Abstract
The myelodysplastic syndromes (MDS) are characterized by cytopenias and risk of progression to acute myeloid leukaemia (AML). Most MDS patients eventually require transfusion of red blood cells for anaemia, placing them at risk of transfusional iron overload. In β-thalassaemia major, transfusional iron overload leads to organ dysfunction and death; however, with iron chelation therapy, organ function is improved, and survival improved to near normal and correlated with the degree of compliance with chelation. In lower-risk MDS, several nonrandomized studies suggest an adverse effect of iron overload on survival and that lowering iron with chelation may minimize this impact. Emerging data indicate that chelation may improve organ function, particularly hepatic function, and a minority of patients may have improvement in cell counts and decreased transfusion requirements. While guidelines for MDS generally recommend chelation in selected lower-risk patients, data from nonrandomized trials suggest iron overload may impact adversely on the outcome of higher-risk MDS and stem cell transplantation (SCT). This effect may be due to increased transplant-related mortality, infection and AML progression, and preliminary data suggest that lowering iron may be beneficial in this patient group. Other areas of active and future investigation include optimizing the monitoring of iron overload using imaging such as T2* MRI and measures of labile iron and oxidative stress; correlating new methods of measuring iron to clinical outcomes; clarifying the contribution of different cellular and extracellular iron pools to iron toxicity; optimizing chelation by using agents that access the appropriate iron pools to minimize the relevant clinical consequences in individual patients; and incorporating measures of quality of life and co-morbidities into clinical trials of chelation in MDS. It should be noted that chelation is costly and potentially toxic, and in MDS should be initiated after weighing potential risks and benefits for each patient until more definitive data are available.
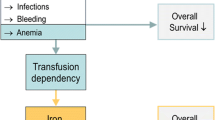
In this review, data on the impact of iron overload in MDS and SCT are discussed; for example, several noncontrolled studies show inferior survival in patients with iron overload in these clinical settings, including an increase in transplant-related mortality and infection risk. Possible mechanisms of iron toxicity include oxidative stress, which can damage cellular components, and the documented impact of lowering iron on organ function with measures such as iron chelation therapy includes an improvement in elevated liver transaminases. Lowering iron also appears to improve survival in both lower-risk MDS and SCT in nonrandomized studies. Selected aspects of iron metabolism, transport, storage and distribution that may be amenable to future intervention and improved removal of iron from important cellular sites are discussed, as are attempts to quantify quality of life and the importance of comorbidities in measures to treat MDS, including chelation therapy.








Similar content being viewed by others
References
Hellstrom-Lindberg E, Schmidt-Mende J, Forsblom AM, et al. Apoptosis in refractory anaemia with ringed sideroblasts is initiated at the stem cell level and associated with increased activation of caspases. Br J Haematol 2001; 112(3): 714–26
Epling-Burnette PK, List AF. Advancements in the molecular pathogenesis of myelodysplastic syndrome. Curr Opin Hematol 2009; 16(2): 70–6
Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89(6): 2079–88
Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25(23): 3503–10
Della Porta MG, Kuendgen A, Malcovati L, et al. Myelodysplastic syndrome (MDS)-specific comorbidity index for predicting the impact of extra-hematological comorbidities on survival of patients with MDS [abstract]. Blood 2008; 112(11): 925–6a
Kantarjian HM, O’Brien S, Ravandi F, et al. Development and validation of a new prognostic model for myelodysplastic syndrome (MDS) that accounts for events not considered by the International Prognostic Scoring System (IPSS) [abstract]. Blood 2008; 112(11): 236–7a
Schanz J, Tuechler H, Sole F, et al. Cytogenetic risk features in MDS: update and present state [abstract]. Blood 2009; 114(22): 1084a
Gondek LP, Tiu R, O’Keefe CL, et al. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood 2008; 111(3): 1534–42
Williamson PJ, Kruger AR, Reynolds PJ, et al. Establishing the incidence of myelodysplastic syndrome. Br J Haematol 1994; 87(4): 743–5
Schiffer CA. Clinical issues in the management of patients with myelodysplasia. Hematology Am Soc Hematol Educ Program 2006: 205–10
Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin+granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120(6): 1037–46
Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106(8): 1794–803
List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 2005; 352(6): 549–57
Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 2002; 20(10): 2429–40
Porter JB. Practical management of iron overload. Br J Haematol 2001; 115(2): 239–52
Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 2005; 23(30): 7594–603
Sanz G, Nomdedeu B, Such E, et al. Independent impact of iron overload and transfusion dependency on survival and leukemic evolution in patients with myelodysplastic syndrome. Blood 2008; 112(11): 238–9a
Olivieri NF, Nathan DG, MacMillan JH, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 1994; 331(9): 574–8
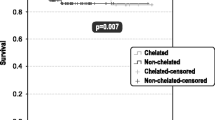
Leitch HA, Leger CS, Goodman TA, et al. Improved survival in patients with myelodysplastic syndrome receiving iron chelation therapy. Clin Leuk 2008; 2(3): 205–11
Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM. Leuk Res 2010; 34(7): 864–70
Fox F, Kündgen A, Nachtkamp K, et al. Matched-pair analysis of 186 MDS patients receiving iron chelation therapy or transfusion therapy only [abstract no. 1747]. Blood 2009; 114(22): 694a
Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 2008; 112(1): 45–52
Ma X, Does M, Raza A, et al. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 2007; 109(8): 1536–42
Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: results of six cross-sectional physician surveys. J Natl Cancer Inst 2008; 100(21): 1542–51
Gupta P, LeRoy SC, Luikart SD, et al. Long-term blood product transfusion support for patients with myelodys-plastic syndromes (MDS): cost analysis and complications. Leuk Res 1999; 23(10): 953–9
Alessandrino EP, Amadori S, Barosi G, et al. Evidence-and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes: a statement from the Italian Society of Hematology. Haematologica 2002; 87(12): 1286–306
Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 2003; 120(2): 187–200
Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006; 91(12): 1588–90
Brittenham GM, Griffith PM, Nienhuis AW, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med 1994; 331(9): 567–73
Davis BA, Porter JB. Long-term outcome of continuous 24-hour deferoxamine infusion via indwelling intravenous catheters in high-risk beta-thalassemia. Blood 2000; 95(4): 1229–36
Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol 1996; 95(1): 26–36
Delea TE, Hagiwara M, Phatak PD. Retrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disorders. Curr Med Res Opin 2009; 25(1): 139–47
Kao JM, McMillan A, Greenberg PL. International MDS risk analysis workshop (IMRAW)/IPSS reanalyzed: impact of cytopenias on clinical outcomes in myelodysplastic syndromes. Am J Hematol 2008; 83(10): 765–70
Raptis A, Duh MS, Wang ST, et al. Treatment of transfusional iron overload in patients with myelodysplastic syndrome or severe anemia: data from multicenter clinical practices. Transfusion 2010; 50(1): 190–9
Takatoku M, Uchiyama T, Okamoto S, et al. Retrospective nationwide survey of Japanese patients with transfusion-dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. Eur J Haematol 2007; 78(6): 487–94
Chee CE, Steensma DP, Wu W, et al. Neither serum ferritin nor the number of red blood cell transfusions affect overall survival in refractory anemia with ringed side-roblasts. Am J Hematol 2008; 83(8): 611–3
Leitch HA, Vickars LM. Supportive care and chelation therapy in MDS: are we saving lives or just lowering iron? Hematology Am Soc Hematol Educ Program 2009: 664–72
Leitch HA. Controversies surrounding iron chelation therapy for MDS. Blood Rev 2011; 25(1): 17–31
Cazzola M, Della Porta MG, Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program 2008: 166–75
Andrews NC. Understanding heme transport. N Engl J Med 2005; 353(23): 2508–9
Andrews NC. Forging a field: the golden age of iron biology. Blood 2008; 112(2): 219–30
Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program 2006: 29–35
Winder A, Lefkowitz R, Ghoti H, et al. Urinary hepcidin excretion in patients with myelodysplastic syndrome (MDS) and myelofibrosis (MF) [abstract]. Blood 2006; 108(11): 740a
Theil EC. Mining ferritin iron: 2 pathways. Blood 2009; 114(20): 4325–6
Cazzola M, Bergamaschi G, Dezza L, et al. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: achievements and prospects. Blood 1990; 75(10): 1903–19
Liu X, Theil EC. Ferritin as an iron concentrator and chelator target. Ann N Y Acad Sci 2005; 1054: 136–40
De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood 2009; 114(20): 4546–51
Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev 2009; 23(3): 95–104
