Abstract
Purpose
We compared the pharmacokinetics of chloroquine in pregnant and nonpregnant women treated for Plasmodium vivax malaria.
Methods
Twelve pregnant women and 15 nonpregnant women of child-bearing age with acute P. vivax malaria were treated with 25 mg chloroquine base/kg over 3 days on the northwestern border of Thailand. Blood concentrations of chloroquine and desethylchloroquine were measured using hydrophilic interaction liquid chromatography coupled with fluorescence detection. Twenty-five women completed the pharmacokinetic study.
Results
Although increasing gestational age was associated with reduced chloroquine \({\text{AUC}}_{0 \to \infty } \), there was no significant difference overall in the pharmacokinetics of chloroquine between pregnant and nonpregnant women. Fever was associated with lower chloroquine \({\text{AUC}}_{0 \to \infty } \) values. Desethylchloroquine area under the curve (AUC) values were not significantly affected by pregnancy.
Conclusions
Pregnancy did not significantly affect blood concentrations of chloroquine or its metabolite, desethylchloroquine, in women with P. vivax malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroquine remains the prophylactic and treatment of choice for Plasmodium vivax, P. malariae, and P. ovale malarias . Pregnancy affects the pharmacokinetic (PK) properties of the majority of antimalarial drugs [1–7]. Optimal dosing is essential to prevent anemia and low birth weight [8, 9]. There are very few PK data on chloroquine (CQ) in pregnancy; two studies involving four and five women, respectively, gave conflicting results [10, 11]. We therefore assessed CQ PKs in pregnant women with acute vivax malaria in an area where resistance to CQ in P. vivax has not yet been documented.
Methods
This study was conducted in an area of low seasonal malaria transmission along the northwestern border of Thailand. In Maela refugee camp, the Shoklo Malaria Research Unit (SMRU) encouraged all pregnant women to attend weekly antenatal clinics (ANC) to detect and treat all parasitemic episodes.
Patients
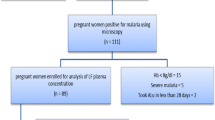
Pregnant women and nonpregnant women of child-bearing age with microscopically confirmed P. vivax infection, fever, or a history of fever, who provided informed consent, were invited to participate. Otherwise healthy pregnant women with a gestational age ≥ 20 weeks and ≤ 32 weeks and a hematocrit of > 25% were enrolled. A full medical history and examination was carried out by a physician and a midwife. Complete blood count, blood glucose, blood group, and parasite count were measured on admission.
Drug regimen
Twenty-five milligrams of CQ base/kg (Chloroquine®, ACDHON CO., Ltd, Bangkok, Thailand) was given over 3 days as 10, 10, and 5 mg/kg doses given strictly, under observation, at 0, 24, and 48 h.
Sampling
Women were admitted to the SMRU inpatient department. A heparinized cannula was inserted into an antecubital vein, and a blood sample (1 ml) was taken immediately before treatment and then at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 h. On days 2, 3, 7, 14, 21, 28, 35, and 42, 1-ml samples were obtained by direct venous sampling. Samples included baseline hematology and biochemistry and follow-up hematology on days 0, 3, 7, 14, 28, and 42.
Ethical review
The study was approved by the Ethical Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand; the Karen Refugee Committee in Mae Sod, Thailand; and the ethics committee of the London School of Hygiene and Tropical Medicine, London, UK.
Follow-up
Malaria smears and axillary temperature measurements were taken daily. After parasite clearance, women were seen weekly and followed up for 6 weeks. If P. vivax malaria recurred, the patient was retreated with CQ. All cases of P. falciparum malaria were treated with quinine or artesunate [2].
Drug assays
A liquid/liquid extraction method using N-hexane/isopropyl alcohol and sodium hydroxide (NaOH), previously validated in our lab, was applied to whole-blood samples (100 µl); 4-aminoquinaldine was used as internal standard, and CQ and desethylchloroquine (DEQ) were quantified using fluorescence detection (excitation 335 nm; emission 390 nm). Compound separation was performed on a silica stationary phase (Waters 3.9 × 390 nm) using a mobile phase composed of acetonitrile/methanol and triethylamine. Linearity, interday, and intraday variability of the method were validated. None of the coefficients of variation for CQ and DEQ was above 10% (data not shown). The lower limits of quantification (LLOQ) for CQ and DEQ were 15 ng/ml whole blood.
