Abstract
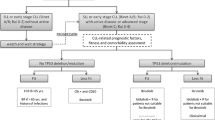
Chronic lymphocytic leukaemia (CLL) is the most common adult leukaemia in Europe and North America. The disease is characterized by proliferation and accumulation of small CD5+ B cells in blood, lymph nodes, spleen, liver and bone marrow. The natural clinical course of CLL is highly variable, and chemotherapy is usually not indicated in early and stable disease. However, patients with progressive and more advanced CLL require treatment. For many years, chlorambucil with or without corticosteroids was used in previously untreated patients with CLL. More recently, purine nucleoside analogues (PNAs) [fludarabine, cladribine and pentostatin] have been included in treatment approaches for this disease, and chlorambucil is no longer the leading standard everywhere. Currently, this drug is rather recommended for the treatment of older, unfit patients with co-morbidities, especially in European countries. Significantly higher overall response (OR) and complete response (CR) rates in patients treated initially with PNAs than in those treated with chlorambucil or cyclophosphamide-based combination regimens have been confirmed in randomized, prospective, multicentre trials. Moreover, PNAs administered in combination with cyclophosphamide produce higher response rates, including CR and molecular CR, compared with PNA as monotherapy.
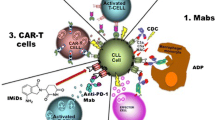
Recent reports suggest that the administration of monoclonal antibodies (mAbs) can significantly improve the course of CLL. At present, two mAbs have the most important clinical value in patients with CLL. The first is rituximab, a human mouse antibody that targets CD20 antigens, and the second is alemtuzumab, a humanized form of a rat antibody active against CD52. Several recent reports suggest that in patients with CLL, rituximab combined with a PNA can increase the OR and CR rates compared with PNA or rituximab alone, with acceptable toxicity. In randomized trials, the combination of rituximab with fludarabine and cyclophosphamide (FC-R regimen) demonstrated higher rates of OR, CR and progression-free survival in patients with previously untreated and relapsed or refractory CLL than fludarabine plus cyclophosphamide (FC regimen). Several reports have confirmed significant activity with alemtuzumab in relapsed or refractory CLL, as well as in previously untreated patients.
Recently, several new agents have been investigated and have shown promise in treating patients with CLL. These treatments include new mAbs, agents targeting the antiapoptotic bcl-2 family of proteins and receptors involved in mediating survival signals from the microenvironment, antisense oligonucleotides and other agents. The most promising are new mAbs directed against the CD20 molecule, lumiliximab and anti-CD40 mAbs. Oblimersen, alvocidib (flavopiridol) and lenalidomide are also being evaluated both in preclinical studies and in early clinical trials. In recent years, a significant improvement in haematopoietic stem cell transplantation (HSCT) procedures in patients with high-risk CLL has been observed. However, the exact role of HSCT, autologous or allogeneic, in the standard management of CLL patients is still undefined.



Similar content being viewed by others
References
Cavenagh JD, Lister TA. Chronic lymphocyte leukemia: diagnosis and management. In: Schiller GJ, editor. Chronic leukemias and lymphomas: biology, pathophysiology, and clinical management. Totowa (NJ): Humana Press Inc., 2003: 23–53
Redaelli A, Laskin BL, Redaelli A, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004; 13(3): 279–87
American Cancer Society. Cancer facts and figures 2008 [online]. Available from URL: http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf [Accessed 2009 Aug 26]
Mauro FR, Foa R. Young patients with chronic lymphocytic leukemia. In: Faguet GB, editor. Chronic lymphocytic leukemia: molecular genetics, biology, diagnosis, and management. Totowa (NJ): Humana Press Inc., 2004: 401–13
Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981; 48(1): 198–206
Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975; 46(2): 219–34
Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999 Sep 15; 94(6): 1840–7
Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999; 94(6): 1848–54
Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343(26): 1910–6
Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003; 348(18): 1764–75
Weinberg JB, Volkheimer AD, Chen Y, et al. Clinical and molecular predictors of disease severity and survival in chronic lymphocytic leukemia. Am J Hematol 2007; 82(12): 1063–70
