Abstract
Background: A novel estradiol-based combined oral contraceptive (COC) is currently available in many countries worldwide, including Europe and the US. Based on previous studies, it is expected that this estradiol-based COC will have a reduced hepatic effect compared with COCs containing ethinylestradiol with regard to proteins controlling the hemostatic balance.
Objective: The aim of this study was to compare the hemostatic effects of the estradiol valerate/dienogest COC with a monophasic low-estrogen dose COC containing ethinylestradiol/levonorgestrel.
Study Design: Healthy women aged 18–50 years were randomized to receive a COC containing estradiol valerate/dienogest (2 days estradiol valerate 3 mg, 5 days estradiol valerate 2mg/dienogest 2 mg, 17 days estradiol valerate 2mg/dienogest 3 mg, 2 days estradiol valerate 1 mg, 2 days placebo) or ethinylestradiol 0.03mg/levonorgestrel 0.15mg in a crossover study design. Women received each treatment for three cycles, with two washout cycles between treatments. The primary efficacy variables were the intra-individual absolute changes in prothrombin fragment 1 + 2 and D-dimer from baseline to cycle three.
Results: Data from 29 women were assessed. Intra-individual absolute changes in prothrombin fragment 1 + 2 and D-dimer from baseline to cycle three were less pronounced with estradiol valerate/dienogest than with ethinylestradiol/ levonorgestrel.
Conclusion: The novel COC containing estradiol valerate/dienogest had similar or less pronounced effects on hemostatic parameters than ethinylestradiol/ levonorgestrel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Combined oral contraceptives (COCs) have become a widely used form of birth control due to their contraceptive efficacy and good tolerability profile. COCs have undergone considerable development since their introduction in the 1960s, including reductions in the dose of synthetic estrogen (i.e. ethinylestradiol)[1–3] and the incorporation of progestogens with more favorable clinical profiles.[4]
However, COCs that contain ethinylestradiol and its prodrug mestranol have been associated with a slight increased risk of cardiovascular events, such as venous thromboembolism.[5,6] Nevertheless, these events occur rarely, and the increased risk of thromboembolism during pregnancy and the puerperium must be considered.[7] Risk factors for thrombotic or thromboembolic events include age, smoking, a positive family history, genetic thrombophilias (e.g. Factor V Leiden mutation), obesity, dyslipoproteinemia, hypertension, migraine, valvular heart disease, atrial fibrillation, prolonged immobilization, major surgery, any surgery to the legs, and major trauma.[8]
It is well established that the hormonal components of COCs have various effects on hemostasis. Moreover, the adverse effects on hemostatic variables are most likely to be influenced by the estrogen type and dose rather than the type of progestogen used. Although hemostatic surrogate parameters are not predictive of the occurrence of thromboembolic events, their evaluation is part of the development process of COCs. Epidemiologic studies are required to evaluate the incidence of venous and arterial thromboembolism in COC users, since these events are so rare that they cannot be accurately assessed in phase III clinical studies.
Several attempts to develop COCs containing 17β-estradiol as the estrogenic component have been made based on the hope that this will (i) improve their tolerability and acceptability; and (ii) broaden the choice for COC users.[9–21]
To prevent the poor cycle control that has been observed with previous 17β-estradiol-containing COCs,[11,16–19,21] a novel COC containing estradiol valerate (1 mg estradiol valerate corresponds to 0.76 mg 17β-estradiol) and dienogest with a fixed estrogen step-down/progestogen step-up dynamic dosing regimen has been developed. Estradiol valerate/dienogest has been shown to be associated with reliable contraceptive efficacy[22,23] and good cycle control.[24] Moreover, 17β-estradiol levels have been shown to be stable throughout the cycle, with estrogen levels remaining comparable with those during the early follicular phase.[25]
Based on studies that have evaluated the effect of 17β-estradiol versus ethinylestradiol on hepatic protein synthesis,[26–29] it is expected that estradiol valerate/dienogest will have a considerably reduced hepatic effect compared with COCs containing ethinylestradiol with regard to proteins controlling the hemostatic balance.
The current study was conducted to quantify the effects of estradiol valerate/dienogest on various key parameters of the coagulation system, indicating changes in the pro-coagulatory, anti-coagulatory, and fibrinolytic activity. A COC containing ethinylestradiol 0.03 mg/levonorgestrel 0.15 mg was used as the comparator COC.