Pharmacokinetic analysis
PK parameters of CQ and its main metabolite, DEQ, were determined using noncompartmental analysis. Peak whole-blood concentrations and tmax were estimated from the first 24 h data. If levels were below the LLOQ, then the terminal phase was characterized by a log linear fit to the last five time points. A sensitivity analysis was conducted by assessing the area under the curve (AUC) in two other ways: by setting all concentration measurements that were not detected to 0 (i.e., assuming clearance of all CQ) and by comparing AUCs that were calculated from 0 to the maximum value of time available. Results were the same regardless of how the “not detected” measurements were managed. Therefore, only results using interpolated values are reported.
Statistical analyses
Chi-square or Fisher’s exact test was used for categorical data. Student’s t test and the Mann–Whitney U test were used for continuous data. Days 28 and 42 cure rates are presented as Kaplan–Meier estimates, and differences between groups were compared using the log rank test or the Wilcoxon-Breslow-Gehan test of equality if the survival lines crossed. Univariate analyses were conducted to check for potential covariates. Variables that had a significant effect on AUC were included in an analysis of variance (ANOVA) that tested for any differences between pregnant and nonpregnant women. All analyses were carried out using STATA, version 9.0 (StataCorp LP, TX, USA).
Results
From 2 March 1998 to 10 August 1998, 12 pregnant women and 15 nonpregnant Karen women of child-bearing age with acute vivax malaria consented to participate. Two nonpregnant women withdrew before completion of the study; their results were not analyzed. Another woman, initially screened as nonpregnant, had a positive pregnancy test on day 35 (i.e., she had been enrolled at 2.4 weeks after her last menstrual period). Her results were analysed in the nonpregnant group as originally assigned. Two women gave birth before 42 days (days 23 and 28). No postpartum samples were analyzed. On admission, there were no significant differences in demographic characteristics between pregnant and nonpregnant women (Table 1).
Pregnant women had lower mean hematocrit, lymphocyte count, and biochemical parameters (i.e., creatinine, blood urea nitrogen (BUN), and albumin) than did nonpregnant women (Table 1), consistent with the physiological changes of pregnancy. The higher bilirubin levels in nonpregnant women may indicate they had more serious malaria infections [12, 13]. Chloroquine was well tolerated and rapidly effective. The cure rate at day 28 (n = 25) was 100% (95% confidence interval (CI) 86.7–100). The day 42 cure rate was comparable in pregnant [83.3% (95% CI 48.2–95.6)], and nonpregnant [69.2% (95% CI 37.3–87.2)] women (p = 0.52).
Pharmacokinetics of chloroquine
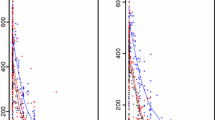
Eight women had late measurements that were below the LLOQ (one pregnant, two values; seven nonpregnant, 12 values). Therefore, these values were extrapolated from the log linear fit to the available concentration measurements from the last five time points. In the univariate and multivariate analyses, for \({\text{AUC}}_{0 \to 3} \), there was no difference between pregnant and nonpregnant women (p = 0.32) (Fig. 1, Table 2). There was no effect of hematocrit (p = 0.11), BMI (p = 0.60), or parasitemia (p = 0.27) on \({\text{AUC}}_{0 \to 3} \). Fever and higher temperatures were associated with lower \({\text{AUC}}_{0 \to 3} \) values (p = 0.02 and p = 0.001, respectively). Among pregnant women, there was no effect of smoking (p = 0.15) or estimated gestational age on \({\text{AUC}}_{0 \to 3} \) (Spearman’s rho = −0.29, p = 0.37). There was no difference in \({\text{AUC}}_{0 \to \infty } \) between pregnant (n = 11) and nonpregnant (n = 11) women (p = 0.99) (Fig. 1); on average, the difference in (log) CQ \({\text{AUC}}_{0 \to \infty } \) between pregnant and nonpregnant women was only 0.2% (95% CI −0.28 to 40). A lower AUC0→∞ was associated with higher temperature (p = 0.006) in the univariate analysis but not with hematocrit (p = 0.91), BMI (p = 0.36), or parasitemia (p = 0.21). Adjusting the model for hematocrit, platelet count, bilirubin, and temperature made no difference to \({\text{AUC}}_{0 \to \infty } \) between the groups (p = 0.26). For pregnant women (n = 11), there was no effect of smoking (p = 1.0) on \({\text{AUC}}_{0 \to \infty } \) , but a higher gestational age at entry to the study was associated with a lower \({\text{AUC}}_{0 \to \infty } \) (Spearman’s rho = −0.67 p = 0.02).
