Abstract
Background: In Japan, when pharmaceutical companies launch a new drug, they are obligated to conduct a post-marketing survey to evaluate the safety and efficacy of the drug in accordance with Good Post-Marketing Surveillance Practice under Article 14-4 (re-examination) of the Pharmaceutical Affairs Law at contracted medical institutions. We report the results of a long-term special survey that we conducted as a post-marketing survey.
Objective: The results of a prospective post-marketing survey that was conducted to assess the safety and efficacy of the b-adrenergic receptor antagonist (β-blocker) Artist® tablets 10 mg, 20mg (carvedilol) in patients with hypertension in Japan, were investigated in order to examine the safety and efficacy of the drug during long-term treatment (18 months).
Patients: Patients were carvedilol-naive and had essential hypertension or renal parenchymal hypertension.
Methods: We performed this survey as a prospective cohort study (special survey) utilizing a centralized registration method over 3 years (starting from April 1994), for an observation period of 18 months of carvedilol treatment.
Results: Sixty-one medical institutions across Japan collected 380 case report forms of patients who received long-term administration of carvedilol, with 363 and 341 cases evaluated for safety and efficacy, respectively. The discontinuation rate was 7.2% and the incidence of adverse drug reactions was 5.23% (19 of 363) in the safety population. There was no significant change in fasting plasma glucose levels from baseline (118.1 ± 46.5mg/dL) to after carvedilol treatment (114.6 ± 43.3 mg/dL) [n = 141; p = 0.310].
In 341 evaluable patients in the efficacy population, decreases in both blood pressure and pulse rate were statistically significant at all assessment points in comparison with baseline data (p < 0.001). Similarly, in hypertensive patients with diabetes mellitus, decreases in blood pressure were statistically significant at all assessment points in comparison with baseline data (p < 0.001).
Conclusions: The results of this study show that carvedilol exerted stable antihypertensive effects leading to favorable blood pressure control throughout long-term treatment, without showing any safety concerns. It was concluded that there were no clinically significant issues in terms of safety or efficacy with the long-term treatment of carvedilol in patients with hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The true goal of hypertension treatment is not simply to decrease blood pressure (BP) but to prevent/improve future development of organ dysfunction, such as stroke and myocardial infarction. β-Adrenergic receptor antagonists (β-blockers) are generally recommended for hypertensive patients with myocardial infarction or ischemic heart disease owing to their pharmacologic actions, such as a decrease in sympathetic nervous system activity and cardioprotective effects. At the same time, β-blockers can be associated with risks, including excessive bradycardia, adverse effects on glucose/lipid metabolism, and occurrence of vasoconstriction. For this reason, in Japan, the number of prescriptions for β-blockers has been decreasing in daily medical practice, although these drugs are aggressively indicated for the treatment of hypertension with angina pectoris, post-myocardial infarction, tachycardia, and/or heart failure. Currently, the most frequently used first-choice medications for hypertension are calcium channel blockers (CCBs) and angiotensin II receptor blockers (ARBs) in daily clinical practice in Japan.
Artist® tablets (carvedilol) were developed with the expectation of an improved adverse drug reaction (ADR) profile. This drug is a nonselective β-blocking agent with vasodilatory (mainly based on an α1-adrenergic receptor antagonist action) and antioxidant effects. Overseas reports have shown that carvedilol does not negatively affect pulse rate or glucose/lipid metabolism.[1,2] In addition, in hypertensive patients with peripheral circulatory disturbance, for whom other β-blockers are contraindicated,[3] carvedilol has been demonstrated to improve coldness of limbs,[4] mainly owing to its reducing effect on peripheral vascular resistance due to the blockade of α1-receptors. With the highest trough : peak ratio (83%) among β-blockers,[5] carvedilol has a long-lasting (24-hour) antihypertensive action, allowing once-daily administration.
Carvedilol was approved and launched in Japan for the following indications in January 1993: the treatment of ‘essential hypertension (mild to moderate), renal parenchymal hypertension, and angina pectoris’. Subsequently, with notable pharmacologic properties different from those of selective β-blockers, carvedilol became the only β-blocker approved for the indication of ‘chronic heart failure’ in Japan in October 2002 based on large-scale international[6,7] and domestic[8] clinical trial data, although many β-blockers had been commercially available before and after the first approval of carvedilol.
Carvedilol has been widely used for patients with hypertension or chronic heart failure, mainly in Internal Medicine and Cardiovascular departments. In Japan, however, there are limited data on the safety and efficacy of the drug for the treatment of hypertensive patients; most of the available data are from clinical trials that had been performed prior to its approval. Furthermore, with the exception of one report from a long-term (≥12 months) clinical trial in 95 subjects[9] that was published in 1990, there are no long-term data on the safety or efficacy of the drug in patients with different background characteristics in daily clinical practice.
