Abstract
Background
Asparagus officinalis L. is often infected by fungi from the Fusarium genus which also contaminate the plant tissues with highly toxic secondary metabolites. To elucidate the plant-pathogen interactions between asparagus and Fusarium oxysporum or F. proliferatum, a fungal mycotoxins profile was assessed together with an impact of the infection on all forms of salicylic acid content.
Methodology
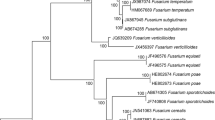
Fungal isolates were identified by their morphological features, species-specific PCR and transcription elongation factor 1a (TEF-1a) sequencing. Mycotoxins were assessed by high-performance liquid chromatography (HPLC). The salicylic acid and its derivatives content was analyzed by the HPLC method combined with fluorometric detection. The levels of free radicals were measured by electron paramagnetic resonance (EPR).
Results
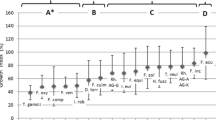
After infection both Fusarium pathogens formed fumonisin B1 and moniliformin. Infection altered salicylic acid biosynthesis and conjugation rates both in the roots and stems when compared with non-inoculated plants. Samples with higher free radical concentrations in stems showed higher concentrations of all forms of salicylic acid.
Conclusions
We postulate that infection by both Fusarium pathogens produces mycotoxins, which may be transported to the upper part of plant. Pathogen attack initiated a plant defense reaction involving increased salicylic acid levels and resulting in increase in free radical levels.
Similar content being viewed by others
Abbreviations
- APx:
-
ascorbate peroxidase
- CAT:
-
catalase
- EPR:
-
electron paramagnetic resonance
- Foa :
-
F. oxysporum f. sp. asparagi
- FR:
-
free radicals
- FBs:
-
fumonisins
- FB1 :
-
fumonisin B1
- HR:
-
hypersensitive reaction
- IR:
-
induced resistance
- MeSA:
-
methyl-salicylates
- MON:
-
moniliformin
- PDA:
-
potato dextrose agar
- PAL:
-
phenylalanine ammonia lyase
- POD:
-
peroxidase
- PR:
-
proteins
- ROS:
-
reactive oxygen species
- SA:
-
salicylic acid
- SAG:
-
salicylic acid glucoside esters
- SOD:
-
superoxide dismutase
- SAR:
-
systemic acquired resistance
- TSA:
-
total content of free and glucoside bound salicylic acid
References
Elmer W.H., Johnson D.A., Mink G.I., Epidemiology and management of the diseases causal to asparagus decline, Plant Dis., 1996, 80, 117–125
Karolewski Z., Waśkiewicz A., Irzykowska L., Bocianowski J., Kostecki M., Goliński P., et al., Fungi presence and their mycotoxins distribution in asparagus spears, Polish J. Environ. Stud., 2011, 20, 911–919
Waśkiewicz A., Irzykowska L., Karolewski Z., Bocianowski J., Kostecki M., Goliński P., et al., Fusarium spp. and mycotoxins present in asparagus spears, Cereal Res. Comm., 2008, 36SB, 405–408
Waśkiewicz A., Goliński P., Karolewski Z., Irzykowska L., Bocianowski J., Kostecki M., et al., Formation of fumonisins and other secondary metabolites by Fusarium oxysporum and F. proliferatum: a comparative study, Food Add. Contam., 2010, 27, 608–615
Weber Z., Kostecki M., von Bargen S., Gossmann M., Waśkiewicz A., Bocianowski J., et al., Fusarium species colonizing spears and forming mycotoxins in field samples of asparagus from Germany and Poland, J. Phytopathol., 2006, 154, 209–216
Irzykowska L., Baturo A., Genetic polymorphism of Fusarium culmorum isolates originating from roots and stem bases of barley, J. Plant Prot. Res., 2008, 48, 303–311
Stępień Ł., Koczyk G., Waśkiewicz A., FUM cluster divergence in fumonisins-producing Fusarium species, Fungal Biol., 2011, 15, 112–123
Stępień Ł., Koczyk G., Waśkiewicz A., Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species, J. Appl. Genet., 2011, 52, 487–496
