Abstract
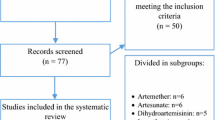
Malaria is a serious parasitic infection, which affects millions of people worldwide. As pregnancy has been shown to alter the pharmacokinetics of many medications, the efficacy and safety of antimalarial drug regimens may be compromised in pregnant women. The objective of this review is to systematically review published literature on the pharmacokinetics of antimalarial agents in pregnant women. A search of MEDLINE (1948-May 2011), EMBASE (1980-May 2011), International Pharmaceutical Abstracts (1970-May 2011), Google and Google Scholar was conducted for articles describing the pharmacokinetics of antimalarials in pregnancy (and supplemented by a bibliographic review of all relevant articles); all identified studies were summarized and evaluated according to the level of evidence, based on the classification system developed by the US Preventive Services Task Force. Identified articles were included in the review if the study had at least one group that reported at least one pharmacokinetic parameter of interest in pregnant women. Articles were excluded from the review if no pharmacokinetic information was reported or if both pregnant and non-pregnant women were analysed within the same group. For quinine and its metabolites, there were three articles (one level II-1 and two level III); for artemisinin compounds, two articles (both level III); for lumefantrine, two articles (both level III); for atovaquone, two articles (both level III); for proguanil, three articles (one level II-1 and two level III); for sulfadoxine, three articles (all level II-1); for pyrimethamine, three articles (all level II-1); for chloroquine and its metabolite, four articles (three level II-1 and one level II-3); for mefloquine, two articles (one level II-1 and one level III); and for azithromycin, two articles (one level II-1 and one level III). Although comparative trials were identified, most of these studies were descriptive and classified as level III evidence. The main findings showed that pharmacokinetic parameters are commonly altered in pregnancy for the majority of recommended agents. Importantly, first-line regimens of artemisinin-based compounds, lumefantrine, chloroquine and pyrimethamine/sulfadoxine may undergo significant changes that could decrease therapeutic efficacy. These changes are usually due to increases in the apparent oral clearance and volume of distribution that commonly occur in pregnant women, and may result in decreased exposure and increased therapeutic failure. In order to assess the clinical implications of these changes and to provide safe and effective dosage regimens, there is an immediate need for dose-optimization studies of all recommended first- and second-line agents used in pregnant women with malaria.


Similar content being viewed by others
References
World Health Organization. World malaria report 2010 [online]. Available from URL: http://www.who.int/malaria/world_malaria_report_2010/en/index.html [Accessed 2011 Aug 29]
World Health Organization. Guidelines for the treatment of malaria. 2nd ed [online]. Available from URL: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf [Accessed 2011 Aug 29]
Nosten F, McGready R, Mutabingwa T. Case management of malaria in pregnancy. Lancet Infect Dis 2007 Feb; 7(2): 118–25
Lobstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet 1997; 33(5): 328–43
Davis TM, Mueller I, Rogerson SJ. Prevention and treatment of malaria in pregnancy. Future Microbiol 2010 Oct; 5(10): 1599–613
Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf 2006 Jan; 1(1): 1–15
US Preventive Services Task Force. Guide to clinical preventive services: report of the US Preventive Services Task Force. 2nd ed. Baltimore (MD): Lippincott Williams & Wilkins, 1996
Muller AE, Mouton JW, Oostvogel PM, et al. Pharmacokinetics of clindamycin in pregnant women in the peripartum period. Antimicrob Agents Chemother 2010 May; 54(5): 2175–81
Abdelrahim II, Adam I, Elghazali G, et al. Pharmacokinetics of quinine and its metabolites in pregnant Sudanese women with uncomplicated Plasmodium falciparum malaria. J Clin Pharm Ther 2007 Feb; 32(1): 15–9
Mirghani R, Elagib I, Elghazali G, et al. Effects of Plasmodium falciparum infection on the pharmacokinetics of quinine and its metabolites in pregnant and non-pregnant Sudanese women. Eur J Clin Pharmacol 2010 Dec; 66(12): 1229–34
Phillips RE, Looareesuwan S, White NJ, et al. Quinine pharmacokinetics and toxicity in pregnant and lactating women with falciparum malaria. Br J Clin Pharmacol 1986 Jun; 21(6): 677–83
McGready R, Stepniewska K, Lindegardh N, et al. The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol 2006 Dec; 62(12): 1021–31
McGready R, Stepniewska K, Ward S, et al. Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol 2006 May; 62(5): 367–71