We report data from a re-analysis of 380 hypertensive patients included in a long-term special survey of Artist® tablets (carvedilol) for hypertensive patients, which was performed between April 1994 and March 1997, in order to encourage the proper use of the drug based on its long-term safety and efficacy. This article also addresses the effect of the drug on glucose metabolism, which can be a concern in patients using β-blockers, by investigating changes in fasting blood glucose, as well as the safety and efficacy of the long-term use of the drug in patients with complications of diabetes mellitus.
Methods
Patients
This survey was conducted in accordance with Good Post-Marketing Surveillance Practice[10] under Article 14-4 (re-examination) of the Pharmaceutical Affairs Law, following a centralized registration method. Patients were carvedilol-naive and had essential hypertension or renal parenchymal hypertension. Among the medical institutions nationwide where the drug was being prescribed, some were selected, mainly their cardiovascular departments, and asked in writing to conduct the survey. After completing a written contract, the physicians in charge filled out a registration form for each patient who started carvedilol treatment and sent it to the registration center via fax. The survey period was from April 1994 to the end of March 1997.
The expected number of case report forms to be collected was 400, which was based on the following reasons gathered during planning of this survey: (i) guidelines for clinical evaluation methods of antihypertensive drugs[11] recommend “at least 300 patients with hypertension whose BP can be controlled well and for whom long-term administration is possible”; (ii) statistical review should involve data from at least 100 eligible patients; and (iii) the dropout rate in a survey with an observation period of 1 year or longer is estimated to be around 30% according to existing academic articles[12] and physicians’ opinions.
Dosage and Administration
Carvedilol was administered at the discretion of physicians participating in the survey and according to the dosage and administration specified in its package insert;[13] “the usual adult dosage for this product for oral use is 10 20 mg of carvedilol once daily, the dosage may be adjusted according to the patients’ ages and symptoms.” Neither prior treatment nor combination drugs were restricted. The standard observation period was 18 months from the start of carvedilol treatment. If patients dropped out from, or discontinued the survey, they were followed up until that time.
Assessments
Patient Demographics
Data collected included patient initials, medical record number, date of birth, sex, pregnancy status (only for women), in- or outpatient status, diagnosis (target disease), duration of disease, presence/absence or names of complications, previous medical history, history of allergy, use of antihypertensive medications before the initiation of carvedilol, and concomitant use of other antihypertensive medications.
Carvedilol Dosage and Administration
The duration of carvedilol treatment, daily dose, reason for dropout/discontinuation of treatment, and compliance status throughout carvedilol treatment were recorded.
Clinical and Laboratory Analyses
Data on systolic blood pressure (SBP)/diastolic blood pressure (DBP), and pulse rate were collected. BP was categorized according to the following three criteria: (i) well controlled; (ii) mostly well controlled; and (iii) poorly controlled. BP control was evaluated by the patients’ physicians at three timepoints: 6, 12, and 18 months after the start of carvedilol treatment.
The laboratory analyses included hematology, blood chemistry, and urinalysis.
Adverse Drug Reactions
The presence/absence of ADR onset after the start of carvedilol treatment, name of the ADR, date of onset, seriousness, progress, medication taken for the ADR, outcome, date of outcome, drug-event relationship, and suspected concomitant medication were recorded for all ADRs.
Diseases that Developed During the Course of Carvedilol Treatment
The name and progression of diseases that developed during the course of carvedilol treatment were recorded.
Data Handling and Collection/Analytical Procedures
The safety population comprised patients who completed a case report form. Excluded were those who (i) received carvedilol during periods other than the survey period; (ii) started the drug before a contract was completed; (iii) provided two case report forms; and (iv) did not revisit the medical institution after the initial visit.
The efficacy population comprised patients from the safety population and excluded patients (i) with unknown target disease; and (ii) who were not evaluated for BP control at all.
The incidence rate of ADRs (the proportion of patients with ADRs) in the safety population was analyzed using Fisher’s direct probability method. Changes in SBP/DBP, pulse rate, and fasting blood glucose from baseline (the start of carvedilol treatment) were analyzed at the last observation time (or at dropout/discontinuation of treatment) using the paired t-test, and in months 6, 12, and 18 according to Dunnett’s multiple comparison test. All statistical tests were performed using a two-sided 5% significance level. The figures are presented as mean ± standard deviation (SD). Patients with unknown or missing data for each background factor were excluded from the analyses.
