Abstract
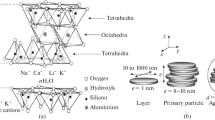
Adsorption of cetyltrimethylammonium bromide (CTAB) onto bituminous coal (BC) and a clay mineral, montmorillonite (MMT), was studied. Simultaneous measurements of the CTAB adsorption and zeta potential determination of the adsorption suspensions were carried out. The adsorption isotherms were found to be of the typical Langmuir type; values of the CTAB adsorption capacities were calculated (a m = 0.65 mmol g−1 for coal and a m = 3.24 mmol g−1 for MMT). The shape of the adsorption isotherms was correlated with zeta potential values at the adsorption equilibrium. The adsorption properties of both sorbents were studied by voltammetry on carbon paste electrodes (CPE) modified with coal-CTAB and MMT-CTAB system, respectively. Open circuit sorption with differential pulse voltammetry was performed in order to compare the sorption properties of the systems with the unmodified sorbents. The Cu2+ adsorption on BC and MMT decreased to approximately 50 % and 40 %, respectively. The surface adsorption mechanism of CTAB on coal based on hydrophilic interactions was proposed. In the case of montmorillonite, the CTAB intercalation is expected via ion exchange into the inter-layer space forming a double- or triple-layer arrangement.
Similar content being viewed by others
References
Başar, C. A., Karagunduz, A., Keskinler, B., & Cakici, A. (2003). Effect of presence of ions on surface characteristics of surfactant modified powdered activated carbon (PAC). Applied Surface Science, 218, 169–174. DOI: 10.1016/S0169-4332(03)00576-2.
Betega de Paiva, L., Morales, A. R., & Valenzuela Díaz, F. R. (2008). Organoclays: Properties, preparation and applications. Applied Clay Science, 42, 8–24. DOI: 10.1016/j.clay.2008.02.006.
Crawford, R. J., & Mainwaring, D. E. (2001). The influence of surfactant adsorption on the surface characterisation of Australian coals. Fuel, 80, 313–320. DOI: 10.1016/S0016-2361(00)00110-1.
Gallardo-Moreno, A. M., González-García, C. M., González-Martín, M. L., & Bruque, J. M. (2004). Arrangement of SDS adsorbed layer on carbonaceous particles by zeta potential determinations. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 249, 57–62. DOI: 10.1016/j.colsurfa.2004.08.051.
Gu, T., Zhu, B. Y., & Rupprecht, H. (1992). Surfactant adsorption and surface micellizations. Progress in Colloid and Polymer Science, 88, 74–85. DOI: 10.1007/BFb0114420.
Hernández, M., Fernández, L., Borrás, C., Mostany, J., & Carrero, H. (2007). Characterization of surfactant/hydrotalcite-like clay/glassy carbon modified electrodes: Oxidation of phenol. Analytica Chimica Acta, 597, 245–256. DOI: 10.1016/j.aca.2007.06.010.
Juang, R.-S., & Wu, W.-L. (2002). Adsorption of sulfate and copper(II) on goethite in relation to the changes of zeta potentials. Journal of Colloid and Interface Science, 249, 22–29. DOI: 10.1006/jcis.2002.8240.
Kooli, F., Liu, Y., Alshahateet, S. F., Messali, M., & Bergaya, F. (2009). Reaction of acid activated montmorillonites with hexadecyl trimethylammonium bromide solution. Applied Clay Science, 43, 357–363. DOI: 10.1016/j.clay.2008.10.006.
Liu, X. Lu, R., Wang, R. C., Zhou, H., & Xu, S. (2007). Interlayer structure and dynamics of alkylammonium-intercalated smectites with and without water: A molecular dynamics study. Clays and Clay Minerals, 55, 554–564. DOI: 10.1346/CCMN.2007.0550602.
Maršálek, R., & Taraba, B. (2008). Adsorption of the SDS on coal. Progress in Colloid and Polymer Science, 135, 163–168. DOI: 10.1007/978-3-540-85134-9.
Mishra, S. K., Kanungo, S. B., & Rajeev (2003). Adsorption of sodium dodecyl benzenesulfonate onto coal. Journal of Colloid and Interface Science, 267, 42–48. DOI: 10.1016/S0021-9797(03)00553-8.
Navrátilová, Z. (2009). Coal as a new carbon paste electrode modifier with sorption properties. Electroanalysis, 21, 1758–1762. DOI: 10.1002/elan.200904657.
Navrátilová, Z., & Kula, P. (2000). Cation and anion exchange on clay modified electrodes. Journal of Solid State Electrochemistry, 4, 342–347. DOI: 10.1007/s100080000126.
Navrátilová, Z., & Vaculíková, L. (2006). Electrodeposition of mercury film on electrodes modified with clay minerals. Chemical Papers, 60, 348–352. DOI: 10.2478/s11696-006-0063-3.
Navrátilová, Z., Wojtowicz, P., Vaculíková, L., & Šugárková, V. (2007). Sorption of alkylammonium cations on montmorillonite. Acta Geodynamica et Geomaterialia, 4(3), 59–65.
Ngameni, E., Tonlé, I. K., Apohkeng, J. T., Bouwé, R. G. B., Jieumboué, A. T, & Walcarius, A. (2006). Permselective and preconcentration properties of a surfactant-intercalated clay modified electrode. Electroanalysis, 18, 2243–2250. DOI: 10.1002/elan.200603654.
Praus, P., Turicova, M., Študentova, S., & Ritz, M. (2006). Study of cetyltrimethylammonium and cetylpyridinium adsorption on montinorillonite. Journal of Colloid and Interface Science, 304, 29–36. DOI: 10.1016/j.jcis.2006.08.038.
Ray, S. S., & Okamoto, M. (2003). Polymer/layered silicate nanocomposites: a review from preparation to processing. Progress in Polymer Science, 28, 1539–1641. DOI: 10.1016/j.progpolymsci.2003.08.002.
Rosen, M. J. (2004). Surfactants and interfacial phenomena (3rd ed.). Hoboken, NJ, USA: Wiley. DOI: 10.1002/0471670561.
Singh, B. P. (1999). The role of surfactant adsorption in the improved dewatering of fine coal. Fuel, 78, 501–506. DOI: 10.1016/S0016-2361(98)00169-0.
Taraba, B., Kula, P., & Gucka, M. (2001). Calorimetric study of interaction between surfactants and coals. In Proceedings of the International Slovak and Czech Calorimetric Seminar, 28 May–1 June 2001 (pp 49–50). Račkova dolina, Slovakia.
Vittal, R., Gomathi, H., & Kim, K.-J. (2006). Beneficial role of surfactants in electrochemistry and in the modification of electrodes. Advances in Colloid and Interface Science, 119, 55–68. DOI: 10.1016/j.cis.2005.09.004.
Wu, H. S., & Pendleton, P. (2001). Adsorption of anionic surfactant by activated carbon: Effect of surface chemistry, ionic strength, and hydrophobicity. Journal of Colloid and Interface Science, 243, 306–315. DOI: 10.1006/jcis.2001.7905.
Wu, S. F., Yanagisawa, K., & Nishizawa, T. (2001). ζ-potential on carbons and carbides. Carbon, 39, 1537–1541. DOI: 10.1016/S0008-6223(00)00275-X.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maršálek, R., Navrátilová, Z. Comparative study of CTAB adsorption on bituminous coal and clay mineral. Chem. Pap. 65, 77–84 (2011). https://doi.org/10.2478/s11696-010-0076-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-010-0076-9