Methods
Study Design
This was a crossover, active-treatment-controlled, randomized, open-label, single-center study conducted between April 2006 and May 2007 (protocol number 310122).[30] The design of the study was selected in adherence to the requirements of the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP).[EMEA/CPMP/EWP/519/98 Rev 1].[31] The study protocol was approved by the local Ethics Committee in the Netherlands. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and the International Conference on Harmonization — Good Clinical Practice. Written informed consent was obtained from all study participants prior to entry into the study.
Study Population
Healthy women aged 18–50 years who required contraception were eligible for inclusion. Exclusion criteria included the following: pregnancy; lactation; occurrence of less than three menstrual cycles following childbirth, abortion, or lactation; known hypersensitivity to any ingredients of the study drugs; known diseases or conditions that might result in the altered absorption, excessive accumulation, impaired metabolism, or altered excretion of the study medication; any known severe systemic disease that might interfere with the conduct of the study or interpretation of the results; uncontrolled thyroid disorders; clinically significant depression; abnormal clinically significant findings that might worsen under hormonal treatment; laboratory values outside inclusion ranges at screening; operations scheduled during the study period; liver disease; vascular and metabolic diseases, or factors that predispose to vascular and metabolic diseases; sickle-cell anemia; known or suspected malignant or premalignant disease; alcohol, drug, or medicine abuse; use of prohibited concomitant medication; use of sex hormones prior to the start of treatment (including oral, transdermal, intrauterine, or intravaginal administration within two cycles prior to the start of treatment; implants within 2 months prior to the study; long-acting progestogens within 6 months prior to visit 1); and a body mass index >30 kg/m2. Cigarette consumption of up to 10 per day was permitted in women aged 18–30 years, while women aged >30 years were required to be non-smokers. The use of additional sex steroids was prohibited.
Study Treatment
The study utilized a crossover design (figure 1). All women were randomized to receive three cycles (84 consecutive days) of estradiol valerate/dienogest (estradiol valerate 3 mg for 2 days, estradiol valerate 2 mg/dienogest 2 mg for 5 days, estradiol valerate 2 mg/dienogest 3 mg for 17 days, estradiol valerate 1 mg for 2 days, and placebo for 2 days) and three cycles (84 consecutive days) of ethinylestradiol/levonorgestrel (ethinylestradiol 0.03 mg/levonorgestrel 0.15 mg for 21 days and placebo for 7 days). Both treatment periods were preceded and separated by a washout period of two cycles.
Design of the crossover study. E 2V/DNG = estradiol valerate/dienogest; = ethinylestradiol/levonorgestrel; EOT = end of treatment; SOT = start of treatment (first day of bleeding); V1 = screening visit; V2 = baseline (days 15–21 of the cycle); V3 = end of treatment period 1 (end of treatment cycle 3).[E2V/DNG = treatment days 74–80; EE/LNG = treatment days 71–77]; V4 = end of washout cycle 1 (days 15–21 of the cycle); V5 = end of washout cycle 2 (days 15–21 of the cycle)/baseline for treatment period 2; V6 = end of treatment period 2 (end of treatment cycle 6).[E2V/DNG = treatment days 158–164; EE/LNG = treatment days 155–161]; V7 = up to 2 weeks after the end of treatment, but at least 2 days after the end of the withdrawal bleeding that followed treatment cycle 6.
Randomization was performed in blocks using a computer-generated list (obtained from the Central Randomization Service of Bayer HealthCare Pharmaceuticals). Women were instructed to start tablet intake on the first day of menstrual bleeding. Tablet intake was recorded using diary cards.
Women were to take any missed tablets as soon as remembered, at the latest with the next administration. For either treatment, if more than one tablet was missed, only the most recently missed tablet (i.e. the one of the immediately preceding day) was to be taken as soon as remembered.
Study Assessments
Citrated (0.11 mmol/L) blood specimens were taken with minimal stasis at baseline (visit 2), at the end of each washout cycle (visits 4 and 5), and at the end of the estrogen/progestogen phase of treatment cycle three (visit 3) and treatment cycle six (visit 6). Specimens were collected at least 2 hours after the previous meal. To minimize the variability of the concentration of high molecular weight blood constituents due to variations in intravascular blood volume, subjects were asked to rest in a supine or sitting position for at least 10 minutes prior to blood collection. The vein was then obstructed for a short time and then released prior to punction for blood collection.
For the preparation of platelet-free citrated plasma (required for the measurement of several hemostatic parameters), samples were centrifuged twice at a force of ≥2500 g for 15 minutes at ambient temperature. For serum preparation (required for the measurement of sex hormone-binding globulin [SHBG]), samples were centrifuged at a force of ≥1200 g for 15 minutes at ambient temperature. Samples were clearly labeled and all necessary transport and storage of blood were performed under appropriate conditions.