The observed ADRs were classified using preferred terms according to the 1996 version of the Japanese Adverse Drug Reaction Terminology.[14]
Results
Patient Number and Disposition
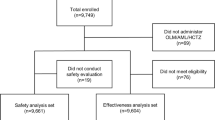
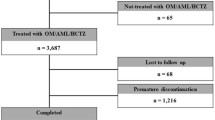
The disposition of patients in the survey is shown in figure 1. A total of 401 patients were registered. Case report forms were collected from 380 patients at 61 medical institutions. Among the 380 patients, ten received carvedilol during periods other than the survey period, two started the drug before a contract was completed, one provided two case report forms, and four failed to revisit the medical institution after the initial visit and were not evaluated for the presence/absence of ADRs; these 17 patients were excluded from the safety analyses and the remaining 363 patients were included in the safety population. In the safety population, one patient had unknown target disease and 21 were not evaluated for efficacy (BP control); these 22 patients were excluded from the efficacy analyses, and the remaining 341 patients were included in the efficacy population.
Of 363 patients in the safety population, 224 (61.7%) continued carvedilol treatment for 18 months or longer, while 139 (38.3%) dropped out or discontinued treatment during the course of the survey. Table I shows patient disposition and the reasons why patients dropped out or discontinued carvedilol treatment. A total of 26 patients (7.2%) discontinued carvedilol treatment for the following reasons: occurrence of ADRs (n = 7); inefficacy (n = 12); and an excessive decrease in BP (n = 7). Among the patients who dropped out from the survey, 86 did not revisit the medical institution for reasons such as 27 had changed hospitals, 14 were too busy, 12 had improvement of subjective symptoms, and eight had moved to a new place. The remaining 27 patients dropped out or discontinued treatment due to other reasons such as the patient’s own judgment/will (n = 6), onset of a new concomitant disease (n = 5), priority in treatment of other complications (n = 4), or introduction of the patient to a new physician (n = 3).
Patient Demographics
The demographic characteristics of the safety population are presented in table II. Mean age at the start of carvedilol treatment was 59.6 ± 12.4 years (range = 13–88 years; median = 61 years). The safety population included 141 elderly patients aged ≥65 years (38.8%), and had almost equal numbers of men and women. A total of 265 patients (73.0%) had existing complications when they commenced carvedilol treatment: 73 (20.1%) had hepatic disease; 72 (19.8%) had heart disease; 58 (16.0%) had diabetes; and 19 (5.2%) had renal disease; some patients had two or more complications. Patients who were receiving other antihypertensive medications (n = 213) accounted for more than half of the safety population (58.7%).
The mean final dosage of carvedilol was 16.2 ± 5.4 mg/day.
Safety
Adverse Drug Reactions
The overall incidence rate of ADRs in the safety population was 5.23% (19 of 363 patients). Table III provides a list of ADRs reported in the safety population. Nineteen patients had 25 ADRs, none of which were considered serious. The most common ADRs (reported in ≥2 patients) were three events each of dizziness, bradycardia, elevated serum cholesterol and increased triglycerides (0.83%, 3 of 363 patients), and two events of increased creatine phosphokinase (0.55%, 2 of 363 patients). None of the 17 patients who were excluded from the safety population reported any ADRs.
Regarding the timing of onset of ADRs following treatment initiation, ten patients had 12 ADRs within 6 months, six had eight ADRs between 6 and 12 months, three had three ADRs between 12 and 18 months, and two had two ADRs after 18 months. None of the patients reported specific ADRs related to the long-term use of carvedilol at any time during the course of treatment that differed from those observed in the clinical trials[15–17] that had been conducted prior to carvedilol approval, or in a drug use result survey of the drug.[18]
Concerning ADRs related to glucose metabolism, one patient had an increased plasma glucose level on day 281 (9.4 months after start of treatment). This event was judged as mild and the patient recovered from the event without any action taken while continuing carvedilol treatment.
During the course of carvedilol treatment, 39 patients experienced 53 new events considered to be concomitant diseases, which were judged not to be related to the drug by their physicians. These included three events of cerebral infarction, and two each of angina pectoris, acute gastroenteritis, and hypercholesterolemia. Thus, development of new concomitant diseases was not considered frequent.
Table IV presents ADRs stratified by selected demographic factors. No factor had a significant association with the incidence of ADRs. The incidence of ADRs in patients with diabetes complications was 6.9% (4 of 58 patients) compared with 5.0% (15 of 300) in those without diabetes complications (p = 0.526).