Wood JC, Enriquez C, Ghugre N, et al. Physiology and pathophysiology of iron cardiomyopathy in thalassemia. Ann N Y Acad Sci 2005; 1054: 386–95
Britton RS, Leicester KL, Bacon BR. Iron toxicity and chelation therapy. Int J Hematol 2002; 76(3): 219–28
Chan LSA, Buckstein R, Reis MD, et al. Iron overload and haematopoiesis in MDS: does blood transfusion promote progression to AML? [abstract]. Blood 2008; 112(11): 928a
Liuzzi JP, Aydemir F, Nam H, et al. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A 2006; 103(37): 13612–7
Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 2003; 9(9): 1187–94
Gao X, Campian JL, Qian M, et al. Mitochondrial DNA damage in iron overload. J Biol Chem 2009; 284(8): 4767–75
Pullarkat V. Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood 2009; 114(26): 5251–5
Nearman ZP, Szpurka H, Serio B, et al. Hemochromatosis-associated gene mutations in patients with myelodysplastic syndromes with refractory anemia with ringed sideroblasts. Am J Hematol 2007; 82(12): 1076–9
Varkonyi J, Tarkovacs G, Karadi I, et al. High incidence of hemochromatosis gene mutations in the myelodysplastic syndrome: the Budapest Study on 50 patients. Acta Haematol 2003; 109(2): 64–7
Steensma DP, Viprakasit V, Hendrick A, et al. Deletion of the alpha-globin gene cluster as a cause of acquired alpha-thalassemia in myelodysplastic syndrome. Blood 2004; 103(4): 1518–20
Brittenham GM, Cohen AR, McLaren CE, et al. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am J Hematol 1993; 42(1): 81–5
Clark PR, St Pierre TG. Quantitative mapping of transverse relaxivity (1/T(2)) in hepatic iron overload: a single spin-echo imaging methodology. Magn Reson Imaging 2000; 18(4): 431–8
St Pierre TG, Clark PR, Chua-Anusorn W. Measurement and mapping of liver iron concentrations using magnetic resonance imaging. Ann N Y Acad Sci 2005; 1054: 379–85
Angelucci E, Brittenham GM, McLaren CE, et al. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med 2000; 343(5): 327–31
Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001; 22(23): 2171–9
Angelucci E, Muretto P, Nicolucci A, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood 2002; 100(1): 17–21
Jensen PD, Jensen FT, Christensen T, et al. Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood 2003; 101(11): 4632–9
Noetzli LJ, Carson SM, Nord AS, et al. Longitudinal analysis of heart and liver iron in thalassemia major. Blood 2008; 112(7): 2973–8
Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood 1997; 89(3): 739–61
Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004; 89(10): 1187–93
Davis BA, O’Sullivan C, Jarritt PH, et al. Value of sequential monitoring of left ventricular ejection fraction in the management of thalassemia major. Blood 2004; 104(1): 263–9
Kirk P, Roughton M, Porter JB, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009; 120(20): 1961–8
Telfer PT, Prestcott E, Holden S, et al. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol 2000; 110(4): 971–7
Jaeger M, Aul C, Sohngen D, et al. Secondary hemochromatosis in polytransfused patients with myelodysplastic syndromes [in German]. Beitr Infusionsther 1992; 30: 464–8
Schafer AI, Cheron RG, Dluhy R, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med 1981; 304(6): 319–24
Chacko J, Pennell DJ, Tanner MA, et al. Myocardial iron loading by magnetic resonance imaging T2* in good prognostic myelodysplastic syndrome patients on long-term blood transfusions. Br J Haematol 2007; 138(5): 587–93
Di Tucci AA, Matta G, Deplano S, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica 2008; 93(9): 1385–8
Konen E, Ghoti H, Goitein O, et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007; 82(11): 1013–6
Papakonstantinou O, Alexopoulou E, Economopoulos N, et al. Assessment of iron distribution between liver, spleen, pancreas, bone marrow, and myocardium by means of R2 relaxometry with MRI in patients with beta-thalassemia major. J Magn Reson Imaging 2009; 29(4): 853–9
Brittenham GM, Farrell DE, Harris JW, et al. Magnetic-susceptibility measurement of human iron stores. N Engl J Med 1982; 307(27): 1671–5
Esposito BP, Breuer W, Sirankapracha P, et al. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood 2003; 102(7): 2670–7
Breuer W, Ronson A, Slotki IN, et al. The assessment of serum nontransferrin-bound iron in chelation therapy and iron supplementation. Blood 2000; 95(9): 2975–82
Gosriwatana I, Loreal O, Lu S, et al. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem 1999; 273(2): 212–20
Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron: implications for non-transferrin-bound iron speciation. Biochim Biophys Acta 2009; 1794(10): 1449–58
Evans RW, Rafique R, Zarea A, et al. Nature of non-transferrin-bound iron: studies on iron citrate complexes and thalassemic sera. J Biol Inorg Chem 2008; 13(1): 57–74
