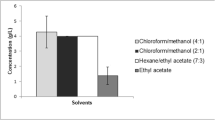
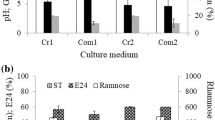
Abstract
The highest yields of biosurfactants were obtained by: (i) Pseudozyma antarctica (107.2 g L−1) cultivated in a medium containing post-refining waste; (ii) Pseudozyma aphidis (77.7 g L−1); and (iii) Starmerella bombicola (93.8 g L−1) both cultivated in a medium with soapstock; (iv)Pichia jadinii (67.3 g L−1) cultivated in a medium supplemented with waste frying oil. It was found that the biosurfactant synthesis yield increased in all strains when the cell surface hydrophobicity reached 70–80 %, enabling the microbial cells to make good contact with hydrophobic substrates. The lowest surface tension of the post-cultivation medium was from 32.0 mN m−1 to 37.8 mN m−1. However, this parameter (which was also determined by a drop collapse assay) was of limited use in monitoring biosurfactant synthesis in this study. The crude glycerol was not a good substrate for biosurfactant synthesis although, in the case of P. aphidis, 67.4 g L−1 of biosurfactants were obtained after cultivation in the medium supplemented with glycerol fraction (GF2). In a low-cost medium containing soapstock and whey permeate or molasses, about 90 g L−1 of mannosylerythritol lipids were synthesised by P. aphidis and approximately 40 g L−1 by P. antarctica.
Similar content being viewed by others
References
Adamczak, M., & Bednarski, W. (2000a). Influence of medium composition and aeration on the synthesis of biosurfactants produced by Candida antarctica. Biotechnology Letters, 22, 313–316. DOI: 10.1023/a:1005634802997.
Adamczak, M., & Bednarski, W. (2000b). Properties and yield of synthesis of mannosylerythritol lipids by Candida antarctica. In S. Bielecki, J. Tramper, & J. Polak (Eds.), Food Biotechnology (pp. 229–234). Amsterdam, The Nertherlands: Elsevier.
Ashby, R. D., & Solaiman, D. K. Y. (2010). The influence of increasing media methanol concentration on sophorolipid biosynthesis from glycerol-based feedstocks. Biotechnology Letters, 32, 1429–1437. DOI: 10.1007/s10529-010-0310-0.
Bednarski, W., Adamczak, M., Tomasik, J., & Płaszczyk, M. (2004). Application of oil refinery waste in the biosynthesis of glycolipids by yeast. Bioresource Technology, 95, 15–18. DOI:10.1016/j.biortech.2004.01.009.
Bednarski, W., Narwojsz, M., Adamczak, M., & Nawotka, R. (2006). Carbon-source-dependent synthesis and composition of biosurfactant synthesized by Pseudozyma antarctica. Environmental Biotechnology, 2, 31–36.
Calvo, C., Manzanera, M., Silva-Castro, G. A., Uad, I., & González-López, J. (2009). Application of bioemulsi-fiers in soil oil bioremediation processes. Future prospects. Science of the Total Environment, 407, 3634–3640. DOI:10.1016/j.scitotenv.2008.07.008.
Cameotra, S. S., & Makkar, R. S. (2004). Recent applications of biosurfactants as biological and immunological molecules. Current Opinion in Microbiology, 7, 262–266. DOI:10.1016/j.mib.2004.04.006.
Coombs, A. (2007). Glycerin bioprocessing goes green. Nature Biotechnology, 25, 953–954. DOI: 10.1038/nbt0907-953.
Daverey, A., & Pakshirajan, K. (2009). Production of sophorolipids by the yeast Candida bombicola using simple and low cost fermentative media. Food Research International, 42, 499–504. DOI:10.1016/j.foodres.2009.01.014.
Desai, J. D., & Banat, I. M. (1997). Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews, 61, 47–64.
Felse, P. A., Shah, V., Chan, J., Rao, K. J., & Gross, R. A. (2007). Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme and Microbial Technology, 40, 316–323. DOI:10.1016/j.enzmictec.2006.04.013.
Fleurackers, S. J. J. (2006). On the use of waste frying oil in the synthesis of sophorolipids. European Journal of Lipid Science and Technology, 108, 5–12. DOI:10.1002/ejlt.200500237.