The hemostatic markers that were assessed are shown in table I.
Primary Target Parameters
The primary target parameters were the intra-individual absolute changes from baseline in thrombin and fibrin turnover, assessed using the markers prothrombin fragment 1 + 2 and D-dimer, after three treatment cycles with estradiol valerate/dienogest and ethinylestradiol/levonorgestrel.
Secondary Target Parameters
The secondary target parameters were the intra-individual absolute changes from baseline in (pro)coagulatory parameters, anti-coagulatory parameters, thrombin and fibrin turnover, and SHBG levels, after three treatment cycles with estradiol valerate/dienogest and ethinylestradiol/levonorgestrel.
Safety
Safety was evaluated by adverse event recording throughout the study, and by general physical and gynecologic examinations (including vital signs, breast palpation, transvaginal ultrasonography, and cytological cervix smear) performed at screening (visit 1) and within 2 weeks after stopping study treatment (visit 7).
Statistics
Analyses of all target parameters and safety parameters were based on the full analysis set (FAS). This included all women who received at least one dose of treatment, and for whom data from the treatment phase were available.
Thirty women were planned to be randomized and, assuming a withdrawal rate of 30% (including all major protocol deviations), approximately 20 women were planned to be included in the per-protocol set analysis. Since this sample size is standard for metabolic studies and based on regulatory requirements, no power calculation was considered necessary.
For each primary target variable, the absolute change from the corresponding baseline value after treatment over three cycles (i.e. value at baseline [visit 2] or after the washout cycle two [visit 5] of the respective treatment period) was to be calculated and analyzed using ANOVA. The significance level of a nominal 5% was adjusted to 2.5% for multiple testing using the Bonferroni method.
For each hemostatic marker listed as a secondary variable, a post hoc explorative analysis was performed. The relative change from baseline to cycle three was determined and an ANOVA with a comparison-wise significance level of 5% was used to test the differences between the two treatments.
Results
Subject Disposition
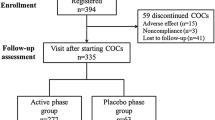
A total of 34 women were screened according to the selection criteria for inclusion in the study. Of these, 32 women were deemed eligible for treatment and were randomized to estradiol valerate/dienogest (n = 16) or ethinylestradiol/levonorgestrel (n = 16). A total of 29 women received at least one unit of any study drugs dependent on their assignment to one of the two treatment sequences and were part of the FAS. In total, 26 women completed three cycles with the estradiol valerate/dienogest treatment and 27 women completed three cycles with the ethinylestradiol/levonorgestrel treatment. The flow of women through the study is shown in figure 2.
Study flow chart showing the number of women screened, randomized, and completing treatment. For sequence A, full analysis set (FAS) was n = 14, per-protocol set (PPS) was n = 13 (one volunteer from FAS with major protocol deviation[s]). For sequence B, FAS was n = 15, PPS was n = 11 (four volunteers from FAS with major protocol deviation[s]). AEs = adverse events; E 2 V/DNG = estradiol valerate/dienogest; EE/LNG = ethinylestradiol/levonorgestrel; GI = gastrointestinal.
The mean age of women in the FAS (n = 29) was 27.4 years, and the mean body mass index was 21.7 kg/m2; the majority (n = 27; 93.1%) of women in the FAS were Caucasian and non-smokers (n = 22; 75.9%). Fifteen (51.7%) women were using oral contraceptives before the study washout period. Nine (31.0%) women reported no prior use of any contraceptive method.
According to diary records, 22 (81.5%) and 21 (72.4%) women took all the required tablets in the estradiol valerate/dienogest and ethinylestradiol/levonorgestrel treatment phases, respectively. There were, respectively, one woman (3.7%) and three women (10.3%) with major treatment deviations in the estradiol valerate/dienogest and ethinylestradiol/levonorgestrel treatment phases. The frequency of minor protocol deviations was comparable under both study treatments, namely four (14.8%) women in the estradiol valerate/dienogest treatment phase and four (13.8%) women in the ethinylestradiol/levonorgestrel treatment phase.