Greenberg PL, Koller CA, Cabantchik ZI, et al. Prospective assessment of effects on iron-overload parameters of deferasirox therapy in patients with myelodysplastic syndromes. Leuk Res 2010; 34(12): 1560–5
Angelluci E. Removal of cardiac iron in MDS. 2008 Global Iron Summit; 2008 Nov 7–8; Athens
Leitch HA, Goodman TA, Wong KK, et al. Improved survival in patients with myelodysplastic syndrome (MDS) receiving iron chelation therapy [abstract]. Blood 2006; 108: 78a
Leitch HA. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk Res 2007; 31 Suppl. 3: S7–9
Rose C, Brechignac S, Vassilief D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly transfused MDS patients: a prospective analysis by the GFM. Blood 2007; 110(11): 80–1a
Novartis. Myelodysplastic syndromes (MDS) event free survival with iron chelation therapy study [ClinicalTrials.gov identifier NCT00940602]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Dec 11]
Mathew P, Tefferi A, Dewald GW, et al. The 5q-syndrome: a single-institution study of 43 consecutive patients. Blood 1993; 81(4): 1040–5
Cazzola M, Barosi G, Gobbi PG, et al. Natural history of idiopathic refractory sideroblastic anemia. Blood 1988; 71(2): 305–12
Goldberg SL, Chen E, Corral M, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol 2010; 28(17): 2847–52
Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood 2007; 109(11): 4663–70
Oliva EN, Dimitrov BD, Benedetto F, et al. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk Res 2005; 29(10): 1217–9
Aldouri MA, Wonke B, Hoffbrand AV, et al. High incidence of cardiomyopathy in beta-thalassaemia patients receiving regular transfusion and iron chelation: reversal by intensified chelation. Acta Haematol 1990; 84(3): 113–7
Anderson LJ, Westwood MA, Holden S, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol 2004; 127(3): 348–55
Huang YC, Chang JS, Wu KH, et al. Regression of myocardial dysfunction after switching from desferrioxamine to deferiprone therapy in beta-thalassemia major patients. Hemoglobin 2006; 30(2): 229–38
Miskin H, Yaniv I, Berant M, et al. Reversal of cardiac complications in thalassemia major by long-term intermittent daily intensive iron chelation. Eur J Haematol 2003; 70(6): 398–403
Tanner MA, Galanello R, Dessi C, et al. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson 2008; 10: 12
Taher A, Sheikh-Taha M, Koussa S, et al. Comparison between deferoxamine and deferiprone (L1) in iron-loaded thalassemia patients. Eur J Haematol 2001; 67(1): 30–4
Gattermann N, Finelli C, Porta MD, et al. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res 2010; 34(9): 1143–50
Del Río Garma J, Fernandez LC, Fonrodona BFJ. Desferrioxamine in the treatment of myelodysplastic syndromes. Haematologica 1997; 82(5): 639–40
Jensen PD, Heickendorff L, Pedersen B, et al. The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol 1996; 94(2): 288–99
Jensen PD, Jensen IM, Ellegaard J. Desferrioxamine treatment reduces blood transfusion requirements in patients with myelodysplastic syndrome. Br J Haematol 1992; 80(1): 121–4
Messa E, Cilloni D, Messa F, et al. Deferasirox treatment improved the hemoglobin level and decreased transfusion requirements in four patients with the myelodysplastic syndrome and primary myelofibrosis. Acta Haematol 2008; 120(2): 70–4
Okabe H, Suzuki T, Omori T, et al. Hematopoietic recovery after administration of deferasirox for transfusional iron overload in a case of myelodysplastic syndrome. Rinsho Ketsueki 2009; 50(11): 1626–9
Badawi MA, Vickars LM, Chase JM, et al. Red blood cell transfusion independence following the initiation of iron chelation therapy in myelodysplastic syndrome. Adv Hematol 2010; 2010: 164045
Smeets ME, Vreugdenhil G, Holdrinet RS. Improvement of erythropoiesis during treatment with deferiprone in a patient with myelofibrosis and transfusional hemosiderosis. Am J Hematol 1996; 51(3): 243–4
Di Tucci AA, Murru R, Alberti D, et al. Correction of anemia in a transfusion-dependent patient with primary myelofibrosis receiving iron chelation therapy with deferasirox (Exjade, ICL670). Eur J Haematol 2007; 78(6): 540–2
Park SJ, Han CW. Complete hematopoietic recovery after continuous iron chelation therapy in a patient with severe aplastic anemia with secondary hemochromatosis. J Korean Med Sci 2008; 23(2): 320–3
Ghoti H, Amer J, Winder A, et al. Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur J Haematol 2007; 79(6): 463–7
Rachmilewitz E, Merkel D, Ghoti H, et al. Improvement of oxidative stress parameters in MDS patients with iron overload treated with deferasirox [abstract]. Blood 2008; 112(11): 924–5a