Changes in Fasting Blood Glucose
This survey was for use of carvedilol in daily clinical practice and did not always require laboratory tests. However, because many β-blockers are likely to adversely affect glucose metabolism, a change from baseline to the last observation time (or dropout/discontinuation of treatment) was examined for fasting blood glucose, an indicator of glucose metabolism alterations. Figure 2 illustrates the change in fasting blood glucose levels in patients with both baseline and last observation values among the 363 patients in the safety population. Mean fasting blood glucose was 118.1 ± 46.5 mg/dL at baseline and 114.6 ± 43.3 mg/dL at the last observation, giving a change in value of -3.5 mg/dL (p = 0.310; not significant). Figure 3 shows changes in fasting blood glucose levels from baseline at each observation timepoint (months 6, 12, and 18). Mean fasting blood glucose was 117.7 ± 44.8 mg/dL at baseline, 117.1 ± 37.9 mg/dL at month 6, 112.4 ± 34.6 mg/dL at month 12 (p < 0.05 vs baseline), and 111.8 ± 39.0 mg/dL at month 18. Thus, no increase in fasting blood glucose was seen throughout the 18-month observation period.
Efficacy
Blood Pressure Control
Assessment of BP control made by the physicians at each timepoint is shown in table V. Of the 341 patients in the efficacy population, 69.8% (n = 238) continued carvedilol treatment for 12 months or longer and were evaluable for BP control. At the month 18 assessment, BP was ‘well controlled’ or ‘mostly well controlled’ for 95.8% of the evaluable patients (228 of 238). BP was judged ‘well controlled’ in 71.4% (170 of 238) and ‘mostly well controlled’ in 24.4% (58 of 238) of evaluable patients.
Changes in Blood Pressure and Pulse Rate in All Patients
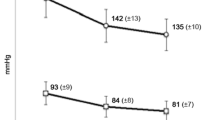
Table VI shows changes in BP and pulse rate from baseline at the last observation time (or at dropout/discontinuation of treatment) for patients among the 341 in the efficacy population who underwent BP or pulse rate measurements. The respective mean values of SBP and DBP were 166.7 ± 17.4 mmHg and 93.6 ± 11.4 mmHg at baseline, and 142.1 ± 17.4 mmHg and 81.5 ± 12.0 mmHg at the last observation; the change in value (the last observation value subtracted by the baseline value) was −24.6 mmHg for SBP and −12.1 mmHg for DBP. Hence, a significant reduction was observed for SBP and DBP (p < 0.001 for both). Mean pulse rate was 74.5 ± 11.0 beats per minute (beats/min) at baseline and 68.7 ± 9.4 beats/min at the last observation, giving a change in value of −5.8 beats/min, indicating a significant decrease in pulse rate (p < 0.001).
Figure 4 illustrates changes in BP and pulse rate from baseline at each observation timepoint during the course of the 18-month treatment. The respective SBP and DBP values were 166.7 ± 17.4 mmHg and 93.6 ± 11.4 mmHg at baseline, which were reduced to 141.9 ± 17.8 mmHg and 81.5 ± 11.2 mmHg in month 6, to 138.6 ± 16.0 mmHg and 79.9 ± 11.7 mmHg in month 12, and to 140.2 ± 14.2 mmHg and 79.7 ± 10.5 mmHg in month 18, suggesting a stable decrease in BP throughout the 18-month treatment period (p < 0.001 for all timepoints). Pulse rate was also reduced throughout the treatment period, as demonstrated by changes from 74.5 ± 11.0 beats/min at baseline to 69.5 ± 8.5 beats/min in month 6, to 68.8 ± 8.6 beats/min in month 12, and to 68.1 ± 8.9 beats/min in month 18 (p < 0.001 for all timepoints).
Changes in Blood Pressure and Pulse Rate in Patients with Diabetes Mellitus Complications
Regarding the 58 patients with diabetes complications among the 341 in the efficacy population for whom measurements were taken, table VII shows changes in BP and pulse rate from baseline to the last observation time (or at dropout/discontinuation of treatment). Mean SBP and DBP were 163.5 ± 17.0 mmHg and 90.4 ± 11.5 mmHg at baseline, and 142.1 ± 20.1 mmHg and 77.7 ± 12.3 mmHg at the last observation; the change in values (the last observation value subtracted by the baseline value) for SBP and DBP were -21.4 mmHg and -12.7 mmHg, respectively. Thus, in this subgroup of patients, there was also a significant decrease in SBP and DBP (p < 0.001 for both). Mean pulse rate was 74.7 ± 9.1 beats/min at baseline and 70.4 ± 10.9 beats/min at the last observation, and the change in value was −4.2 beats/min, indicating a significant decrease in pulse rate (p = 0.031).