Inamdar KV, Bueso-Ramos CE. Pathology of chronic lymphocytic leukemia: an update. Ann Diagn Pathol 2007; 11(5): 363–89
Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87(12): 4990–7
Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111(12): 5446–56
Del Giudice I, Chiaretti S, Tavolaro S, et al. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood 2009; 114(3): 638–46
Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. N Engl J Med 1998; 338(21): 1506–14
CLL Trialsts Collaborative Group. Chemotherapeutic options in chronic lymphocytic leukaemia: a meta-analysis of the randomized trials. J Natl Cancer Inst 1999 May 19; 91(10): 861–8
Eichhorst B, Hallek M, Dreyling M; ESMO Guidelines Working Group. Chronic lymphocytic leukemia: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008; 19 Suppl. 2: 60–2
Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med 1995; 333(16): 1052–7
Robak T. Recent progress in the management of chronic lymphocytic leukemia. Cancer Treat Rev 2007; 33(8): 710–8
Wendtner CM, Eichhorst BF, Hallek MJ. Advances in chemotherapy for chronic lymphocytic leukemia. Semin Hematol 2004; 41(3): 224–33
Nabhan C, Gartenhaus RB, Tallman MS. Purine nucleoside analogues and combination therapies in B-cell chronic lymphocytic leukemia: dawn of a new era. Leuk Res 2004; 28(5): 429–42
Robak T. Therapy of chronic lymphocytic leukemia with purine nucleoside analogues: facts and controversies. Drugs Aging 2006; 22(12): 985–1012
Oscier D, Fegan C, Hillmen P, et al. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Hematol 2004; 125(3): 294–317
Johnson S, Smith AG, Loffter H, et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin and prednisone (CAP) for treatment of advanced stage chronic lymphocytic leukemia. The French Cooperative Group on CLL. Lancet 1996; 347(9013): 1432–8
Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000; 343(24): 1750–7
Leporrier M, Chevret S, Cazin B, et al. Randomized comparison of fludarabine, CAP and CHOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood 2001; 98(8): 2319–25
Robak T, Błoński JZ, Kasznicki M, et al. Cladribine with prednisone versus chlorambucil with prednisone as first-line therapy in chronic lymphocytic leukemia report of a prospective, randomized, multicenter trial. Blood 2000; 96(8): 2723–9
Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006; 107(3): 885–91
Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E 2997. J Clin Oncol 2007; 25(7): 793–8
Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007; 370(9583): 230–9
Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first line therapy for patients requiring treatment for chronic lymphocytic leukemia. J Clin Oncol 2007; 25(35): 5616–23
Robak T. Monoclonal antibodies in the treatment of chronic lymphoid leukemias. Leuk Lymphoma 2004; 45(2): 205–19
Jaksic B, Brugiatelli M, Krc I, et al. High dose chlorambucil versus Binet’s modified cyclophosphamide, doxorubicin, vincristine and prednisone regimen in the treatment of patients with advanced B-cell chronic lymphocytic leukemia: results of an international multi-center randomized trial. Cancer 1997; 79(11): 2107–14
Robak T, Błoński JZ, Kasznicki M, et al. Comparison of cladribine plus prednisone with chlorambucil plus prednisone in patients with chronic lymphocytic leukemia: final report of the Polish Adult Leukemia Group (PALG CLL1). Med Sci Monit 2005; 11(10): 171–9
Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009; 50(2): 171–8
Eichhorst BF, Busch R, Stilgenbauer S, et al. First line therapy with fludarabine compared to chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocyticleukemia. Blood 2009; 114(16): 3382–91
Knauf WU, Lissichkov T, Aldaound A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009; 27(26): 4378–84
Robak T, Kasznicki M. Alkylating agents and nucleoside analogues in the treatment of B-cell chronic lymphocytic leukemia. Leukemia 2002; 16: 1015–27
French Cooperative Group on Chronic Lymphocytic Leukemia. A randomized clinical trial of chlorambucil versus COP in stage B chronic lymphocytic leukemia. Blood 1990; 75(7): 1422–5
Raphael B, Andersen JW, Silber R, et al. Comparison of chlorambucil and prednisone versus cyclophosphamide, vincristine and prednisone as initial treatment for chronic lymphocytic leukemia: long-term follow-up of an Eastern Cooperative Oncology Group randomized clinial trial. J Clin Oncol 1991; 9(5): 770–6
Kimby E, Melstedt H. Chlorambucil/prednisone versus CHOP in symptomatic chronic lymphocytic leukemias of B-cell type: a randomized trial. Leuk Lymphoma 1991; 5 Suppl. 1: 93–6
Hansen MM, Andersen E, Birgeus H, et al. CHOP versus prednisone+chlorambucil in chronic lymphocytic leukemia: preliminary results of a randomized multicenter study. Nouv Rev Fr Hematol 1998; 30(5–6): 433–6