Szuber N, Buss JL, Soe-Lin S, et al. Alternative treatment paradigm for thalassemia using iron chelators. Exp Hematol 2008; 36(7): 773–85
Cortelezzi A, Cattaneo C, Sarina B, et al. Efficacy of N-acetylcysteine and all-trans retinoic acid in restoring in vitro effective hemopoiesis in myelodysplastic syndromes. Leuk Res 2000; 24(2): 129–37
Messa E, Defilippi I, Roetto A, et al. Deferasirox is the only iron chelator acting as a potent NFKB inhibitor in myelodysplastic syndromes [abstract]. Blood 2008; 112(11): 923a
Ren X, Dorrington KL, Maxwell PH, et al. Effects of desferrioxamine on serum erythropoietin and ventilatory sensitivity to hypoxia in humans. J Appl Physiol 2000; 89(2): 680–6
Alessandrino EP, Della Porta MG, Bacigalupo A, et al. Prognostic impact of pre-transplantation transfusion history and secondary iron overload in patients with myelodysplastic syndrome undergoing allogeneic stem cell transplantation: a GITMO study. Haematologica 2010; 95(3): 476–84
Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007; 109(10): 4586–8
Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2008; 42(12): 799–805
Armand P, Kim HT, Cutler CS, et al. A prognostic score for patients with acute leukemia or myelodysplastic syndromes undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14(1): 28–35
Platzbecker U, Bornhauser M, Germing U, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS). Biol Blood Marrow Transplant 2008; 14(11): 1217–25
Sorror ML, Storer BE, Schoch G, et al. Low albumin, high ferritin, and thrombocytopenia before transplant predict non-relapse mortality (NRM) independent of the Hematopoietic Cell Transplantation Comorbidity Index (HCTCI) [abstract]. Blood 2009; 114(22): 271a
Altes A, Remacha AF, Sarda P, et al. Early clinical impact of iron overload in stem cell transplantation: a prospective study. Ann Hematol 2007; 86(6): 443–7
Mahindra A, Bolwell B, Sobecks R, et al. Elevated ferritin is associated with relapse after autologous hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transplant 2008; 14(11): 1239–44
Kim YR, Kim JS, Cheong JW, et al. Transfusion-associated iron overload as an adverse risk factor for transplantation outcome in patients undergoing reduced-intensity stem cell transplantation for myeloid malignancies. Acta Haematol 2008; 120(3): 182–9
Mahindra A, Sobecks R, Rybicki L, et al. Elevated pretransplant serum ferritin is associated with inferior survival following nonmyeloablative allogeneic transplantation. Bone Marrow Transplant 2009; 44(11): 767–8
Maradei SC, Maiolino A, de Azevedo AM, et al. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood 2009; 114(6): 1270–5
Ozyilmaz E, Aydogdu M, Sucak G, et al. Risk factors for fungal pulmonary infections in hematopoietic stem cell transplantation recipients: the role of iron overload. Bone Marrow Transplant 2010; 45(10): 1528–33
Iqbal M, Creger RJ, Fox RM, et al. Laparoscopic liver biopsy to evaluate hepatic dysfunction in patients with hematologic malignancies: a useful tool to effect changes in management. Bone Marrow Transplant 1996; 17(4): 655–62
Azar N, Valla D, Abdel-Samad I, et al. Liver dysfunction in allogeneic bone marrow transplantation recipients. Transplantation 1996; 62(1): 56–61
Alessandrino EP, Della Porta MG, Bacigalupo A, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 2008; 112(3): 895–902
Lee JW, Kang HJ, Kim EK, et al. Effect of iron overload and iron-chelating therapy on allogeneic hematopoietic SCT in children. Bone Marrow Transplant 2009; 44(12): 793–7
Altes A, Remacha AF, Sureda A, et al. Iron overload might increase transplant-related mortality in haematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 29(12): 987–9
Kanamori H, Tachibana T, Takasaki H, et al. Elevated serum ferritin predicts the delay of engraftment and high incidence of blood stream infection within 100 days after allogeneic hematopoietic stem cell transplantation [abstract]. Blood 2009; 114: 467a
Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15(2): 195–204
Mattiuzi G, Amin HM, Kantarjian H, et al. Baseline serum ferritin predicts rate of infection in patients with acute myelogenous leukemia and high-risk myelodysplastic syndrome [abstract]. Blood 2009; 114(22): 644a
Sahlstedt L, Ebeling F, von Bonsdorff L, et al. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol 2001; 113(3): 836–8
Sahlstedt L, von Bonsdorff L, Ebeling F, et al. Non-transferrin-bound iron in haematological patients during chemotherapy and conditioning for autologous stem cell transplantation. Eur J Haematol 2009; 83(5): 455–9
Carmine TC, Evans P, Bruchelt G, et al. Presence of iron catalytic for free radical reactions in patients undergoing chemotherapy: implications for therapeutic management. Cancer Lett 1995; 94(2): 219–26