Folch, J., Lees, M., & Sloane Stanley, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Giannopoulos, A., Makri, A., & Aggelis, G. (2011). Production of biosurfactants from yeasts cultivated on glycerol. In FEBS Workshop: Microbial Lipids from Genomics to Lipidomics, May 13–15, 2010 (pp. 92). Vienna, Austria: Graz University of Technology.
Glenns, R. N., & Cooper, D. G. (2006). Effect of substrate on sophorolipid properties. Journal of the American Oil Chemists’ Society, 83, 137–145. DOI: 10.1007/s11746-006-1186-y.
Kitamoto, D., Isoda, H., & Nakahara, T. (2002). Functions and potential applications of glycolipid biosurfactants — from energy-saving materials to gene delivery carriers —. Journal of Bioscience and Bioengineering, 94, 187–201. DOI: 10.1016/s1389-1723(02)80149-9.
Kitamoto, D., Morita, T., Fukuoka, T., Konishi, M., & Imura, T. (2009). Self-assembling properties of glycolipid biosurfactants and their potential applications. Current Opinion in Colloid & Interface Science, 14, 315–328. DOI:10.1016/j.cocis.2009.05.009.
Kuiper, I., Lagendijk, E. L., Pickford, R., Derrick, J. P., Lamers, G. E. M., Thomas-Oates, J. E., Lugtenberg, B. J. J., & Bloemberg, G. V. (2004). Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Molecular Microbiology, 51, 97–113. DOI: 10.1046/j.1365-2958.2003.03751.x.
Makkar, R. S., Cameotra, S. S., & Banat, I. M. (2011). Advances in utilization of renewable substrates for biosurfactant production. AMB Express, 1, 5. DOI: 10.1186/2191-0855-1-5.
Morita, T., Konishi, M., Fukuoka, T., Imura, T., & Kitamoto, D. (2007a). Microbial conversion of glycerol into glycolipid biosurfactants, mannosylerythritol lipids, by a basidiomycete yeast, Pseudozyma antarctica JCM 10317T. Journal of Bioscience and Bioengineering, 104, 78–81. DOI: 10.1263/jbb.104.78.
Morita, T., Konishi, M., Fukuoka, T., Imura, T., & Kitamoto, D. (2007b). Physiological differences in the formation of the glycolipid biosurfactants, mannosylerythritol lipids, between Pseudozyma antarctica and Pseudozyma aphidis. Applied Microbiology and Biotechnology, 74, 307–315. DOI: 10.1007/s00253-006-0672-3.
Pagliaro, M., Ciriminna, R., Kimura, H., Rossi, M., & Della Pina, C. (2009). Recent advances in the conversion of bioglycerol into value-added products. European Journal of Lipid Science and Technology, 111, 788–799. DOI:10.1002/ejlt.200800210.
Papanikolaou, S., Fick, M., & Aggelis, G. (2004). The effect of raw glycerol concentration on the production of 1,3-propanediol by Clostridium butyricum. Journal of Chemical Technology and Biotechnology, 79, 1189–1196. DOI: 10.1002/jctb.1103.
Pinzon, N. M., Aukema, K. G., Gralnick, J. A., & Wackett, L. P. (2011). Nile red detection of bacterial hydrocarbons and ketones in a high-throughput format. mBio, 2, e00109–11. DOI: 10.1128/mbio.00109-11.
Rodrigues, L., Banat, I. M., Teixeira, J., & Oliveira, R. (2006). Biosurfactants: potential applications in medicine. Journal of Antimicrobial Chemotherapy, 57, 609–618. DOI: 10.1093/jac/dkl024.
Rosenberg, E., & Ron, E. Z. (1999). High- and low-molecularmass microbial surfactants. Applied Microbiology and Biotechnology, 52, 154–162. DOI: 10.1007/s002530051502.
Rosenberg, M., Gutnick, D., & Rosenberg, E. (1980). Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiology Letters, 9, 29–33. DOI: 10.1111/j.1574-6968.1980.tb05599.x.
Rymowicz, W., Rywińska, A., & Gładkowski, W. (2008). Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chemical Papers, 62, 239–246. DOI: 10.2478/s11696-008-0018-y.
