J. 1994. Metal complexes of bovine lactoferrin inhibit in Vitro replication of herpes simplex virus type 1 and 2. BioMetals 11(2): 89–94. DOI: 10.1023/A:1009217709851
Mattsby-Baltzer I., Roseanu A., Motas C., Elverfors J., Engberg I. & Hanson L.A. 1996. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr. Res. 40(2): 257–262. PMID:8827774
Mercier A., Gauthier S.F. & Fliss I. 2004. Immunomodulating effects of whey proteins and their enzymatic digests. Int. Dairy J. 14(3): 175–183. DOI: http://dx.doi.org/10.1016/j.idairyj.2003.08.003
Middleton N., Jelen P. & Bell G. 2004. Whole blood and mononuclear cell glutathione response to dietary whey protein supplementation in sedentary and trained male human subjects. Int. J. Food Sci. Nut. 55(2): 131–141. DOI:10.1080/096374080410001666504
Nunes E.A., Bonatto S.J., de Oliveira H.H., Rivera N.L., Maiorka A., Krabbe E.L., Tanhoffer R.A., Fernandes L.C. 2008. The effect of dietary supplementation with 9-cis:12-trans and 10-trans:12-cis conjugated linoleic acid (CLA) for nine months on serum cholesterol, lymphocyte proliferation and polymorphonuclear cells function in Beagle dogs. Res. Vet. Sci. 84(1): 62–67. DOI: http://dx.doi.org/10.1016/j.rvsc.2007.03.010
Plotnick G.D., Corretti M.C. & Vogel R.A. 1997. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single highfat meal. JAMA 278(20): 1682–1686. PMID: 9388088
Redwan E.R. & Tabll A. 2007. Camel lactoferrin markedly inhibits Hepatitis CVirus genotype 4 infection of human peripheral blood leukocytes. J. Immunoassay Immunochem. 28(3): 267–277. DOI:10.1080/15321810701454839
Rivat C., Rodrigues S., Bruyneel E., Piétu G., Robert A., Redeuilh G., Bracke M., Gespach C. & Attoub S. 2005. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) — and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res. 65(1): 195–202. PMID: 15665295
Rusu D., Drouin R., Pouliot Y., Gauthier S. & Poubelle P.E. 2010. A bovine whey protein extract stimulates human neutrophils to generate bioactive IL−1Ra through a NF-kappaBand MAPK-dependent mechanism. J. Nutr. 140(2): 382–391. DOI: 10.3945/jn.109.109645
Shargorodsky M., Debby O., Matas Z. & Zimlichman R. 2010. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr. Metab. 7: 55. DOI: 10.1186/1743-7075-7-55
Shen K., Lili J.Y.C., Qianming Y. & Zhengtao W. 2011. Influence of glutathione levels and activity of glutathione-related enzymes in the brains of tumor-bearing mice. BioSci Trends 5(1): 30–37. DOI:10.5582/bst.2011.v5.1.30
Shimada K., Fujii T., Anai S., Fujimoto K. & Konishi N. 2011. ROS generation via NOX4 and its utility in the cytological diagnosis of urothelial carcinoma of the urinary bladder. BMC Urol. 11: 22. DOI: 10.1186/1471-2490-11-22
Shinoda I., Takase M., Fukuwatari Y., Shimamura S., Köller M. & König W. 1996. Effects of lactoferrin and lactoferricin on the release of interleukin 8 from human polymorphonuclear leukocytes. Biosci. Biotechnol. Biochem. 60(3): 521–523. PMID:8901116
Ustunol Z. & Wong C. 2010. Effect of nonfat dry milk and major whey components on interleukin-6 and interleukin-8 production in human intestinal epithelial-like Caco-2 cells. J. Dairy Sci. 93(6): 2311–2304. DOI:10.3168/jds.2009-2607
Wolff H., Neubert U., Volkenandt M., Zöchling N., Schlüpen E.M., Bezold G. & Meurer M. 1994. Detection of Chlamydia trachomatis in semen by antibody-enzyme immunoassay compared with polymerase chain reaction, antigen-enzyme immunoassay, and urethral cell culture. Fertil. Steril. 62(2): 1250–1254. PMID: 7957993
Wong C.W. & Watson D.L. 1995. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 62(2): 359–368. DOI:10.1017/S0022029900031058
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebaid, H., Badr, G. & Metwalli, A. Immunoenhancing property of dietary un-denatured whey protein derived from three camel breeds in mice. Biologia 67, 425–433 (2012). https://doi.org/10.2478/s11756-012-0014-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-012-0014-0