Effects on Hemostasis
Primary Target Parameters
Thrombin and Fibrin Turnover (Activation Markers)
During treatment with estradiol valerate/dienogest, no intra-individual change was seen in the levels of prothrombin 1 + 2, whereas a slight increase was observed with ethinylestradiol/levonorgestrel, although mean levels in both treatment groups remained in the reference interval (table II). The differences between the two treatments were not statistically significant. Mean baseline levels of D-dimer were almost comparable at the start of each treatment period with the respective study treatment. A significantly (p = 0.01; 95% CI -217.22, -11.77) smaller increase in D-dimer levels in the estradiol valerate/dienogest group (from 203.0 ± 94.1 ng/mL to 237.4 ± 101.6 ng/mL) than in the ethinylestradiol/levonorgestrel group (from 201.8 ± 73.5 ng/mL to 352.6 ± 217.8 ng/mL) was observed, but mean levels in both groups also remained in the reference interval. The intra-individual relative change was 37.3% ± 69.3% and 88.1% ± 99.3% for estradiol valerate/dienogest and ethinylestradiol/levonorgestrel, respectively (table II).
Secondary Target Parameters
Thrombin and Fibrin Turnover (Activation Markers)
Following treatment in both groups, prothrombin levels were above the normal range; however, intra-individual increases were more pronounced in the ethinylestradiol/levonorgestrel group (table II).
Pro-Coagulatory Parameters
Changes in pro-coagulatory markers with treatment were generally less pronounced with estradiol valerate/dienogest than with ethinylestradiol/levonorgestrel (table III). Mean fibrinogen levels were elevated above the normal range in both treatment groups, although the mean intra-individual increase was greater in the ethinylestradiol/levonorgestrel group (table III). Mean levels of factor VII activity increased minimally in the estradiol valerate/dienogest group and slightly more pronounced in the ethinylestradiol/levonorgestrel group; however, mean levels remained within the reference range. Almost no changes in the mean levels of factor VIII were observed in either treatment group (table III).
Anti-Coagulatory Parameters
Only minor changes were observed in most anti-coagulatory markers. The mean levels of antithrombin III activity, protein C activity, protein S activity, and activated protein C (APC) resistance remained generally stable and within the reference range in both treatment groups (table III). However, APC sensitivity ratio levels were slightly above the proposed reference range[32] in the ethinylestradiol/levonorgestrel group. The difference between the two treatments in mean individual relative change from baseline to cycle three was statistically significant (p = 0.0006).[table III].
As is common with any population, at baseline, a small and comparable proportion of women with both treatments had values outside the reference ranges for the parameters measured. Although there were no statistical differences between treatments in the majority of parameters measured, at cycle three, the proportion of women who had values outside of the reference range was higher with ethinylestradiol/levonorgestrel treatment than with estradiol valerate/dienogest treatment.
Sex-Hormone Binding Globulin (SHBG) Levels
Following three treatment cycles, mean SHBG concentrations increased by comparable levels in each treatment group (from 48.80 ± 19.34 nmol/L at baseline to 72.61 ± 27.95 nmol/L at cycle three with estradiol valerate/dienogest, and from 51.73 ± 21.27 nmol/L to 72.60 ± 30.12 nmol/L with ethinylestradiol/levonorgestrel). At the end of the respective treatments, there were two women with increased values and one woman with a decreased value that were outside of the reference range, in each of the two treatment groups.
Safety
Fifty-one adverse events were reported by 20 women (74.1%) in the estradiol valerate/dienogest group, and 61 adverse events by 20 women (69.0%) in the ethinylestradiol/levonorgestrel group. The most commonly reported adverse events in the estradiol valerate/dienogest and ethinylestradiol/levonorgestrel groups were headache (11 cases in 6 women [22.2%]; and 14 cases in 11 women [37.9%], respectively) and nasopharyngitis (5 cases in 5 women [18.5%]; and 6 cases in 5 women [17.2%], respectively [table IV]).
No adverse events were considered definitely or probably related to either study drug. Moreover, there were no serious adverse events or deaths reported during the study.
The mean systolic and diastolic blood pressure values were generally stable throughout the study visits. The results of the cervical smear screening were normal for all but one woman (3.4%) who had an abnormal result (cervical dysplasia) at final examination. There were no positive results for pregnancy testing during the study, and no pregnancy was reported.
Discussion
The findings from this study show that estradiol valerate/dienogest has a similar or less of an impact on hemostatic parameters than a monophasic COC containing ethinylestradiol/levonorgestrel over three cycles of treatment. This was manifested in a significantly smaller increase in D-dimer levels (37% vs 88% for estradiol valerate/dienogest and ethinylestradiol/levonorgestrel, respectively) and by less pronounced increases in the levels of prothrombin and fibrinogen. The APC resistance, as measured by the partial thromboplastin time, remained unchanged in both groups, whereas the mean APC sensitivity ratio (measured using the Rosing test[32] ) showed a distinctly and statistically significant greater increase in the ethinylestradiol/levonorgestrel group. It should be noted that both tests reflect the protein C mediated anti-coagulatory activity in plasma, but the Rosing test shows a higher sensitivity towards concentration changes of the proteins involved. Although no significant differences between treatments were observed in the majority of parameters measured, at cycle three, the proportion of women who had several values outside of the reference range was higher with ethinylestradiol/levonorgestrel treatment than with estradiol valerate/dienogest treatment.