Figure 5 shows changes in BP and pulse rate from baseline at each observation time point in patients with diabetes complications. There was also a stable reduction in BP throughout the 18-month treatment period in this subgroup of patients, as demonstrated by the changes in SBP and DBP from 163.5 ± 17.0 mmHg and 90.4 ± 11.5 mmHg at baseline to 142.2 ± 19.5 mmHg and 80.5 ± 11.1 mmHg in month 6, 138.8 ± 18.8 mmHg and 79.6 ± 13.2 mmHg in month 12, and 137.4 ± 12.4 mmHg and 76.1 ± 8.8 mmHg in month 18 (p < 0.001 for all timepoints), respectively. Mean pulse rate was 74.6 ± 9.0 beats/min at baseline, 68.4 ± 7.7 beats/min in month 6, 70.3 ± 11.4 beats/min in month 12, and 69.3 ± 8.1 beats/min in month 18. Overall, pulse rate was also significantly reduced from baseline in this subgroup (p < 0.001 for month 6; p < 0.05 for month 18), although it was transiently increased at month 12. In terms of the change in values for BP and pulse rate between baseline and the last observation, as well as changes in BP and pulse rate during the 18-month treatment period, the 58 patients with diabetes mellitus complications had similar results to those seen in the 341 patients in the efficacy population.
Changes (arithmetic mean ± SD) in (a) blood pressure (BP) and (b) pulse rate from baseline at each observation timepoint during the 18-month treatment period in patients with diabetes mellitus complications in the efficacy population. DBP = diastolic BP; SBP = systolic BP; * p < 0.05, *** p < 0.001 (Dunnett’s multiple comparison).
Achievement Rate of Target Blood Pressure
Figure 6 illustrates the achievement rate of target BP (<140/90 mmHg) at months 6, 12, and 18 in the efficacy population. The achievement rate for target BP was 2.6% at baseline, 41.0% in month 6, 48.2% in month 12, and 44.3% in month 18, with an achievement rate of ≥40% from month 6 onwards from the start of carvedilol treatment.
Discussion
The primary goal in the treatment of hypertension is to maintain target BP for as long as possible to prevent cardiovascular events and death. For optimal evaluation of antihypertensive medications, it is important to examine long-term effectiveness, while investigating long-term tolerability and long-term adherence. Regarding data on the long-term use of carvedilol, a 12-month clinical trial[9] had been conducted to confirm the safety and efficacy of the drug in 95 subjects prior to the approval of the drug. The clinical trial included patients with selected background characteristics who had been eligible for follow-up in a long-term phase; these patients received carvedilol under strict control by their physicians.
In terms of estimating the safety and efficacy of long-term use of carvedilol in daily medical practice, there were several limitations in using these data. For example, the findings would not allow the estimation of BP control status or adherence to the drug during actual treatment, as well as results regarding longer-term use, or use in patients with different concomitant diseases, such as underlying diseases or diabetes complications. Therefore, we conducted this special survey during a period from April 1994 to March 1997 to investigate the safety and efficacy of long-term use of carvedilol.
In this survey, a total of 380 case report forms were collected from patients with different background characteristics who were being treated in daily medical practice at 61 medical institutions (mainly in the Internal Medicine and Cardiovascular departments).
Among the 363 patients in the safety population, 139 (38.3%) dropped out from or discontinued carvedilol treatment, including 26 (7.2%) because of the occurrence of ADRs, inefficacy, or an excessive decrease in BP. This rate was lower than the discontinuation rates (8.3–21.9%) reported with long-term use of other antihypertensive drugs in daily clinical practice.[19–21]
In terms of the overall incidence of ADRs, this survey had a rate of 5.23% (19 of 363 patients), which was more than 50% lower than that (11.70%, 11 of 94 patients) reported in a 12-month, long-term clinical trial[9] conducted prior to the approval of the drug. In addition, none of the patients in this survey reported serious ADRs at any time. When ADRs were stratified by selected demographic factors, there was no significant association with ADR incidence rate for any factor, suggesting that carvedilol alone or in combination with other antihypertensive drugs is safe for patients with varying background characteristics, even when used on a long-term basis.