Pharmacokinetics of desethylchloroquine
Two women (both pregnant) had no DEQ measurements, which left ten pregnant and 13 nonpregnant women for comparison. Cmax and tmax did not differ between pregnant and nonpregnant women (p = 0.29 and p = 0.33, respectively). The ratio of CQ to DEQ \({\text{AUC}}_{0 \to 3} \) was also not different [median 4.89 (range 3.21–6.28) and 4.39 (2.34–8.77), respectively; p = 0.66]. In the univariate analysis, the CQ to DEQ AUC0→3 ratio was significantly correlated with parasitemia on admission (p = 0.003) but not with temperature (p = 0.22), fever (p = 0.14), or BMI (p = 0.50). Inclusion of parasitemia in the model made no difference in the CQ to DEQ \({\text{AUC}}_{0 \to 3} \) ratio between pregnant and nonpregnant women (p = 0.70).
DEQ levels were reliably detected for 14 days. There was no difference in median \({\text{AUC}}_{0 \to 14} \) or the median ratio of CQ to DEQ \({\text{AUC}}_{0 \to 14} \) between pregnant and nonpregnant women [for median \({\text{AUC}}_{0 \to 14} \): 29,841 (range 17,533–46,849) vs. 29,556 (10,145–64,102), p = 0.65 and 3.78 (2.42–5.14) vs. 3.50 (2.47–5.48), p = 0.41 for median CQ:DEQ ratio]. The CQ:DEQ \({\text{AUC}}_{0 \to 14} \) ratio was not associated with temperature (p = 0.72), parasitemia (p = 0.56), fever (p = 0.42), or BMI (p = 0.86) at admission. In pregnant women (n = 10), the CQ:DEQ \({\text{AUC}}_{0 \to 14} \) ratio did not correlate significantly with smoking (p = 0.09) or estimated gestational age (p = 0.49) and did not correlate with the CQ \({\text{AUC}}_{0 \to 14} \) (Spearman’s rho = 0.08, p = 0.75).
Discussion
This study found no significant differences in the pharmacokinetic parameters of CQ and its main metabolite DEQ in pregnant women and nonpregnant women treated for acute vivax malaria. Lower CQ \({\text{AUC}}_{0 \to \infty } \) and \({\text{AUC}}_{0 \to 3} \) values were associated with fever on admission, and a lower \({\text{AUC}}_{0 \to \infty } \) was significantly associated with greater estimated gestational age. There was also no difference in the DEQ \({\text{AUC}}_{0 \to 3} \), \({\text{AUC}}_{0 \to 14} \) , or CQ:DEQ \({\text{AUC}}_{0 \to 14} \) ratio between pregnant and nonpregnant women, although there was wide interindividual variation in the concentration measurements between women in both groups.
Previous studies of antimalarial drug pharmacokinetics have reported lower plasma concentrations in pregnant women for mefloquine, sulfadoxine, dihydroartemisinin, artemether, lumefantrine, atovaquone, proguanil, and cycloguanil [1—5]. CQ appears different in that its pharmacokinetic properties are not significantly affected by pregnancy, although there was evidence that AUC values decreased as gestation increased, compatible with a pregnancy-related expansion in the apparent volume of distribution (Vd). CQ has unusual pharmacokinetic properties. It has very long terminal half-life and an enormous Vd [14, 10, 11]. Previous pharmacokinetic studies in pregnancy measured plasma concentrations, which has limitations, as CQ is concentrated in platelets and granulocytes and then variably released from these cells in blood samples [15]. On the other hand, because large within-group variations can result in an apparent small between-groups variation, the wide interindividual variation in CQ concentrations can make it more difficult to detect moderate to small differences between groups.
A previous study reported higher plasma DEQ concentrations in pregnant women compared with nonpregnant Nigerian women [11]. Our study found no significant differences in DEQ for median peak whole-blood concentration, time-to-peak concentration, \({\text{AUC}}_{0 \to 3} \), or the CQ:DEQ \({\text{AUC}}_{0 \to 14} \) ratio among pregnant and nonpregnant Karen women. The CQ:DEQ \({\text{AUC}}_{0 \to 14} \) was also not correlated with CQ \({\text{AUC}}_{0 \to 14} \) values, which suggests that metabolic biotransformation was not a major determinant of CQ clearance.