Waśkiewicz A., Stępień Ł., Wilman K., Kachlicki P., Diversity of pea-associated F. proliferatum and F. verticillioides populations revealed by FUM1 sequence analysis and fumonisin biosynthesis, Toxins, 2013, 5, 488–503
Seefelder W., Gossman M., Humpf H.U., Analysis of fumonisin B1 in Fusarium proliferatum — infected asparagus spears and garlic bulbs from Germany by liquid chromatography-electrospray ionization mass spectrometry, J. Agr. Food Chem., 2002, 50, 2778–2781
Waśkiewicz A., Beszterda M., Goliński P., Occurrence of fumonisins in food — an interdisciplinary approach to the problem, Food Control, 2012, 26, 491–499
Marasas W.F.O., Discovery and occurrence of the fumonisins: a historical perspective, Environ. Health Perspect., 2001, 109, 239–243
Ueno Y., Iijima K., Wang S.D., Sugiura Y., Sekijima M., Tanaka T., et al., Fumonisins as a possible contributory risk factor for primary liver cancer: a 3-year study of corn harvested in Haiman, China by HPLC and ELISA, Food Chem. Toxicol., 1997, 35, 1143–1150
Missmer S.A., Suarez L., Felkner M., Wang E., Merrill Jr A.H., Rothman K.J., et al., Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border, Environ. Health Perspect., 2006, 114, 237–241
Fincham J.E., Marasas W.F.O., Taljaard J.J., Kriek N.P., Badenhorst C.J., Gelderblom W.C., et al., Atherogenic effects in a non-human primate of Fusarium moniliforme cultures added to a carbohydrate diet, Atherosclerosis, 1992, 94, 13–25
International Agency for Research on Cancer (IARC). Fumonisin B1. IARC Monographs on the evaluation of the carcinogenic risks to humans: Some traditional herbal medicines, some mycotoxins, naphthalene and styrene, IARC, Lyon, France, 2002
Pineda-Valdes G., Bullerman L.B., Thermal stability of moniliformin at varying temperature, pH, and time in an aqueous environment, J. Food Prot., 2000, 63, 1598–1601
Commission of European Communities, Commission recommendation of 17 August, 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding, Offi J Eur Union, 23, L229/7, 2006
Delaney T.P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., et al., A central role of salicylic acid in plant disease resistance, Science, 1994, 266, 1247–1250
Molodchenkova O.O., Adamovskaya V.G., Levitskii Y.A., Gontarenko O.V., Sokolov V.M., Maize response to salicylic acid and Fusarium moniliforme, Appl. Biochem. Microbiol., 2002, 38, 381–385
Vasyukova N.I., Ozeretskovskaya O.L., Induced plant resistance and salicylic acid: a review, Appl. Biochem. Microbiol., 2007, 43, 367–373
Enyedi A.J., Yalpani N., Silverman P., Raskin I., Localization, conjugation and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus, Proc. Nat. Acad. Sci. USA, 1992, 89, 2480–2484
O’Donnell P.J., Jones J.B., Antoine F.R., Cialdi J., Klee H.J., Ethylene-dependent salicylic acid regulates an expended cell death response to a plant pathogen, Plant J., 2001, 25, 315–323
Yalpani N., Silverman P., Wilson T.M., Kleier D.A., Raskin I., Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco, Plant Cell, 1991, 3, 809–818
Coquoz J.L., Buchala A., Métraux J.P., The biosynthesis of salicylic acid in potato plants, Plant Physiol., 1998, 117, 1095–1101
Meuwly P., Molders W., Buchala A., Metraux J.P., Local and systemic biosynthesis of salicylic acid in infected cucumber plants, Plant Physiol., 1995, 109, 1107–1114
Pancheva T., Popova L., Uzunova A., Effect of salicylic acid in growth and photosynthesis in barley plants, J. Plant Physiol., 1996, 149, 57–63
Raskin I., Role of salicylic acid in plants, Ann. Rev. Plant Physiol. Plant Mol. Biol., 1992, 43, 439–462
Raskin I., Ehmann A., Melander W., Meeuse B., Salicylic acid — a natural inducer of heat production in Arum lilies, Science, 1987, 237, 1545–1556