Gordon LI, Brown SG, Tallman MS, et al. Sequential changes in serum iron and ferritin in patients undergoing high-dose chemotherapy and radiation with autologous bone marrow transplantation: possible implications for treatment related toxicity. Free Radic Biol Med 1995; 18(3): 383–9
Harrison P, Marwah SS, Hughes RT, et al. Non-transferrin bound iron and neutropenia after cytotoxic chemotherapy. J Clin Pathol 1994; 47(4): 350–2
Halliwell B, Aruoma OI, Mufti G, et al. Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy: therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett 1988; 241(1–2): 202–4
Cetin T, Arpaci F, Yilmaz MI, et al. Oxidative stress in patients undergoing high-dose chemotherapy plus peripheral blood stem cell transplantation. Biol Trace Elem Res 2004; 97(3): 237–47
Wayner DD, Burton GW, Ingold KU, et al. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta 1987; 924(3): 408–19
Evens AM, Mehta J, Gordon LI. Rust and corrosion in hematopoietic stem cell transplantation: the problem of iron and oxidative stress. Bone Marrow Transplant 2004; 34(7): 561–71
Kontoghiorghes GJ, Weinberg ED. Iron: mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev 1995; 9(1): 33–45
van Asbeck BS, Marx JJ, Struyvenberg A, et al. Functional defects in phagocytic cells from patients with iron overload. J Infect 1984; 8(3): 232–40
Hole PS, Pearn L, Tonks AJ, et al. Ras-induced reactive oxygen species promote growth factor-independent proliferation in human CD34+ hematopoietic progenitor cells. Blood 2010; 115(6): 1238–46
Rassool FV, Gaymes TJ, Omidvar N, et al. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res 2007; 67(18): 8762–71
Hann HW, Stahlhut MW, Blumberg BS. Iron nutrition and tumor growth: decreased tumor growth in iron-deficient mice. Cancer Res 1988; 48(15): 4168–70
Yuan J, Lovejoy DB, Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective anti-tumor activity: in vitro and in vivo assessment. Blood 2004; 104(5): 1450–8
Jiang Y, Xue ZH, Shen WZ, et al. Desferrioxamine induces leukemic cell differentiation potentially by hypoxia-inducible factor-1 alpha that augments transcriptional activity of CCAAT/enhancer-binding protein-alpha. Leukemia 2005; 19(7): 1239–47
Eberhard Y, McDermott SP, Wang X, et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood 2009; 114(14): 3064–73
Fu D, Richardson DR. Iron chelation and regulation of the cell cycle: 2 mechanisms of posttranscriptional regulation of the universal cyclin-dependent kinase inhibitor p21CIP1/WAF1 by iron depletion. Blood 2007; 110(2): 752–61
Liang SX, Richardson DR. The effect of potent iron chelators on the regulation of p53: examination of the expression, localization and DNA-binding activity of p53 and the transactivation of WAF 1. Carcinogenesis 2003; 24(10): 1601–14
Ohyashiki JH, Kobayashi C, Hamamura R, et al. The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD 1. Cancer Sci 2009; 100(5): 970–7
Richardson DR. Molecular mechanisms of iron uptake by cells and the use of iron chelators for the treatment of cancer. Curr Med Chem 2005; 12(23): 2711–29
Greene BT, Thorburn J, Willingham MC, et al. Activation of caspase pathways during iron chelator-mediated apoptosis. J Biol Chem 2002; 277(28): 25568–75
Callens C, Coulon S, Naudin J, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med 2010; 207(4): 731–50
Busca A, Falda M, Manzini P, et al. Iron overload in patients receiving allogeneic hematopoietic stem cell transplantation: quantification of iron burden by a superconducting quantum interference device (SQUID) and therapeutic effectiveness of phlebotomy. Biol Blood Marrow Transplant 2010; 16(1): 115–22
Majhail NS, Lazarus HM, Burns LJ. A prospective study of iron-overload management in allogeneic hematopoietic-cell transplant survivors. Biol Blood Marrow Transplant 2010; 16(6): 832–7
Rose C, Ernst O, Hecquet B, et al. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (SCT). Haematologica 2007; 92(6): 850–3
Kamble RT, Selby GB, Mims M, et al. Iron overload manifesting as apparent exacerbation of hepatic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12(5): 506–10
McKay PJ, Murphy JA, Cameron S, et al. Iron overload and liver dysfunction after allogeneic or autologous bone marrow transplantation. Bone Marrow Transplant 1996; 17(1): 63–6
Tomas JF, Pinilla I, Garcia-Buey ML, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant 2000; 26(6): 649–55
Brown SA, Goringe A, Fegan C, et al. Parenteral glutamine protects hepatic function during bone marrow transplantation. Bone Marrow Transplant 1998; 22(3): 281–4
Colombo AA, Alessandrino EP, Bernasconi P, et al. N-acetylcysteine in the treatment of steroid-resistant acute graft-versus-host-disease: preliminary results: Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Transplantation 1999; 68(9): 1414–6
Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol 2008; 83(11): 858–61
Gattermann N, Porter J, Lopes LF, et al. Consensus statement on iron overload in myelodysplastic syndromes. Hematol Oncol Clin North Am 2005; 19 Suppl. 1: 18–25
Novartis. Efficacy and safety of oral deferasirox (20 mg/kg/d) in pts 3 to 6 months after allogeneic hematopoietic cell transplantation who present with iron overload [ClinicalTrials.gov identifier NCT00654589]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Dec 10]
Greenberg PL, Baer MR, Bennett JM, et al. Myelodys-plastic syndromes clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006; 4(1): 58–77
Mittelman M, Lugassy G, Merkel D, et al. Iron chelation therapy in patients with myelodysplastic syndromes: consensus conference guidelines. Isr Med Assoc J 2008; 10(5): 374–6
Suzuki T, Tomonaga M, Miyazaki Y, et al. Japanese epidemiological survey with consensus statement on Japanese guidelines for treatment of iron overload in bone marrow failure syndromes. Int J Hematol 2008; 88(1): 30–5
Valent P, Krieger O, Stauder R, et al. Iron overload in myelodysplastic syndromes (MDS)-diagnosis, management, and response criteria: a proposal of the Austrian MDS platform. Eur J Clin Invest 2008; 38(3): 143–9
Wells RA, Leber B, Buckstein R, et al. Iron overload in myelodysplastic syndromes: a Canadian consensus guideline. Leuk Res 2008; 32(9): 1338–53
Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol 2008; 88(1): 24–9
Santini V, Alessandrino PE, Angelucci E, et al. Clinical management of myelodysplastic syndromes: update of SIE, SIES, GITMO practice guidelines. Leuk Res 2010; 34(12): 1576–88
Giagounidis A, Heptinstall K, Jasmin D, et al. Detection and treatment of iron overload in red blood cell (RBC) transfusion-dependent myelodysplastic syndromes (MDS): a European survey [abstract]. Blood 2009; 114: 4830
Remacha AF, Arrizabalaga B, Del Canizo C, et al. Iron overload and chelation therapy in patients with low-risk myelodysplastic syndromes with transfusion requirements. Ann Hematol 2010; 89(2): 147–54
Cohen AR. New advances in iron chelation therapy. Hematology Am Soc Hematol Educ Program 2006: 42–7
Porter JB, Jaswon MS, Huehns ER, et al. Desferrioxamine ototoxicity: evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. Br J Haematol 1989; 73(3): 403–9
Davis BA, Porter JB. Results of long term iron chelation treatment with deferoxamine. Adv Exp Med Biol 2002; 509: 91–125
Cohen AR, Glimm E, Porter JB. Effect of transfusional iron intake on response to chelation therapy in beta-thalassemia major. Blood 2008; 111(2): 583–7
Vichinsky E. Clinical application of deferasirox: practical patient management. Am J Hematol 2008; 83(5): 398–402
Borgna-Pignatti C, Franchini M, Gandini G, et al. Subcutaneous bolus injection of deferoxamine in adult patients affected by onco-hematologic diseases and iron overload. Haematologica 1998; 83(9): 788–90
Franchini M, Gandini G, de Gironcoli M, et al. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload. Blood 2000; 95(9): 2776–9
Franchini M, Gandini G, Veneri D, et al. Safety and efficacy of subcutaneous bolus injection of deferoxamine in adult patients with iron overload: an update. Blood 2004; 103(2): 747–8
Borgna-Pignatti C, Cohen A. Evaluation of a new method of administration of the iron chelating agent deferoxamine. J Pediatr 1997; 130(1): 86–8
Di Gregorio F, Romeo MA, Pizzarelli G, et al. An alternative to continuous subcutaneous infusion of desferrioxamine in thalassaemic patients. Br J Haematol 1997; 98(3): 601–2
Jensen PD, Jensen FT, Christensen T, et al. Evaluation of transfusional iron overload before and during iron chelation by magnetic resonance imaging of the liver and determination of serum ferritin in adult non-thalassaemic patients. Br J Haematol 1995; 89(4): 880–9
Porter JB, Srichairatanakool S, Nathan DG, et al. Relative efficacy of subcutaneous infusion and bolus injections of deferoxamine in the removal of non-transferrin-bound iron (TBI) [abstract]. Blood 1998; 92 Suppl. 1: 668a
Yarali N, Fisgin T, Duru F, et al. Subcutaneous bolus injection of deferoxamine is an alternative method to subcutaneous continuous infusion. J Pediatr Hematol Oncol 2006; 28(1): 11–6
Porter JB, Rafique R, Srichairatanakool S, et al. Recent insights into interactions of deferoxamine with cellular and plasma iron pools: implications for clinical use. Ann N Y Acad Sci 2005; 1054: 155–68
Toliyat T, Jorjani M, Khorasanirad Z. An extended-release formulation of desferrioxamine for subcutaneous administration. Drug Deliv 2009; 16(7): 416–21
Glickstein H, El RB, Shvartsman M, et al. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood 2005; 106(9): 3242–50