Siloto, R. M. P., Truksa, M., He, X. H., McKeon, T., & Weselake, R. J. (2009). Simple methods to detect triacylglycerol biosynthesis in a yeast-based recombinant system. Lipids, 44, 963–973. DOI: 10.1007/s11745-009-3336-0.
Singh, A., Van Hamme, J. D., & Ward, O. P. (2007). Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnology Advances, 25, 99–121. DOI:10.1016/j.biotechadv.2006.10.004.
Smyth, T. J. P., Perfumo, A., Marchant, R., & Banat, I. (2010). Isolation and analysis of low molecular weight microbial glycolipids. In K. N. Timmis (Ed.), Handbook of hydrocarbon and lipid microbiology (pp. 3705–3723). Heidelberg, Germany: Springer-Verlag.
Sobrinho, H. B. S., Rufino, R. D., Luna, J. M., Salgueiro, A. A., Campos-Takaki, G. M., Leite, L. F. C., & Sarubbo, L. A. (2008). Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochemistry, 43, 912–917. DOI:10.1016/j.procbio.2008.04.013.
Takahashi, M., Morita, T., Wada, K., Hirose, N., Fukuoka, T., Imura, T., & Kitamoto, D. (2011). Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. Journal of Oleo Science, 60, 267–273.
Tang, S., Boehme, L., Lam, H., & Zhang, Z. S. (2009). Pichia pastoris fermentation for phytase production using crude glycerol from biodiesel production as the sole carbon source. Biochemical Engineering Journal, 43, 157–162. DOI:10.1016/j.bej.2008.09.020.
Thanomsub, B., Pumeechockchai, W., Limtrakul, A., Arunrattiyakorn, P., Petchleelaha, W., Nitoda, T., & Kanzaki, H. (2007). Chemical structures and biological activities of rhamnolipids produced by Pseudomonas aeruginosa B189 isolated from milk factory waste. Bioresource Technology, 98, 1149–1153. DOI:10.1016/j.biortech.2005.10.045.
Thavasi, R., Jayalakshmi, S., Balasubramanian, T., & Banat, I. M. (2007). Biosurfactant production by Corynebacterium kutscheri from waste motor lubricant oil and peanut oil cake. Letters in Applied Microbiology, 45, 686–691. DOI: 10.1111/j.1472-765x.2007.02256.x.
Transparency Market Research (2012). Biosurfactants market — global scenario, raw material and consumption trends, industry analysis, size, share and forecasts, 2011–2018. Retreived December 18, 2012, from http://www.transparencymarketresearch.com/biosurfactants-market.html
Van Hamme, J. D., Singh, A., & Ward, O. P. (2006). Physiological aspects. Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnology Advances, 24, 604–620. DOI: 10.1016/j.biotechadv.2006.08.001.
Vasileva-Tonkova, E., & Gesheva, V. (2004). Potential for biodegradation of hydrocarbons by microorganisms isolated from Antarctic soils. Zeitschrift für Naturforschung C, Journal of Biosciences, 59, 140–145.
Walter, V., Syldatk, C., & Hausmann, R. (2010). Screening concepts for the isolation of biosurfactant producing microorganisms. In R. Sen (Ed.), Biosurfactants (pp. 1–13). New York, NY, USA: Springer.
Willke, T., & Vorlop, K. D. (2004). Industrial bioconversion of renewable resources as an alternative to conventional chemistry. Applied Microbiology and Biotechnology, 66, 131–142. DOI: 10.1007/s00253-004-1733-0.
Willumsen, P. A., & Karlson, U. (1996). Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation, 7, 415–423. DOI: 10.1007/bf00056425.
Yin, H., Qiang, J., Jia, Y., Ye, J. S., Peng, H., Qin, H. M., Zhang, N., & He, B. Y. (2009). Characteristics of biosurfactant produced by Pseudomonas aeruginosa S6 isolated from oil-containing wastewater. Process Biochemistry, 44, 302–308. DOI:10.1016/j.procbio.2008.11.003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dzięgielewska, E., Adamczak, M. Evaluation of waste products in the synthesis of surfactants by yeasts. Chem. Pap. 67, 1113–1122 (2013). https://doi.org/10.2478/s11696-013-0349-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0349-1