Abstract
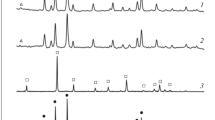
The present study concerns the electrochemical synthesis of basic copper carbonate nanoparticles by oxidation of metallic copper on the anode in an aqueous bicarbonate solution. This simple and one-step preparation can be considered as green synthesis. The scanning electron microscopy (SEM) analysis indicates that average particle size of the product is in the range of about 70 nm. On the other hand, basic copper carbonate micro-powder has been prepared, by mixing solutions of copper(II) sulphate and sodiu bicarbonate. The SEM analysis showed that the size of particles prepared in the same way is in the range of about 1 µm. In another part of this study, the thermal decomposition of micro and nanoparticles of copper carbonate produced by various methods was studied in air using TG-DTA techniques. The results of thermal study show that the decomposition of both samples occurs in single step. Also, the TG-DTA analysis of the nanoparticles indicates that the main thermal degradation occurs in the temperature range of 245–315°C. However, microparticles of Cu(OH)2 · CuCO3 decomposed endothermally in the temperature range of 230–330°C.

Similar content being viewed by others
References
W. Gottfried et al., U.S. Patent 4, 659, 555 (1987)
A.P. Martina, U.S. Patent 6, 228, 191 (2001)
W.L. Masterson, C.N. Hurley, Chemistry: Principles and Reactions, 5th edition (Thomson Learning Inc, USA 2004) 498
M.A. Hiskey, D.L. Naud, U.S. Patent 6, 599, 379 (2003)
G. Hawley, Condensed Chemical Dictionary, Van Nostrand Reinhold Company, 20th edition (1981) ISBN: 0-442-23244-6
C.D. Hodgman, Handbook of Chemistry and Physics, 43th edition (CRC Press, Ohio, 1962)
S.A.A. Mansour, J. Therm. Anal. 42 (1994) 1251
S.W. Moon, Korean patent no: PCT/KR2001/001329, 2002
N. Koga, J.M. Criado, H. Tanaka. Thermochim. Acta 340–341, 387 (1999)
U. Teipel, Energetic Material (Wiley-VCH Verlag, Germany, 2002)
M. Fathollahi, S.M. Pourmortazavi, S.G. Hosseini, Combust. Flame 138, 304 (2004)
A. Robertson, U. Erb, G. Palumbo, NanoStruct. Mat. 12, 1035 (1999)
U. Erb, A. M. El-Sherik, G. Palumbo, K.T. Aust, NanoStruct. Mat. 2, 383 (1993)
J.L. Camalet, J.C. Lacroix, S. Aeiyach, K. Chane-Ching, P.C. Lacaze, Synthetic Metals 93, 133 (1998)
P.K. Khanna, B.K. Das, Materials Letters 58, 1030 (2004)
M.I. Schimmel, N.R. de. Tacconi, K. Rajeshwar, J. Electroanal. Chem. 453, 187 (1998)
Y. Gui, C. Xie, Q. Zhang, M. Hu, J. Yu, Z. Weng, J. Crystal Growth 289, 663 (2006)
D. Brevet, Y. Mugnier, S. Samreth, Electrochim. Acta 48, 3419 (2003)
S.G. Hosseini, S.M. Pourmortazavi, S.S. Hajimirsadeghi, Combust. Flame 141, 322 (2005)
W.M. Shaheen, M.M. Selim, Thermochim. Acta 322, 117 (1998)
M. Odlyha, N.S. Cohen, G.M. Foster, R.H. West, Thermochim. Acta 365, 53 (2000)
P.J. Haines, Thermochim. Acta 340–341, 285 (1999)
M. Dinamani, P.V. Kamath, Mater. Res. Bull. 36, 2043 (2001)
ASTM E967, Standard Practice for Temperature Calibration of Differential Scanning Calorimeters and Differential Thermal Analyzers, American Society for Testing and Materials (Philadelphia, PA, 1997)
D. Dollimore, T.J. Taylor, Thermal Analysis, Proceeding of the Sevent ICTA (Wiley-Heyden, New York, 1982) 636
N.A. Hassan, W.M. Shaheen, M.M. Selim, International Conference on Chemistry and Its Role in Development, April 1997, Mansoura, Egypt (Mansoura University, Egypt, 1997)
H. Henmi, T. Hirayama, S. Shanmugarajah, N. Mitzutani, M. Kato, Thermochim. Acta 96, 145 (1985)
H. Henmi, T. Hirayama, S. Shanmugarajah, N. Mitzutani, M. Kato, Thermochim. Acta 106, 263 (1986)
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pourmortazavi, S.M., Kohsari, I. & Hajimirsadeghi, S.S. Electrosynthesis and thermal characterization of basic copper carbonate nanoparticles. cent.eur.j.chem. 7, 74–78 (2009). https://doi.org/10.2478/s11532-008-0094-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11532-008-0094-4