It is generally well accepted that the influence of COCs on coagulation and fibrinolysis depends mainly on the estrogen component of the regimen. The reduced impact of estradiol valerate/dienogest on hemostatic parameters observed in the current study is probably due to the inclusion of estradiol valerate instead of ethinylestradiol as the estrogenic component. Estradiol valerate is considerably less potent than ethinylestradiol in terms of hepatic protein synthesis induction, as demonstrated in clinical studies assessing SHBG, angiotensinogen, and hemostatic parameters.[26–29] However, changes observed with COCs reflect not only influences of the estrogen component, but also those of the progestogen component; indeed, progestogen can affect the binding of testosterone or cortisol to transport proteins[33] and the clearance time from serum.[34]
The findings of the current study are also consistent with those of a study that compared the metabolic effects of estradiol valerate/dienogest with a triphasic regimen of ethinylestradiol/levonorgestrel.[35] In the comparative study with triphasic ethinylestradiol/levonorgestrel, levels of factors associated with activation of coagulation increased slightly with both study preparations, but generally remained well within the normal reference range. Observed changes were consistently less pronounced with estradiol valerate/dienogest relative to ethinylestradiol/levonorgestrel. Furthermore, increases in the levels of SHBG were observed in both groups, with increases being less marked in women who received estradiol valerate/dienogest compared with women who received ethinylestradiol/levonorgestrel. Despite the fact that levels of SHBG increased in the estradiol valerate/dienogest group, mean levels remained within the normal range, whereas in the ethinylestradiol/levonorgestrel group, the mean levels of SHBG increased to exceed the normal range.[35] It is, however, questionable whether measuring SHBG levels is the most appropriate way of assessing estrogen-induced effects of COCs. SHBG levels increase with use of COCs, with the observed net effect resulting from effects exerted by both components. Estrogen increases SHBG levels in a dose-dependent fashion. Moreover, at the dose required to produce similar effects on follicle-stimulating hormone suppression, ethinylestradiol increases levels of SHBG to a greater extent than 17β-estradiol.[28] SHBG levels are decreased by androgens and some progestogens with partial androgenic activity, including levonorgestrel. In contrast, dienogest, drospirenone, and cyprotenone acetate do not counteract the estrogen-induced increases of SHBG.[34] Nevertheless, in the current study, increases in SHBG were more pronounced with ethinylestradiol/levonorgestrel than with estradiol valerate/dienogest. This is possibly consistent with the inclusion of estradiol valerate instead of ethinylestradiol as the estrogenic component, with estradiol valerate being less potent than ethinylestradiol in terms of hepatic protein synthesis induction.[29]
A prudent approach is necessary when attempting to interpret surrogate parameters for clinical outcomes. Hemostaseologic surrogate parameters have not been proven to capture the modifying effect of COCs on the risk of thromboembolic events.[36–38] Biomarkers and surrogate endpoints have important roles to play in exploratory research and development trials, but cannot be used clinically until validated, no matter how intuitively appealing the biomarker may be.[31,37] For a surrogate endpoint to be an effective substitute for the clinical outcome, effects of the intervention on the surrogate must reliably predict the overall effect on the clinical outcome. However, the validity of a surrogate endpoint has rarely been rigorously established.[39]
Therefore, it is important that caution is applied in the interpretation of the observed differences in surrogate hemostatic parameters between estradiol valerate/dienogest and ethinylestradiol/levonorgestrel since these parameters have not been proven to be predictive of the occurrence of actual clinical events. Nevertheless, the current study is a useful comparison of the impact on hemostatic parameters of a novel dynamically dosed COC containing estradiol valerate/dienogest with that of the established COC regimen containing ethinylestradiol/levonorgestrel.
As such, a COC containing estradiol valerate at dosages of 1–3 mg (as used in the current study) may induce less estrogen-related responses in the liver than currently available ethinylestradiol-containing COCs. However, further clinical and epidemiologic studies will be needed to confirm whether this translates into better clinical outcomes over the long term.