For over 90% of the 341 patients in the efficacy population, BP was ‘well controlled’ or ‘mostly well controlled’ at any observation time (months 6, 12, and 18) as judged by the patients’ physicians. Both SBP and DBP were significantly reduced from baseline at the last observation (p < 0.001 for both). Stable decreases in SBP and DBP were shown for any observation timepoint during the 18-month treatment period (p < 0.001 for all timepoints). Therefore, carvedilol was demonstrated to effectively control BP over 18 months without the development of tolerance to its antihypertensive effect. For achievement of target BP (<140/90 mmHg) as specified in several current guidelines,[3,22–24] rates were calculated using data from patients’ SBP and DBP values at months 6, 12, and 18. The achievement rate was over 40% at all observation timepoints. According to research results in hypertensive patients published in 2000,[25,26] the achievement rate was 41.5% in patients being treated at Cardiovascular Internal Medicine departments/clinics and 40.5% in those treated at hypertensive or diabetes care departments/clinics, which is similar to the rate observed in this survey. At the start of this survey, which had an observation period from 1994 to 1997, the target BP was <150/90 mmHg according to guidelines for clinical evaluation of antihypertensive drugs.[11]
Regarding pulse rate, a significant reduction from baseline was found at the last observation (p < 0.001). Pulse rate was consistently decreased at all observation times during the 18-month treatment period (p < 0.001 for all timepoints). However, the decreases were slight and bradycardia was not observed as a serious ADR. Some epidemiologic studies[27,28] have shown a positive association between pulse rate values and cardiovascular mortality in patients with hypertension: the higher the pulse rate, the higher the mortality rate. Hence, it is important to manage not only BP but also pulse rate in order to prevent cardiovascular events. An increase in pulse rate is due to sympathetic hyperactivity. The blockade of β1-receptors by β-blockers can decrease pulse rate and cardiac contractility, resulting in a reduction in myocardial oxygen consumption. Current evidence suggests that reduced BP can be associated with a decreased risk of cardiovascular events, so called ‘the lower the better’, in hypertensive individuals. Generally, other classes of antihypertensive drugs cannot avoid the problem of an increase in pulse rate due to the baroreceptor reflex related to decreased BP, hence β-blockers are the only antihypertensive drug class that can reduce BP and pulse rate simultaneously, resulting in a decrease in myocardial oxygen consumption. In this survey, carvedilol was confirmed to significantly reduce both BP and pulse rate throughout the 18-month treatment period when used in daily medical practice. As supported by this fact, the drug can be characterized to be appropriate for hypertensive patients with tachycardia or coronary artery disease. It is expected that long-term continuous treatment with the drug can improve the prognosis of patients with cardiovascular disease and prevent cardiovascular events.
It is known that many β-blockers have a negative impact on individuals with impaired glucose tolerance or insulin resistance such as those with diabetes. For this reason, many physicians tend to be hesitant to prescribe β-blockers to hypertensive patients with diabetes. A study to compare the effects on insulin sensitivity between carvedilol and metoprolol showed that carvedilol increased the insulin sensitivity index by 9% while metoprolol decreased it by 14%.[1] In addition, in a large-scale clinical trial in the US (GEMINI [The Glycemic Effects in Diabetes Mellitus: Carvedilol-Metoprolol Comparison in Hypertensives]), carvedilol was also compared with metoprolol and reported to have no effect on glycosylated hemoglobin, but did improve insulin resistance while managing BP well.[2] Another report showed that carvedilol had no negative impact on glucose metabolism but decreased the new onset of diabetes compared with other β-blockers.[29] Further to these data, in this survey we investigated the long-term effects of carvedilol on fasting blood glucose levels and its long-term safety in hypertensive patients with diabetes, as well as its safety and efficacy for long-term use in hypertensive patients in general. Although 19 patients had 25 ADRs among the 363 patients evaluated for safety, only one reported a mild increase in plasma glucose level as an ADR related to glucose metabolism; this patient recovered without any action while continuing carvedilol treatment. There was no significant difference in the incidence of ADRs between the patients with and without diabetes complications. No significant difference in fasting blood glucose was found between the levels measured before and after carvedilol treatment. No increase in fasting blood glucose was seen at any observation time (assessed at 6-month intervals) throughout the 18-month treatment period, with a significant reduction in month 12 (p < 0.05). Therefore, long-term use of carvedilol was safe in hypertensive patients with diabetes, without negatively affecting glucose metabolism, at least during the course of this survey.
In the large-scale clinical UKPDS (United Kingdom Prospective Diabetes Study), it was demonstrated that tight BP control in addition to strict control of blood glucose can reduce the risk of diabetes-related events in patients with complications of hypertension and diabetes.[30] Decreased risks of diabetes-related events and mortality were also seen as a result of tight BP control with the use of an angiotensin-converting enzyme inhibitor and a β-blocker (atenolol) in the UKPDS.[31] Accordingly, it can be concluded that the effect of β-blockers on glucose metabolism would not be clinically relevant and that these drugs could be relatively safe in patients with complications of hypertension and diabetes. Considering an adverse effect on insulin resistance, vasodilating β-blockers that improve insulin resistance are recommended and should be initiated at lower doses in these patients. In this survey, the 341 patients evaluable for efficacy included only a small number (n = 58) of patients with diabetes complications. In terms of the change in values for BP and pulse rate between before and after carvedilol treatment, and the changes in these parameters during the course of the 18-month treatment, the subgroup of patients with diabetes was similar to the overall efficacy population. The drug was confirmed to have long-term efficacy in patients with diabetes. Thus, as evidenced, not only by overseas data but also by this survey, carvedilol was well tolerated and effective for the treatment of hypertension complicated with diabetes, without having an adverse effect on glucose metabolism. Long-term use of the drug was also suggested to be useful to achieve the therapeutic goal of tight control of BP and blood glucose.
