Abstract
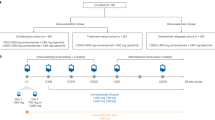
Joining cetuximab, sorafenib, afatinib, intedanib, and crizotinib in phase III development for non-small cell lung cancer (NSCLC) are ramucirumab (developed by ImClone, a subsidiary of Lilly), necitumumab (developed by ImClone and Bristol-Myers Squibb), and tivantinib (ARQ 197, developed by ArQule and Daiichi Sankyo).
Necitumumab is a second-generation anti-EGFR monoclonal antibody (mAb) similar to cetuximab. Enrollment has been stopped in one of two necitumumab phase III trials because of safety concerns.
Ramucirumab is an anti-VEGFR2 mAb targeting the same pathway as bevacizumab. Although the phase II safety data for ramucirumab appear better than the data for necitumumab, fewer phase III data are available.
Tivantinib is a highly selective, orally available MET tyrosine kinase inhibitor. MET is overexpressed in 61% of NSCLC cases. Although tivantinib is the last of the three agents discussed here to enter phase III, its phase II results are the most robust.
Similar content being viewed by others
Notes
The inThought Approvability Index (IAI) is a dynamic tool that assesses the progress of a drug candidate through clinical development, evaluating the strength of clinical data and trial design, benchmarked against historical parameters and the likelihood of maintaining forward momentum. Points are assigned for specific line items relating to safety, efficacy, and other factors in each phase of clinical development. Possible points total 100 upon drug approval and are allocated in each phase according to the historical approval rate of similar drugs, such that the current points of a drug relate to its probability of approval. In addition, a letter grade is assigned and reflects the momentum of a drug candidate in its current phase, with ‘A’ indicating significantly above average/likely to progress, ‘C’ indicating average, and ‘F’ indicating significantly below average/unlikely to progress.
References
Yee D. Erbitux and figitumumab for NSCLC: a tale of two monoclonal antibodies. inThought Research, 2010 Aug 5
Yee D. Poor track record for kinase inhibitors in NSCLC: focus on Nexavar and Sutent. inThought Research, 2010 Aug 17
Yee D. New kinase inhibitors target NSCLC: Tovok, Vargatef, and Crizotinib. inThought Research, 2010 Nov 4
ImClone LLC. First-line treatment of patients with stage IV nonsquamous non-small cell lung cancer with necitumumab (IMC-11F8) and pemetrexed-cisplatin (INSPIRE) [ClinicalTrials.gov identifier NCT00982111]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00982111 [Accessed 2011 Jun 7]
ImClone LLC. First-line treatment of participants with stage IV squamous non-small cell lung cancer with necitumumab and gemcitabine-cisplatin (SQUIRE) [ClinicalTrials.gov identifier NCT00981058]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/show/NCT00981058 [Accessed 2011 Jun 7]
Bristol-Myers Squibb. Lilly, Bristol-Myers Squibb stop enrollment in one of two phase III lung cancer trials of necitumumab [media release]. 2011 Feb 2 [online]. Available from URL: http://www.bms.com/news/press_releases/pages/default.aspx [Accessed 2011 Jun 7]
Yee D. Receptor kinase inhibitors target NSCLC: two antibodies and a small-molecule c-Met inhibitor. inThought Research, 2011 Feb 25
Acknowledgments and Disclosures
The original inThought™ Research Report[7] this article is adapted from is available on request from biodrugs@adis.co.nz. Wolters Kluwer inThought™ Research Reports provide unbiased analysis and ideas based on the needs and direction of clients. Analyses include a proprietary methodology for quantifying the probability of FDA approval as well as a forecasting revenue methodology for both approved and developmental drugs and medical devices. The material herein, while not guaranteed, is based on information believed to be reliable and accurate. inThought™ do not (a) give investment advice; or (b) advocate the sale or purchase of any security or investment. The material herein is not to be deemed an offer or solicitation on our part with respect to the sale or purchase of any securities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Adapted and reproduced from Yee D. Receptor Kinase Inhibitors Target NSCLC: Two Antibodies and a Small-Molecule c-Met Inhibitor. inThought Research, 2011 Feb 25
Rights and permissions
About this article
Cite this article
Yee, D. Receptor Kinase Inhibitors Target NSCLC. BioDrugs 25, 271–273 (2011). https://doi.org/10.2165/11592720-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11592720-000000000-00000