Since the estradiol valerate/dienogest COC is new to the market, epidemiologic study data on cardiovascular safety are currently not available. Moreover, the information from clinical trials is limited, based on the rarity of events such as venous thromboembolism and the limited number of patients investigated in phase III trials. Therefore, it is reassuring that estradiol valerate/dienogest has a similar or less pronounced hemostatic impact than the well established monophasic ethinylestradiol/levonorgestrel, a COC that has been comprehensively studied and extensively marketed. Furthermore, results of a double-blind, randomized study suggest that a monophasic COC combining nomegestrol acetate 2.5 mg with 17β-estradiol 1.5 mg might have less of an impact on hemostatic parameters compared with levonorgestrel 0.10 mg/ethinylestradiol 0.02 mg.[40] The overall magnitude of changes in hemostatic parameters is comparable with those observed with estradiol valerate/dienogest in the current study as shown, for example, by a similar increase in SHBG and by similarly less pronounced increases in prothrombin fragment 1 + 2 and fibrinogen and thrombin generation (determined either by the APC sensitivity ratio[32] or by APC resistance as measured by the endogenous thrombin generation method).[40,41] This suggests that COCs comprising either 17β-estradiol or estradiol valerate in equimolar doses may have very similar effects on hemostatic parameters.
It is possible that lower doses of ethinylestradiol (e.g. 0.02 mg) may have reduced hemostatic effects compared with COCs containing 0.03 mg of ethinylestradiol, such as the comparator used in our study.[42–45] However, whether the impact on hemostatic effects is further reduced with even lower doses of ethinylestradiol than 0.02 mg, down to 0.015 mg, may be questionable,[46] and may also be associated with increased bleeding irregularities; overall incidences of breakthrough bleeding and/or spotting of 21%[1] and 29%[47] with 0.015 mg ethinylestradiol have been reported.
An international prospective, controlled, non-interventional cohort active surveillance study (INAS-SCORE [International Active Surveillance Study–Safety of Contraceptives: Role of Estrogens]), which is currently underway, was designed to assess the risks of short- and long-term use of estradiol valerate/dienogest and of established COCs in a study population that is representative of actual users of the individual preparations. A total of 50 000 new users of a COC (starters or switchers) will be followed-up over a 3- to 5-year period to allow documentation of approximately 150 000 women-years. The main clinical outcomes of interest for the short- and long-term follow-up are cardiovascular events, primarily deep venous thrombosis, pulmonary embolism, acute myocardial infarction, and cerebrovascular accidents.
In the current study, there were no safety concerns raised with estradiol valerate/dienogest, and both study treatments were similarly well tolerated. None of the adverse events during the study were rated as serious and only three adverse events in one subject led to premature study discontinuation in the ethinylestradiol/levonorgestrel group. Exposure to either of the study treatments was not associated with severe, rare side effects of COCs; there were no cases of venous or arterial thromboembolism reported.
Conclusion
In conclusion, a dynamically dosed estrogen step-down/progestogen step-up estradiol valerate/dienogest COC regimen had a minimal impact on hemostatic parameters and was well tolerated. Findings in both treatment groups support a positive benefit-risk profile of these COCs.
References
Gestodene Study Group 322. The safety and contraceptive efficacy of a 24-day low-dose oral contraceptive regimen containing gestodene 60 microg and ethinylestradiol 15 microg. Eur J Contracept Reprod Health Care 1999; 4 Suppl. 2: 9–15
Bannemerschult R, Hanker JP, Wunsch C, et al. A multicenter, uncontrolled clinical investigation of the contraceptive efficacy, cycle control, and safety of a new low dose oral contraceptive containing 20 micrograms ethinyl estradiol and 100 micrograms levonorgestrel over six treatment cycles. Contraception 1997; 56 (5): 285–90
Endrikat J, Jaques MA, Mayerhofer M, et al. A twelve-month comparative clinical investigation of two low-dose oral contraceptives containing 20 micrograms ethinylestradiol/75 micrograms gestodene and 20 micrograms ethinylestradiol/150 micrograms desogestrel, with respect to efficacy, cycle control and tolerance. Contraception 1995; 52 (4): 229–35
Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update 2006; 12 (2): 169–78
Combined oral contraceptives: a statement by the committee on safety of drugs. Br Med J 1970; 2 (5703): 231–2
Bottiger LE, Boman G, Eklund G, et al. Oral contraceptives and thromboembolic disease: effects of lowering oestrogen content. Lancet 1980; I (8178): 1097–101
Blickstein I. Thrombophilia and women’s health: an overview. Obstet Gynecol Clin North Am 2006; 33 (3): 347–56
Lowe GD. Common risk factors for both arterial and venous thrombosis. Br J Haematol 2008; 140 (5): 488–95
Astedt B, Jeppsson S, Liedholm P, et al. Clinical trial of a new oral contraceptive pill containing the natural oestrogen 17 beta-oestradiol. Br J Obstet Gynaecol 1979; 86 (9): 732–6
Astedt B, Svanberg L, Jeppsson S, et al. The natural oestrogenic hormone oestradiol as a new component of combined oral contraceptives. Br Med J 1977; 1 (6056): 269
Csemiczky G, Dieben T, Coeling Bennink HJ, et al. The pharmacodynamic effects of an oral contraceptive containing 3 mg micronized 17 beta-estradiol and 0.150 mg desogestrel for 21 days, followed by 0.030 mg desogestrel only for 7 days. Contraception 1996; 54 (6): 333–8
Hirvonen E, Allonen H, Anttila M, et al. Oral contraceptive containing natural estradiol for premenopausal women. Maturitas 1995; 21 (1): 27–32
Hirvonen E, Stenman UH, Malkonen M, et al. New natural oestradiol/cyproterone acetate oral contraceptive for premenopausal women. Maturitas 1988; 10 (3): 201–13
Hoffmann H, Moore C, Zimmermann H, et al. Approaches to the replacement of ethinylestradiol by natural 17betaestradiol in combined oral contraceptives. Exp Toxicol Pathol 1998; 50 (4–6): 458–64
Kovacs L, Hoffmann H. A new low-dose oral contraceptive containing ethinylestradiol, estradiol and dienogest: first experience of its clinical use. In: Elstein M, editor. Extragenital effects of contraceptives. 4th Congress of the European Society of Contraception, Barcelona, Spain, June 1996. Pearl River (NY): The Parthenon Publishing Group Inc., 1997: 39–44
Schubert W, Cullberg G. Ovulation inhibition with 17 betaestradiol cyclo-octyl acetate and desogestrel. Acta Obstet Gynecol Scand 1987; 66 (6): 543–7
Serup J, Bostofte E, Larsen S, et al. Natural oestrogens for oral contraception. Lancet 1979; II (8140): 471–2
Hirvonen E, Vartiainen E, Kulmala Y. A multicenter trial with a new OC using natural estradiol and cyproterone acetate for women over 35 [abstract]. Adv Contracept 1990; 6 (4): 248
Kivinen S, Saure A. Efficacy and tolerability of a combined oral contraceptive containing 17 β-estradiol and desogestrel [abstract]. Eur J Contracept Reprod Health Care 1996; 1: 183
Hoffmann H, Moore C, Kovacs L, et al. Alternatives for the replacement of ethinylestradiol by natural 17b-estradiol in dienogest-containing oral contraceptives. Drugs Today 1999; 35 (Suppl. C): 105–13
Wenzl R, Bennink HC, van Beek A, et al. Ovulation inhibition with a combined oral contraceptive containing 1mg micronized 17 beta-estradiol. Fertil Steril 1993; 60 (4): 616–9
Endrikat J, Parke S, Trummer D, et al. Ovulation inhibition with four variations of a four-phasic estradiol valerate/ dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception 2008; 78 (3): 218–25
Palacios S, Wildt L, Parke S, et al. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a phase III trial. Eur J Obstet Gynecol Reprod Biol 2009; 149 (1): 57–62
Ahrendt HJ, Makalova D, Parke S, et al. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception 2009; 80 (5): 436–44
Lu M, Uddin A, Foegh M, et al. Pharmacokinetics and pharmacodynamics of a new four-phasic estradiol valerate and dienogest oral contraceptive [abstract]. Obstet Gynecol 2007; 109 (4 Suppl.): 61S
Helgason S. Estrogen replacement therapy after the menopause: estrogenicity and metabolic effects. Acta Obstet Gynecol Scand Suppl 1982; 107: 1–29
Lindberg UB, Crona N, Stigendal L, et al. A comparison between effects of estradiol valerate and low dose ethinyl estradiol on haemostasis parameters. Thromb Haemost 1989; 61 (1): 65–9
Mashchak CA, Lobo RA, Dozono-Takano R, et al. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol 1982; 144 (5): 511–8
Wiegratz I, Lee JH, Kutschera E, et al. Effect of four oral contraceptives on hemostatic parameters. Contraception 2004; 70 (2): 97–106
BayerHealthCare Pharmaceuticals. Impact of SHT00658ID as compared to a monophasic contraceptive containing ethinylestradiol and levonorgestrel (SH D01155E) on hemostatic parameters [ClinicalTrials.gov identifier NCT00318799]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2011 Apr 5]