In conclusion, carvedilol appears to maintain good BP control over a long time, while not being associated with significant safety issues in patients with different background characteristics, such as complications of diabetes.
Conclusions
We conducted a long-term special survey of Artist® tablets for the treatment of Japanese hypertensive patients over 3 years (from April 1994 to March 1997), following a centralized registration method. A total of 380 case report forms were collected from 61 medical institutions nationwide. The safety and efficacy of long-term use of carvedilol were investigated in 363 patients evaluable for safety, and 341 for efficacy. The results are summarized in the following six points:
-
1.
A total of 224 patients (61.7%) continued carvedilol treatment over 18 months, and 139 (38.3%) prematurely dropped out from the survey or discontinued treatment. Carvedilol-associated discontinuation occurred in 26 patients (7.2%), comprising seven patients because of ADRs, 12 patients because of inefficacy, and seven patients because of an excessive decrease in BP.
-
2.
The overall incidence rate of ADRs was 5.23% (19 of 363 patients); no patients reported serious ADRs at any time during the course of carvedilol treatment.
-
3.
No risk factor for occurrence of ADRs was found when incidence was stratified by the selected background factors of the patients.
-
4.
There was no significant difference in fasting blood glucose levels before and after carvedilol treatment. No increase in this parameter was seen at any time during the 18-month treatment period.
-
5.
Both BP and pulse rate were significantly reduced by treatment with carvedilol over 18 months (p < 0.001 for both).
-
6.
The long-term safety and efficacy of carvedilol were confirmed even in patients with hypertension and diabetes.
Carvedilol can be concluded to be useful for the treatment of hypertensive patients with different background characteristics who use the drug on a long-term basis.
References
Jacob S, Rett K, Wicklmayr M, et al. Differential effect of chronic treatment with two beta-blocking agents on insulin sensitivity: the carvedilol-metoprolol study. J Hypertens 1996; 14: 489–94
Bakris GL, Fonseca V, Katholi RE, et al. Metabolic effects of carvedilol vs. metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 2004; 292: 2227–36
The Japanese Society of Hypertension & Guidelines Subcommittee of Japanese Society of Hypertension. 2004 guidelines for the management of hypertension [in Japanese]. Life Science Publishing Co., Ltd.; Tokyo, 2004
Marchi F, Ciriello G. Efficacy of carvedilol in mild to moderate essential hypertension and effects on microalbuminuria: a multicenter, randomized, open-label, controlled study versus atenolol. Adv Ther 1995; 12: 212–21
White WB, Lund-Johansen P, Omvik P. Twenty-four-hour blood pressure load as a surrogate end-point in assessing antihypertensive therapy. J Hypertens 1993; 11: S75–80
Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334: 1349–55
Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–8
Hori M, Sasayama S, Kitabatake A, et al. Low-dose carvedilol improves left ventricular function and reduces cardiovascular hospitalization in Japanese patients with chronic heart failure: The Multicenter Carvedilol Heart Failure Dose Assessment (MUCHA) trial. Am Heart J 2004; 147: 324–30
Goto Y, Tanabe T, Ikeda M, et al. Efficacy and safety of long-term administration of carvedilol (DQ-2466) for the treatment of essential hypertension [in Japanese]. Jpn J Clin Exp Med 1990; 67: 965–84
Good post-marketing surveillance practice [online; in Japanese]. Available from URL: http://www.hourei.mhlw.go.jp/cgi-bin/t_docframe2.cgi?MODE=hourei&DMODE=SEARCH&SMODE=NORMAL&KEYWORD=%90%bb%91%a2%94%cc%94%84%8c%e3%92%b2%8d%b8&EFSNO=597&FILE=FIRST&POS=0&HITSU=83 [Accessed 2011 May 24]
Working group for study on establishment of clinical evaluation methods of antihypertensive drugs. Guideline for clinical evaluation methods of antihypertensive drugs [in Japanese]. Pharmaceutical Regulatory Science 1989; 20: 902–14
Kato I, Kumagai M, Kato C, et al. Clinical study of longterm use of nipradilol: post marketing surveillance by Midori Medical Association. Ther Res 1994; 15: 1189–99
Artist® tablets 1.25-mg, Artist® Tablets 2.5-mg, Artist® Tablets 10-mg, and Artist® Tablets 20-mg [package inserts in Japanese]. Daiichi Sankyo Co., Ltd.; Tokyo, 2007