Shvartsman M, Kikkeri R, Shanzer A, et al. Non-transferrin-bound iron reaches mitochondria by a chelator-inaccessible mechanism: biological and clinical implications. Am J Physiol Cell Physiol 2007; 293(4): C1383–94
Glickstein H, El RB, Link G, et al. Action of chelators in iron-loaded cardiac cells: Accessibility to intracellular labile iron and functional consequences. Blood 2006; 108(9): 3195–203
Le NT, Richardson DR. Iron chelators with high anti-proliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 2004; 104(9): 2967–75
Lovejoy DB, Richardson DR. Iron chelators as anti-neo-plastic agents: current developments and promise of the PIH class of chelators. Curr Med Chem 2003; 10(12): 1035–49
Whitnall M, Howard J, Ponka P, et al. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A 2006; 103(40): 14901–6
Pinchon DJ, Stanworth SJ, Doree C, et al. Quality of life and use of red cell transfusion in patients with myelodys-plastic syndromes: a systematic review. Am J Hematol 2009; 84(10): 671–7
Jansen AJ, Essink-Bot ML, Beckers EA, et al. Quality of life measurement in patients with transfusion-dependent myelodysplastic syndromes. Br J Haematol 2003; 121(2): 270–4
Steensma DP, Heptinstall KV, Johnson VM, et al. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): results of a large internet-based survey. Leuk Res 2008; 32(5): 691–8
Gabrilove J, Paquette R, Lyons RM, et al. Phase 2, singlearm trial to evaluate the effectiveness of darbepoetin alfa for correcting anaemia in patients with myelodysplastic syndromes. Br J Haematol 2008; 142(3): 379–93
Buckstein R, Alibhai S, Lam A, et al. Transfusion dependence and low hemoglobin have the greatest impact on quality of life (QOL) in MDS patients: a tertiary care cross sectional and longitudinal study. Blood 2009; 114(22): 986–7a
Wang R, Gross CP, Halene S, et al. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res 2009; 33(12): 1594–8
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–83
Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–9
Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol 2007; 25(27): 4246–54
Zipperer E, Pelz D, Nachtkamp K, et al. The hematopoietic stem cell transplantation comorbidity index is of prognostic relevance for patients with myelodysplastic syndrome. Haematologica 2009; 94(5): 729–32
Sperr WR, Wimazal F, Kundi M, et al. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann Oncol 2010; 21(1): 114–9
Della Porta MG, Malcovati L. Clinical relevance of extra-hematologic comorbidity in the management of patients with myelodysplastic syndrome. Haematologica 2009; 94(5): 602–6
Frytak JR, Henk HJ, De Castro CM, et al. Estimation of economic costs associated with transfusion dependence in adults with MDS. Curr Med Res Opin 2009; 25(8): 1941–51
Karnon J, Tolley K, Oyee J, et al. Cost-utility analysis of deferasirox compared to standard therapy with desferrioxamine for patients requiring iron chelation therapy in the United Kingdom. Curr Med Res Opin 2008; 24(6): 1609–21
Tolley K, Oliver N, Miranda E, et al. Cost effectiveness of deferasirox compared to desferrioxamine in the treatment of iron overload in lower-risk, transfusion-dependent myelodysplastic syndrome patients. J Med Econ 2010; 13(3): 559–70
Leitch HA. Iron chelation therapy in MDS: does it improve survival? Leuk Res 2010; 34(7): 852–3
Leitch HA, Chase JM, Goodman TA, et al. Improved survival in red blood cell transfusion dependent patients with primary myelofibrosis (PMF) receiving iron chelation therapy. Hematol Oncol 2010; 28(1): 40–8
Leitch HA. Delineating parameters of iron overload in MDS patients treated with deferasirox. Leuk Res 2010; 34(12): 1556–7
Acknowledgements
Dr Linda M. Vickars is the long-term Director of the Home Hemosiderosis Program of the province of British Columbia (BC). Her efforts on behalf of BC patients requiring iron chelation therapy have made this review and other studies possible.[19,37,38,86,87,107,216–218]
The author is grateful to Dr Rena Buckstein of the Myelodysplastic Syndromes Program, Odette Cancer Centre, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada. Much of the section on quality of life and co-morbidities in MDS was summarized from a grant proposal prepared by Dr Buckstein.
Conflict of interest: The author has received research funding and honoraria from Novartis Canada.
Off-label use of medications: Off-label use of deferiprone and deferasirox are discussed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leitch, H.A. Optimizing Therapy for Iron Overload in the Myelodysplastic Syndromes. Drugs 71, 155–177 (2011). https://doi.org/10.2165/11585280-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11585280-000000000-00000