Abstract
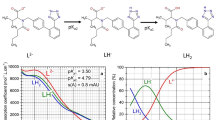
The mixed dissociation constant of naphazoline is determined at various ionic strengths I [mol dm−3] in the range of 0.01 to 0.26 and at temperatures of 25°C and 37°C using ESAB and HYPERQUAD regression analysis of the potentiometric titration data. A strategy of efficient experimentation is proposed in a protonation constant determination, followed by a computational strategy for the chemical model with a protonation constant determination. Two group parameters, L 0 and H T were ill-conditioned in the model and their determination is therefore uncertain. These group parameters, L 0 and H T, can significantly influence a systematic error in the estimated common parameter pKa and they always should be refined together with pK a. The thermodynamic dissociation constant pK Ta was estimated by nonlinear regression of {pK a, I} data at 25°C and 37°C: for naphazoline pK Tal = 10.41(1) and 10.13(2). Goodness-of-fit tests for various regression diagnostics enabled the reliability of the parameter estimates to be found.

Similar content being viewed by others
References
S. Casado-Terrones, J.F. Fernandez-Sanchez, B.C. Diaz, A.S. Carretero, A. Fernandez-Gutierrez, Journal of Pharmaceutical and Biomedical Analysis 38, 785 (2005)
A. Goodmann-HiIlman, T. Rall, A. Nier, P. Taylor, the Pharmacologycal Basic of Therapeutic (McGraw-Hill, New York, 1996)
P. Chocholous, D. Satinsky, P. Solich, Talanta 70, 408 (2006)
S. Goenechea, Journal of Chromatography A 36, 375 (1968)
H.C. Goicoechea, A.C. Olivieri, Analytica Chimica Acta 453, 289 (2002)
K.M. Kelani, Journal of AOAC International 81, 1128 (1998)
M. Massaccesi, Pharmaceutica Acta Helvetiae 62, 302 (1987)
R. Bocic, C. Vallejos, A.A. lvarez-Luege, F. Lo’pez, J. AOAC Int. 75, 902 (1992)
S.C. Ruckmick, D.F. Marsh, S.T. Duong, Journal of Pharmaceutical Sciences, 84, 502 (1995)
A.F. Marchesini, M.R. Williner, V.E. Mantovani, J.C. Robles, H.C. Goicoechea, Journal of Pharmaceutical and Biomedical Analysis 31, 39 (2003)
J.M. Lemus Gallego, J. Pérez Arroyo, Journal of Chromatography B 784, 39 (2003)
J.M. Lemus Gallego, J. Pérez Arroyo, Journal of Separation Science 26, 947 (2003)
S. Khalil, Microchimica Acta 130, 181 (1999)
J.L. Manzoori, M. Amjadi, Indian J. Chem. A 42, 2988 (2003)
S.L. McCall, J.D. Winefordner, Analytical Chemistry 55, 391 (1983)
A.S. Carretero, C.C. Blanco, B.C. Diaz, A.F. Gutierrez, Analyst 123, 1069 (1998)
A.F. Gutierrez, A.S. Carretero, B.C. Diaz, C.C. Blanco, Applied Spectroscopy 53, 741 (1999)
I. Shoukrallah, Acta Pharmaceutica Jugoslavica 41, 107 (1991)
S.M. Sabry, M.H. Abdel-Hay, M.H. Barary, T.S. Belal, Journal of Pharmaceutical and Biomedical Analysis 22, 257 (2000)
G. Santoni, P. Mura, S. Pinzauti, P. Gratteri, E. Laporta, International Journal of Pharmaceutics 50, 75 (1989)
J.M.L. Gallego, J.P. Arroyo, Journal of Separation Science 26, 947 (2003)
A. Salinas-Castillo, A.S. Carretero, A. Fernandez-Gutierrez, Analytical and Bioanalytical Chemistry 376, 1111 (2003)
H.C. Goicoechea, A.C. Olivieri, Analyst 126, 1105 (2001)
Y. Liang, Q. Huang, Yaowu Fenxi Zazhi 5, 81 (1985)
M. Meloun, T. Syrovy, A. Vrana, Talanta 62, 511 (2004)
C. Rigano, M. Grasso, S. Sammartano, Annali Di Chimica 74, 537 (1984)
C. De Stefano, P. Princi, C. Rigano, S. Sammartano, Annali di Chimica 77, 643 (1987)
S. Capone, A. Derobertis, C. Destefano, S. Sammartano, R. Scarcella, Talanta 34, 593 (1987)
P. Gans, A. Sabatini, A. Vacca, Talanta 43, 1739 (1996)
M. Meloun, J. Havel, E. Högfeldt, Computation of solution equilibria: a guide to methods in potentiometry, extraction, and spectrophotometry (Ellis Horwood Chichester, England, 1988)
M. Meloun, M. Bartos, E. Hogfeldt, Talanta 35, 981 (1988)
M. Meloun, J. Militký, M. Forina, Chemometrics for analytical chemistry. PC-aidedregression and related mathods (Ellis Horwood, Chichester, 1994) Volume 2
M. Meloun, J. Militký, M. Forina, Chemometrics for analytical chemistry. PC-Aided Statistical Data Analysis (Ellis Horwood, Chichester, 1992) Volume 1
P.M. May, D.R. Williams, P.W. Linder, R.G. Torrington, Talanta 29, 249 (1982)
M. Meloun, V. Riha, J. Zacek, Chemicke Listy 82, 765 (1988)
T.S.S. Ltd., (TriloByte Statistical Software Ltd., Pardubice, Czech republic, 1990 and 1999)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Meloun, M., Ferenčíková, Z., Netolická, L. et al. The thermodynamic dissociation constant of naphazoline by the regression analysis of potentiometric data. cent.eur.j.chem. 9, 66–74 (2011). https://doi.org/10.2478/s11532-010-0117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-010-0117-9