Editorial supervisor: Ministry of Health, Labor and Welfare. Adverse drug reaction terminology [in Japanese]. Yakugyo Jiho Co., Ltd.; Tokyo, 1996
Kumahara Y, Ogihara T, Goto Y, et al. Effects of carvedilol (DQ-2466) at administration alone in patients with essential hypertension: multicenter, open clinical trial [in Japanese]. Rinshou to Kenkyu 1989; 66: 3968–82
Takeda T, Kohno M, Ishii M, et al. Effects of carvedilol in patients with renal parenchymal hypertension: multicenter, open clinical trial [in Japanese]. Rinshou to Kenkyu 1990; 67: 312–24
Goto Y, Tanabe T, Ikeda M, et al. Examination of safety and efficacy with the long-term treatment of carvedilol (DQ-2466) in patients with essential hypertension [in Japanese]. Rinshou to Kenkyu 1989; 66: 3968–82
Mori Y, Nishikawa Y, Iizuka T, et al. Artist® tablets (carvedilol) for hypertensive patients in Japan: results of a drug use survey. Drugs R&D 2011; 11 (2): 171–90
Kanagawa study group of the treatment of elderly patients with hypertension. Safety and efficacy of long-term use of benidipine hydrochloride in elderly patients with hypertension [in Japanese]. Geriat Med 1997; 35: 989–1007
Kuramoto K, Ishii T, Yazaki Y, et al. Efficacy and safety of long-term administration of Longes Tablets (lisinopril) for the treatment of hypertension: a large-scale post-marketing surveillance study [in Japanese]. J Blood Pressure 1998; 5: 1001–19
Kanagawa Selectol study group. Long-term effect of a β-blocker celiprolol for the treatment of elderly patients with essential hypertension [in Japanese]. Jpn J Clin Exp Med 1999; 76: 1635–47
World Health Organization, International Society of Hypertension Writing Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–92
Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–52
Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105–87
Yamamoto Y, Sonoyama K, Matsubara K, et al. The status of hypertension management in Japan in 2000. Hypertens Res 2000; 25: 717–25
Katayama S, Inaba M, Morita T, et al. Blood pressure control in Japanese hypertensives with or without type 2 diabetes mellitus. Hypertens Res 2000; 23: 601–5
Gillman MW, Kannel WB, Belanger A, et al. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J 1993; 215: 1148–54
Hozawa A, Ohkubo T, Kikuya M, et al. Prognostic value of home heart rate for cardiovascular mortality in the general population: the Ohasama study. Am J Hypertens 2004; 17: 1005–10
Torp-Pedersen C, Metra M, Charlesworth A, et al. Effects of metoprolol and carvedilol on pre-existing and new onset diabetes in patients with chronic heart failure: data from the Carvedilol Or Metoprolol European Trial (COMET). Heart 2007; 93: 968–73
UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–13
UK Prospective Diabetes Study (UKPDS) Group. Efficacy of atenolol and captopril in reduction risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998; 317: 713–20
Acknowledgements
The authors would like to thank the physicians for their support for the long-term special survey of Artist® tablets and for providing valuable data. We also thank Nila Bhana, MSc, of inScience Communications, a Wolters Kluwer business, who provided medical writing support funded by Daiichi Sankyo Co., Ltd., Tokyo, Japan.
This study was supported for funding, data collection, and statistical analysis by Daiichi Sankyo Co., Ltd., Tokyo, Japan. Daiichi Sankyo Co., Ltd. was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the article. In particular, Yasuhiro Nishikawa, MD, provided intellectual advice for the study concept, scientific interpretation of the results, and approval of the article. The authors have no conflicts of interest to declare.
Tomoko Iizuka is currently employed by Pharmacovigilance Department, Daiichi Sankyo Co., Ltd., Tokyo, Japan; Yasuhiro Nishikawa is currently employed by Global Project Management Department, Daiichi Sankyo Co., Ltd., Tokyo, Japan; and Masahiro Komiya is currently employed by Daiichi Sankyo Healthcare Co., Ltd., Tokyo, Japan.
This study was originally published in Japanese in the Journal of Clinical Therapeutics & Medicines [2007; 23 (12): 1073-87]. The study has been reproduced here in English with kind permission of the publisher, Rinsho Iyaku Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Iizuka, T., Nishikawa, Y., Mori, Y. et al. Artist® Tablets (Carvedilol) for Hypertensive Patients in Japan. Drugs R D 11, 191–205 (2011). https://doi.org/10.2165/11592460-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11592460-000000000-00000