Klessig D.F., Malamy J., The salicylic acid signal in plants, Plant Mol. Biol., 1994, 26, 1439–1458
Eichhorn H., Klinghammer M., Becht P., Tenhaken R., Isolation of a novel ABC-transporter gene from soybean induced by salicylic acid, J. Exp. Bot., 2006, 57, 2193–2201
Durner J., Klessig D.F., Salicylic acid is a modulator of tobacco and mammalian catalases, J. Biol. Chem., 1996, 271, 28492–28501
Rao M., Paliyath G., Ormrod D., Murr D., Watkins C., Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes, Plant Physiol., 1997, 115, 137–149
Rasmussen J.B., Hammerschmidt R., Zook M.N., Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv. Syringae, Plant Physiol., 1991, 97, 1342–1347
Troshina N.B., Yarullina L.G., Valeev A.S., Maksimov I.V., Salicylic acid induces resistance to Septoria nodorum Berk. in wheat, Plant Physiol., 2007, 34, 451–456
Baker C.J., Orlandi E.W., Active oxygen in plant pathogenesis, Ann. Rev. Phytopathol., 1995, 33, 299–321
Egan M.J., Talbot N.J., Genomes, free radicals and plant cell invasion: recent developments in plant pathogenic fungi, Curr. Opin. Plant Biol., 2008, 11, 367–372
Hipelli S., Rohnert U., Koske D., Elstner E.F., Schneider W., Free radicals in pathogenesis: health-promoting functions of plant- and milkderived antioxidants, Monatsschr Kinderheilk, 1998, Suppl 1, S63–S72
Bhattacharjee S., Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants, Curr. Sci., 2005, 89, 1113–1121
Kempe S., Metz H., Mäder K., Application of Electron Paramagnetic Resonance (EPR) spectroscopy and imaging in drug delivery research — chances and challenges, Eur. J. Pharm. Biopharm., 2010, 74, 55–56
Morsy M.A., Khaled M.M., Novel EPR characterization of the antioxidant activity of tea leaves, Spectrochimica Acta Part A, 2002, 58, 1271–1277
Jonas M., Concepts and methods of ESR dating, Radiat Measur, 1997, 27, 943–973
Booth C., The Genus Fusarium, CABI, Kew, Surrey, 1971
Gerlach W., Nirenberg H., The genus Fusarium. A Pictorial Atlas, Mitt Biol Bundesanst Land Forstwirtschaft, Berlin-Dahlem, 1982
Kwaśna H., Chełkowski J., Zajkowski P., Fungi (Mycota), XXII., Polish Academy of Sciences, Warsaw, Cracow, 1991
Barnett H.L., Hunter B.B., Illustrated genera of imperfect fungi, APS Press, St. Paul, Minnesota, USA, 1998
Mulè G., Susca A., Stea G., Moretti A., Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences, FEMS Microbiol. Lett., 2004, 230, 235–240
Mulè G., Susca A., Stea G., Moretti A., Corrigendum to “Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences”. [FEMS Microbiol. Lett. 230 (2004) 235–240], FEMS Microbiol. Lett., 2004, 232, 229
Irzykowska L., Bocianowski J., Waśkiewicz A., Weber Z., Karolewski Z., Goliński P., et al., Genetic variation of Fusarium oxysporum isolates forming fumonisin B1 and moniliformin, J. Appl. Genet., 2012, 53, 237–247
Sydenham E.W., Thiel P.G., Marasas W.F.O., Shephard G.S., Van Schalkwyk D.J., Natural occurrence of some Fusarium mycotoxins in com from low and high esophageal cancer prevalent areas of the Transkei, southern Africa, J. Agr. Food Chem., 1990, 38, 1900–1903
Goliński P., Waśkiewicz A., Wiśniewska H., Kiecana I., Mielniczuk E., Gromadzka K., et al., Reaction of winter wheat (Triticum aestivum L.) cultivars to infection with Fusarium spp.: mycotoxin contamination in grain and chaff, Food Add. Contam., 2010, 27, 1015–1024
Sharman M., Gilbert J., Chełkowski J., A survey of the occurrence of the mycotoxin moniliformin in cereal samples from sources worldwide, Food Add. Contam., 1991, 4, 459–466
Payne R., Murrey D., Harding S., Baird D., Soutou D., Lane P., GenStat for Windows (7th edition) — Introduction, VSN International, Oxford, UK, 2003
