Abstract
The UK National Institute for Health and Clinical Excellence (NICE) has used its Single Technology Appraisal (STA) programme to assess several drugs for cancer. Typically, the evidence submitted by the manufacturer comes from one short-term randomized controlled trial (RCT) demonstrating improvement in overall survival and/or in delay of disease progression, and these are the pre-eminent drivers of cost effectiveness.
We draw attention to key issues encountered in assessing the quality and rigour of the manufacturers’ modelling of overall survival and disease progression. Our examples are two recent STAs: sorafenib (Nexavar®) for advanced hepatocellular carcinoma, and azacitidine (Vidaza®) for higher-risk myelodysplastic syndromes (MDS).
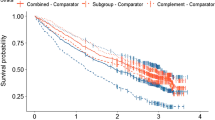
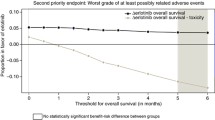
The choice of parametric model had a large effect on the predicted treatment-dependent survival gain. Logarithmic models (log-Normal and loglogistic) delivered double the survival advantage that was derived from Weibull models. Both submissions selected the logarithmic fits for their basecase economic analyses and justified selection solely on Akaike Information Criterion (AIC) scores. AIC scores in the azacitidine submission failed to match the choice of the log-logistic over Weibull or exponential models, and the modelled survival in the intervention arm lacked face validity. AIC scores for sorafenib models favoured log-Normal fits; however, since there is no statistical method for comparing AIC scores, and differences may be trivial, it is generally advised that the plausibility of competing models should be tested against external data and explored in diagnostic plots.
Function fitting to observed data should not be a mechanical process validated by a single crude indicator (AIC). Projective models should show clear plausibility for the patients concerned and should be consistent with other published information. Multiple rather than single parametric functions should be explored and tested with diagnostic plots. When trials have survival curves with long tails exhibiting few events then the robustness of extrapolations using information in such tails should be tested.






Similar content being viewed by others
References
National Institute for Health and Clinical Excellence. Guide to the single technology (STA) process. London: NICE, 2006 Sep 19 [online]. Available from URL: http://www.nice.org.uk/page.aspx?o=STAprocessguide [Accessed 2010 Sep 1]
Bagust A, Greenhalgh J, Boland A, et al. Cetuximab for recurrent and/or metastatic squamous cell carcinoma of the head and neck: a NICE single technology appraisal. Pharmacoeconomics 2010; 28 (6): 439–48
Tappenden P, Chilcott J, Ward S, et al. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006; 42: 2867–75
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–32
National Institute for Health and Clinical Excellence. Single technology appraisal of Vidaza® (azacitidine): manufacturer/sponsor submission of evidence. London: NICE, 2009 Mar 20 [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=download&o=45026 [Accessed 2010 Sep 1]
National Institute for Health and Clinical Excellence. Bayer HealthCare (Bayer Schering Pharma). Single technology appraisal (STA) of sorafenib (Nexavar®) for the treatment of hepatocellular carcinoma (HCC). London: NICE, 2009 Jan 14 [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=folder&o=44106 [Accessed 2010 Sep]
Connock M, Round J, Bayliss S, et al. Sorafenib for the treatment of advanced hepatocellular carcinoma. Health Technol Assess 2010; 14 Suppl. 1: 17–21
Edlin R, Connock M, Tubeuf S, et al. Azacitidine for the treatment of myelodysplastic syndrome, chronic myelomonocytic leukaemia and acute myeloid leukaemia. Health Technol Assess 2010; 14 Suppl. 1: 69–74
Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet 2002; 359: 1309–10
Machin D, Cheung YB, Parmar MKB. Survival analysis: a practical approach. 2nd ed. Chichester: John Wiley and Sons Ltd., 2006: 120–2
Hosmer Jr DW, Lemeshow S. Applied survival analysis regressionmodelling of time to event data. 1st ed. New York: John Wiley, 1999: 123–7
Acknowledgements
The authors acknowledge the very constructive suggestions of an anonymous referee.
This project stems from work funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project numbers 08/75/01 and 08/84/01). See the Health Technology Assessment programme website for further project information (http://www.hta.ac.uk). This summary of issues was compiled after the NICE Appraisal Committee’s review.
The views and opinions expressed are those of the authors and do not necessarily reflect those of NICE or the Department of Health, UK.
The authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connock, M., Hyde, C. & Moore, D. Cautions Regarding the Fitting and Interpretation of Survival Curves. Pharmacoeconomics 29, 827–837 (2011). https://doi.org/10.2165/11585940-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11585940-000000000-00000