In conclusion, in this study of Karen women with P. vivax malaria, pregnancy did not significantly affect blood concentrations of CQ or its metabolite, DEQ, when compared with nonpregnant women. Weight-based standard doses are appropriate for treating malaria in pregnancy.
References
McGready R, Stepniewska K, Lindegardh N, Ashley EA, La Y, Singhasivanon P, White NJ, Nosten F (2006) The pharmacokinetics of artemether and lumefantrine in pregnant women with uncomplicated falciparum malaria. Eur J Clin Pharmacol 62(12):1021–1031
Ward SA, Sevene EJ, Hastings IM, Nosten F, McGready R (2007) Antimalarial drugs and pregnancy: safety, pharmacokinetics, and pharmacovigilance. Lancet Infect Dis 7(2):136–144
McGready R, Stepniewska K, Edstein MD, Cho T, Gilveray G, Looareesuwan S, White NJ, Nosten F (2003) The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol 59(7):545–552
McGready R, Stepniewska K, Seaton E, Cho T, Cho D, Ginsberg A, Edstein MD, Ashley E, Looareesuwan S, White NJ, Nosten F (2003) Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol 59(7):553–557
McGready R, Stepniewska K, Ward SA, Cho T, Gilveray G, Looareesuwan S, White NJ, Nosten F (2006) Pharmacokinetics of dihydroartemisinin following oral artesunate treatment of pregnant women with acute uncomplicated falciparum malaria. Eur J Clin Pharmacol 62(5):367–371
Na-Bangchang K, Manyando C, Ruengweerayut R, Kioy D, Mulenga M, Miller GB, Konsil J (2005) The pharmacokinetics and pharmacodynamics of atovaquone and proguanil for the treatment of uncomplicated falciparum malaria in third-trimester pregnant women. Eur J Clin Pharmacol 61(8):573–582
Nosten F, Karbwang J, White NJ, Honeymoon, Na Bangchang K, Bunnag D, Harinasuta T (1990) Mefloquine antimalarial prophylaxis in pregnancy: dose finding and pharmacokinetic study. Br J Clin Pharmacol 30(1):79–85
Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, Hkirijaroen L, Looareesuwan S, White NJ (1999) Effects of Plasmodium vivax malaria in pregnancy. Lancet 354(9178):546–549
Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ (1991) Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg 85(4):424–429
Fakeye TO, Fehintola FA, Ademowo OG, Walker O (2002) Therapeutic monitoring of chloroquine in pregnant women with malaria. West Afr J Med 21(4):286–287
Chukwuani MC, Bolaji OO, Onyeji CO, Makinde ON, Ogunbona FA (2004) Evidence for increased metabolism of chloroquine during the early third trimester of human pregnancy. Trop Med Int Health 9(5):601–605
Selvam R, Mathews ST (1992) Biochemical alterations in Plasmodium vivax-infected malarial patients before and after radical treatment. Indian J Malariol 29(2):103–111
Tangpukdee N, Thanachartwet V, Krudsood S, Luplertlop N, Pornpininworakij K, Chalermrut K, Phokham S, Kano S, Looareesuwan S, Wilairatana P (2006) Minor liver profile dysfunctions in Plasmodium vivax, P. malaria and P. ovale patients and normalization after treatment. Korean J Parasitol 44(4):295–302
Krishna S, White NJ (1996) Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clin Pharmacokinet 30(4):263–299
Bergqvist Y, Domeij-Nyberg B (1983) Distribution of chloroquine and its metabolite desethyl-chloroquine in human blood cells and its implication for the quantitative determination of these compounds in serum and plasma. J Chromatogr 272(1):137—148
Acknowledgements
We express our sincere thanks to the pregnant and nonpregnant women, doctors, midwives, medics, nurses, lab technicians, home visitors, drivers, and logistical and administrative teams of SMRU and MORU who made this detailed work possible, and special thanks to the study monitor Michele van Vugt. Thanks also to Niklas Lindegardh and Joel Tarning, who provided many insightful comments and suggestions during the preparation of the manuscript. This study was part of the Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme, funded by the Wellcome Trust of Great Britain.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lee, S.J., McGready, R., Fernandez, C. et al. Chloroquine pharmacokinetics in pregnant and nonpregnant women with vivax malaria. Eur J Clin Pharmacol 64, 987–992 (2008). https://doi.org/10.1007/s00228-008-0500-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-008-0500-z