Tarning J, McGready R, Lindegardh N, et al. Population pharmacokinetics of lumefantrine in pregnant women treated with artemether-lumefantrine for uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother 2009 Sep; 53(9): 3837–46
McGready R, Stepniewska K, Edstein MD, et al. The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol 2003 Oct; 59(7): 545–52
Na-Bangchang K, Manyando C, Ruengweerayut R, et al. The pharmacokinetics and pharmacodynamics of atovaquone and proguanil for the treatment of uncomplicated falciparum malaria in third-trimester pregnant women. Eur J Clin Pharmacol 2005 Sep; 61(8): 573–82
Wangboonskul J, White NJ, Nosten F, et al. Single dose pharmacokinetics of proguanil and its metabolites in pregnancy. Eur J Clin Pharmacol 1993 Jan; 44(3): 247–51
Karunajeewa H, Salman S, Mueller I, et al. Pharmacokinetic properties of sulfadoxine-pyrimethamine in pregnant women. Antimicrob Agents Chemother 2009 Oct; 53(10): 4368–76
Green MD, Eijk AMV, Kuile FOV, et al. Pharmacokinetics of sulfadoxine-pyrimethamine in HIV-infected and uninfected pregnant women in Western Kenya. J Infect Dis 2007; 196: 1403–8
Nyunt MM, Adam I, Kayentao K, et al. Pharmacokinetics of sulfadoxine and pyrimethamine in intermittent preventive treatment of malaria in pregnancy. Clin Pharmacol Ther 2010 Feb; 87(2): 226–34
Karunajeewa H, Salman S, Mueller I, et al. Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy. Antimicrob Agents Chemother 2010 Mar; 54(3): 1186–92
Lee SJ, McGready R, Fernandez C, et al. Chloroquine pharmacokinetics in pregnant and nonpregnant women with vivax malaria. Eur J Clin Pharmacol 2008 Oct; 64(10): 987–92
Chukwuani MC, Bolaji OO, Onyeji CO, et al. Evidence for increased metabolism of chloroquine during the early third trimester of human pregnancy. Trop Med Int Health 2004 May; 9(5): 601–5
Massele A, Kilewo C, Aden Abdi Y, et al. Chloroquine blood concentrations and malaria prophylaxis in Tanzanian women during the second and third trimesters of pregnancy. Eur J Clin Pharmacol 1997; 52: 299–305
Na Bangchang K, Davis TM, Looareesuwan S, et al. Mefloquine pharmacokinetics in pregnant women with acute falciparum malaria. Trans R Soc Trop Med Hyg 1994; 88(3): 321–3
Nosten F, Karbwang J, White NJ, et al. Mefloquine antimalarial prophylaxis in pregnancy: dose finding and pharmacokinetic study. Br J Clin Pharmacol 1990 Jul; 30(1): 79–85
Salman S, Rogerson SJ, Kose K, et al. Pharmacokinetic properties of azithromycin in pregnancy. Antimicrob Agents Chemother 2010 Jan; 54(1): 360–6
Ramsey P. Maternal and transplacental pharmacokinetics of azithromycin. Am J Obstet Gynecol 2003 Mar; 188(3): 714–8
McGready R, Tan SO, Ashley E, et al. A randomised controlled trial of artemether-lumefantrine versus artesunate for uncomplicated plasmodium falciparum treatment in pregnancy. PLoS Med 2008 Dec; 5(12): e253
White N, van Vugt M, Ezzet F. Clinical pharmacokinetics and pharmacodynamics of artemether-lumefantrine. Clin Pharmacokinet 1999 Aug; 37(2): 105–25
Ward S, Sevene EJP, Hastings IM, et al. Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis 2007 Feb; 7(2): 136–44
McGready R, White NJ, Nosten F. Parasitological efficacy of antimalarials in the treatment and prevention of falciparum malaria in pregnancy 1998 to 2009: a systematic review. BJOG 2011 Jan; 118(2): 123–35
Newton P, Suputtamongkol Y, Teja-Isavadharm P, et al. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob Agents Chemother 2000 Apr; 44(4): 972–7
Fakeye TO, Fehintola FA, Ademowo OG, et al. Therapeutic monitoring of chloroquine in pregnant women with malaria. West Afr J Med 2002 Oct–Dec; 21(4): 286–7
Nosten F, Vincenti M, Simpson J, et al. The effects of mefloquine treatment in pregnancy. Clin Infect Dis 1999 Apr; 28(4): 808–15
McGready R, Stepniewska K, Seaton E, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol 2003 Oct; 59(7): 553–7
Parikh S, Rosenthal PJ. Intermittent preventive therapy for malaria in pregnancy: is sulfadoxine pyrimethamine the right drug? Clin Pharmacol Ther 2010 Feb; 87(2): 160–2
Tagbor HK, Chandramohan D, Greenwood B. The safety of amodiaquine use in pregnant women. Expert Opin Drug Saf 2007 Nov; 6(6): 631–5
Brabin BJ, Eggelte TA, Parise M. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf 2004; 27(9): 633–48
Acknowledgements
No sources of funding were used to prepare this review. The authors have no potential conflicts of interest to declare that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.2165/11598400-000000000-00000.
Rights and permissions
About this article
Cite this article
Wilby, K.J., Ensom, M.H.H. Pharmacokinetics of Antimalarials in Pregnancy. Clin Pharmacokinet 50, 705–723 (2011). https://doi.org/10.2165/11594550-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11594550-000000000-00000