He C.Y., Wolyn D.J., Potential role for salicylic acid in induced resistance of asparagus roots to Fusarium oxysporum f. Sp. Asparagi, Plant Pathol., 2005, 54, 227–232
Mandal S., Hazra B., Sarkar R., Biswas S., Mandal N., Hemidesmus indicus, an age old plant: study of its in vitro antioxidant and free radical scavenging potentials, Pharmacologyonline, 2009, 1, 604–617
Borowiak K., Rucińska-Sobkowiak R., Rymer K., Gwóźdź E.A., Zbierska J., Biochemical markers of tropospheric ozone: experimentation with testplants, Polish J. Ecol., 2009, 57, 3–14
Chong J., Baltz R., Schmitt C., Beffa R., Fritig B., Saindrenan P., Downregulation of a pathogen-responsive tobacco UDP-Glc: phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress and weakens virus resistance, Plant Cell, 2002, 14, 1093–1107
Lee H.I., Leon J., Raskin I., Biosynthesis and metabolism of salicylic acid, Proc. Nat. Acad. Sci. USA, 1995, 92, 4076–4079
Malamy J., Carr J.P., Klessig D.F., Raskin I., Salicylic acid: likely endogenous signal in the resistance response of tobacco to viral infection, Science, 1990, 250, 1002–1004
Malamy J., Klessig D.F., Salicylic acid and plant disease resistance, Plant J., 1992, 2, 643–654
Mölders W., Buchala A., Métraux J.P., Transport of salicylic acid in tobacco necrosis virus-infected cucumber plants, Plant Physiol., 1996, 112, 787–792
Enyedi A.J., Raskin I., Induction of UDP-glucose: salicylic acid glucosyltransferase activity in tobacco mosaic virus-inoculated tobacco (Nicotiana tabacum) leaves, Plant Physiol., 1993, 101, 1375–1380
Hennig J., Malamy J., Grynkiewicz G., Indulski J., Klessig D., Interconversion of the salicylic acid and its glucoside in tobacco, Plant J., 1993, 4, 593–600
Shulaev V., Silverman P., Raskin I., Airborne signaling by methyl salicylate in plant pathogen resistance, Nature, 1997, 385, 718–721
Prell H.H., Day P., Plant-fungal pathogen interaction, A classical and molecular view, 2001, 214
Edgar C.I., McGrath K.C., Dombrecht B., Manners J.M., Maclean D.J., Schenk P.M.P., et al., Salicylic acid mediates resistance to the vascular wilt pathogen Fusarium oxysporum in the model host Arabidopsis thaliana, Austr. Plant Pathol., 2006, 35, 581–591
Abbas H.K., Boyette C.D., Hoagland R.E., Phytotoxicity of Fusarium, other fungal isolates, and of the phytotoxins fumonisin, fusaric acid and moniliformin to jimsonweed, Phytoprotect., 1995, 76, 17–25
Doehlert D.C., Knutson C.A., Vesonder R.F., Phytotoxic effects of fumonisin B1 on maize seedling growth, Mycopathol., 1994, 127, 117–121
Van Asch M.A.J., Rijkenberg F.H., Coutinho T.A., Phytotoxicity of fumonisins B1, moniliformin, and T-2 toxin to corn callus cultures, Postharv. Pathol. Mycotox., 1992, 82, 1330–1333
Vesonder R.F., Labeda D.P., Peterson R.E., Phytotoxic activity of selected water-soluble metabolites of Fusarium against Lemna minor L. (Duckweed), Mycopathol., 1992, 118, 185–189
Schmidt-Heydt M., Magan N., Geisen R., Stress induction of mycotoxin biosynthesis genes by abiotic factors, FEMS Microbiol. Lett., 2008, 228, 142–149
Dellinger B., Lomnicki S., Khachatryan L., Maskos Z., Hall R.W., Adounkpe J., et al., Formation and stabilization of persistent free radicals, Proc Combus Inst, 2007, 31, 521–528
Tian L., Koshland C.P., Yano J., Yachandra V.K., Yu I.T.S., Lee S.C., et al., Carbon-centered free radicals in particulate matter emissions from wood and coal combustion, Energy Fuels, 2009, 23, 2523–2526
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Waśkiewicz, A., Irzykowska, L., Drzewiecka, K. et al. Plant-pathogen interactions during infection process of asparagus with Fusarium spp.. cent.eur.j.biol. 8, 1065–1076 (2013). https://doi.org/10.2478/s11535-013-0217-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-013-0217-6