European Medicines Agency (EMEA). Committee for Medicinal Products for Human Use (CHMP): guideline on clinical investigation of steroid contraceptives inwomen, 2005 [online]. Available from URL: http://www.ema.europa.eu/pdfs/human/ewp/051998en.pdf [Accessed 2010 Jun 22]
Rosing J, Tans G, Nicolaes GA, et al. Oral contraceptives and venous thrombosis: different sensitivities to activated protein C in women using second- and third-generation oral contraceptives. Br J Haematol 1997; 97 (1): 233–8
Oettel M, Breitbarth H, Elger W, et al. The pharmacological profile of dienogest. Eur J Contracept Reprod Health Care 1999; 4 (Suppl. 1): 2–13
Oettel M, Carol W, Elger W, et al. A 19-norprogestin without 17alfa-ethinyl group II: dienogest from a pharmacodynamic point of view. Drugs Today 1995; 31 (7): 517–36
Parke S, Nahum GG, Mellinger U, et al. Metabolic effects of a new 4-phasic oral contraceptive containing estradiol valerate and dienogest [abstract]. Obstet Gynecol 2008; 111 (4 Suppl.): 12S
Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 2005; 24 (1): 67–78
Grimes DA, Schulz KF, Raymond EG. Surrogate end points in women’s health research: science, protoscience, and pseudoscience. Fertil Steril 2010; 93 (6): 1731–4
Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol 2009; 29 (3): 332–6
Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996; 125 (7): 605–13
Gaussem P, Alhenc-Gelas M, Thomas JL, et al. Haemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17beta-estradiol, compared with those of levonorgestrel/ethinyl estradiol: a double-blind, randomised study. Thromb Haemost 2011; 105 (3): 560–7
Tans G, van Hylckama Vlieg A, Thomassen MC, et al. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol 2003; 122 (3): 465–70
Endrikat J, Klipping C, Gerlinger C, et al. A double-blind comparative study of the effects of a 23-day oral contraceptive regimen with 20 microg ethinyl estradiol and 75 microg gestodene and a 21-day regimen with 30 microg ethinyl estradiol and 75 microg gestodene on hemostatic variables, lipids, and carbohydrate metabolism. Contraception 2001; 64 (4): 235–41
Kluft C, Endrikat J, Mulder SM, et al. A prospective study on the effects on hemostasis of two oral contraceptives containing drospirenone in combination with either 30 or 20 microg ethinyl estradiol and a reference containing desogestrel and 30 microg ethinyl estradiol. Contraception 2006; 73 (4): 336–43
Winkler UH, Holscher T, Schulte H, et al. Ethinylestradiol 20 versus 30 micrograms combined with 150 micrograms desogestrel: a large comparative study of the effects of two low-dose oral contraceptives on the hemostatic system. Gynecol Endocrinol 1996; 10 (4): 265–71
Winkler UH, Schindler AE, Endrikat J, et al. A comparative study of the effects of the hemostatic system of two monophasic gestodene oral contraceptives containing 20 micrograms and 30 micrograms ethinylestradiol. Contraception 1996; 53 (2): 75–84
van der Mooren MJ, Klipping C, van Aken B, et al. A comparative study of the effects of gestodene 60 microg/ ethinylestradiol 15 microg and desogestrel 150 microg/ ethinylestradiol 20 microg on hemostatic balance, blood lipid levels and carbohydrate metabolism. Eur J Contracept Reprod Health Care 1999; 4 (Suppl. 2): 27–35
Gestodene Study Group 324. Cycle control, safety and efficacy of a 24-day regimen of gestodene 60 microg/ ethinylestradiol 15 microg and a 21-day regimen of desogestrel 150 microg/ethinylestradiol 20 microg. Eur J Contracept Reprod Health Care 1999; 4 Suppl. 2: 17–25
Acknowledgements
The study was funded by Bayer HealthCare Pharmaceuticals, Berlin, Germany, the manufacturer of Qlaira®/Natazia® (estradiol valerate/dienogest). Medical writing services from Lyndal Staples and Danielle Turner of inScience Communications, a Wolters Kluwer business, were funded by Bayer HealthCare Pharmaceuticals. CK and ID are directors of the company that performed the current study. SP, UM, and MS are employees of Bayer HealthCare Pharmaceuticals. WJ is medical director of the company that performed the laboratory analyses for the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Klipping, C., Duijkers, I., Parke, S. et al. Hemostatic Effects of a Novel Estradiol-Based Oral Contraceptive. Drugs R D 11, 159–170 (2011). https://doi.org/10.2165/11591200-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11591200-000000000-00000