French Cooperative Group on Chronic Lymphocytic Leukemia. Long term results of CHOP regimen in stage C chronic lymphocytic leukemia. Br J Haematol 1989; 73(3): 334–40
Knauf W. Bendamustine in the treatment of chronic lymphocytic leukemia. Expert Rev Anticancer Ther 2009; 9(2): 165–74
Kalaycio M. Bendamustine: a new look at an old drug. Cancer 2009; 115(3): 473–9
Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 2008; 14(1): 309–17
Keating MJ, O’Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood 1998; 92(4): 1165–71
Steurer M, Pall G, Richards S, et al. Single agent purine analogues for the treatment of chronic lymphocytic leukemia: a systematic review and meta analysis. Cancer Treat Rev 2006; 32(5): 377–89
Rossi JF, van Hoof A, De Boeck K, et al. Efficacy and safety of oral fludarabine phosphate in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2004; 22(7): 1260–7
Boogaerts MA. Oral fludarabine therapy in chronic lymphocytic leukemia-increased convenience. Hematology J 2004; 5 Suppl. 1: S31–7
Robak T, Bloñski JZ, Kasznicki M, et al. Cladribine with or without prednisone in the treatment of previously treated and untreated B-cell chronic lymphocytic leukemia: updated results of the multicentre study of 378 patients. Br J Haematol 2000; 108(2): 357–68
Karlsson KA, Strömberg M, Jönsson V, et al. Cladribine (CdA) gives longer response duration than fludarabine (F) and high-dose intermittent chlorambucil (Chl) as first-line treatment of symptomatic chronic lymphocytic leukemia (CLL). First results from the international randomized phase III trial [abstract no. 630]. Blood 2007; 110(11): 194
O’Brien SM, Kantarjian HM, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol 2001; 19(5): 1414–20
Hallek M, Schmitt B, Wilhelm M, et al. Fludarabine plus cyclophosphamide is an efficient treatment for advanced chronic lymphocytic leukaemia (CLL): results of a phase II study of the German CLL Study Group. Br J Haematol 2001; 114(2): 342–8
Robak T, Blonski JZ, Gora-Tybor J, et al. Cladribine alone and in combination with cyclophosphamide or cyclophosphamide plus mitoxantrone in the treatment of progressive chronic lymphocytic leukemia: report of prospective, multi-center, randomized trial of the Polish Adult Leukemia Group (PALG CLL2). Blood 2006; 108(2): 473–9
Robak T, Blonski JZ, Jamroziak K, et al. Randomized comparison of cladribine plus cyclophosphamide with fludarabine plus cyclophosphamide in untreated patients with chronic lymphocytic leukemia: report of the Polish Adult Leukemia Group (PALG-CLL3) [abstract no. 2103]. Blood 2008; 112(11): 732
Hallek M, Fingerle-Rowson G, Fink AM, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia (CLL) [abstract no. 325]. Blood 2008; 112(11): 125
Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: results from the US Intergroup Phase III trial E 2997. J Clin Oncol 2007; 25(7): 799–804
Tsimberidou AM, Keating MJ, Giles FJ, et al. Fludarabine and mitoxantrone for patients with chronic lymphocytic leukemia. Cancer 2004; 100(12): 2583–91
Bosch F, Ferrer A, Villamor N, et al. Fludarabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia: high response rate and disease eradication. Clin Cancer Res 2008; 14(1): 155–61
Robak T, Błoński JZ, Kasznicki M, et al. Cladribine combined with cyclophosphamide is highly effective in the treatment of chronic lymphocytic leukemia. Hematol J 2002; 3(5): 244–50
Robak T, Blonski JZ, Wawrzyniak E, et al. Activity of cladribine combined with cyclophosphamide in frontline therapy for chronic lymphocytic leukemia with 17p13.1/TP53 deletion: report from the Polish Adult Leukemia Group. Cancer 2009; 115(1): 94–100
Robak T, Błoński JZ, Kasznicki M, et al. Cladribine combined with cyclophosphamide and mitoxantrone as frontline therapy in chronic lymphocytic leukemia. Leukemia 2001; 15(10): 1510–6
Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol 2001; 19(8): 2153–64
O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukaemia. J Clin Oncol 2001; 19(8): 2165–70
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23(18): 4079–88
Tam CS, O’Brien S, Wierda W, et al. Long term results of the fludarabine, cyclophosphamide and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008; 112(4): 975–80
Faderl S, Wierda W, O’Brien S, et al. Fludarabine, cyclophosphamide, mitoxantrone plus rituximab (FCM-R) in frontline CLL <70 years. Leuk Res. Epub 2009 Jul 29
Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 2007; 109(2): 405–11
Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective analysis of CALGB 9712 and CALGB 9011. Blood 2005; 105(1): 49–53
Tsimberidou AM, Tam C, Abruzzo LV, et al. Chemo-immunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer 2009; 115(2): 373–80
Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009; 27(4): 498–503
Bosch F, Abrisqueta P, Villamor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol. Epub 2009 Aug 24
Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 2007; 109(11): 2291–8
Gribben JG, Hallek M. Rediscovering alemtuzumab: current and emerging therapeutic roles. Br J Haematol 2009; 144(6): 818–31
Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. Epub 2009 Aug 20
Osterborg A, Dyer MJ, Bunjes D, et al. Phase II multi-center study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J Clin Oncol 1997; 15(4): 1567–74
Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99(10): 3554–61
Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002; 100(3): 768–73
Osterborg A, Fassas AS, Anagnostopoulos A, et al. Humanized CD52 monoclonal antibody Campath-1H as first line treatment in chronic lymphocytic leukemia. Br J Haematol 1996; 93(1): 151–3
Wierda WG, O’Brien SM, Faderl SH, et al. CFAR, an active frontline regimen for high-risk patients with CLL, including those with del 17p [abstract no. 2095]. Blood (ASH Annual Meeting Abstracts) 2008; 112(11): 729
Osterborg A, Foa R, Bezares RF, et al. Management guidelines for the use of alemtuzumab in chronic lymphocytic leukemia. Leukemia. Epub 2009 Jul 23
Keating M, Coutré S, Rai K, et al. Management guidelines for use of alemtuzumab in B-cell chronic lymphocytic leukemia. Clin Lymphoma 2004; 4(4): 220–7
Robak T. Alemtuzumab for B-cell chronic lymphocytic leukemia. Expert Rev Anticancer Ther 2008; 8(7): 1033–51
Thursky KA, Worth LJ, Seymour JF, et al. Spectrum of infection risk and recommendations for prophylaxis and screening among patients lymphoproliferative disorders treated with alemtuzumab. Br J Haematol 2006; 132(1): 3–12
Lin TS, Flinn IW, Lucas MS, et al. Filgrastim and alemtuzumab (Campath-1H) for refractory chronic lymphocytic leukemia. Leukemia 2005; 19(7); 1207–10
Lundin J, Porwit-MacDonald A, Rossmann ED, et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukemia. Leukemia 2004; 18(3); 1207–10
Alinari L, Lapalombella R, Andritsos L, et al. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 2007; 26(25): 3644–53
O’Brien S, Ravandi F, Riehl T, et al. Valganciclovir prevents cytomegalovirus reactivation in patients receiving alemtuzumab-based therapy. Blood 2008; 111(4): 1816–9
Rai KR, Byrd JC, Peterson BL, et al. A phase II trial of fludarabine followed by alemtuzumab (Campath-1H) in previously untreated chronic lymphocytic leukemia (CLL) patients with active disease. Cancer and Leukemia Group B (CALGB) Study 199 [abstract no. 772]. Blood 2002; 100 Suppl. 1: 205
Montillo M, Tedeschi A, Miqueleiz S, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol 2006; 24(15): 2337–42
Wendtner CM, Ritgen M, Schweighofer CD, et al. Consolidation with alemtuzumab in patients with chronic lymphocytic leukemia (CLL) in first remission: experience on safety and efficacy within a randomized multicenter phase III trial of the German CLL Study Group (GCLLSG). Leukemia 2004; 18(6): 1093–101
Schweighofer CD, Ritgen M, Eichhorst BF, et al. Consolidation with alemtuzumab improves progression-free survival in patients with chronic lymphocytic leukaemia (CLL) in first remission: long-term follow-up of a randomized phase III trial of the German CLL Study Group (GCLLSG). Br J Haematol 2009; 144(1): 95–8
Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low Grade Lymphoma Study Group (GLSG). Blood 2006; 108: 4003–8
Cartron G, Solal-Céligny P. Maintenance therapy for lowgrade lymphomas: has the time come? Curr Opin Oncol 2007; 19: 425–32
Thomas DA, O’Brien SM, Kantarjian HM, et al. Retreatment with fludarabine: outcome of 203 patients with relapsed or refractory CLL treated with salvage therapy [abstract no. PO84]. Hematol Cell Ther 2000; 42: 92
Robak T, Błoński JZ, Kasznicki M, et al. Re-treatment with cladribine-based regimens in relapsed patients with B-cell chronic lymphocytic leukemia. Eur J Haematol 2002; 69(1): 27–36
Juliusson G, Liliemark J. Retreatment of chronic lymphocytic leukemia with 2-chlorodeoxyadenosine (CdA) at relapse following CdA induced remission: no acquired resistance. Leuk Lymphoma 1994; 13(1–2): 75–80
Tam CS, O’Brien S, Lerner S, et al. The natural history of fludarabine-refractory chronic lymphocytic leukemia patients who fail alemtuzumab or have bulky lymphadenopathy. Leuk Lymphoma 2007; 48(10): 1931–9
Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer 2009; 115(13): 2824–36
Robak T, Blonski JZ, Kasznicki M, et al. The effect of subsequent therapies in patients with chronic lymphocytic leukemia previously treated with prednisone and either cladribine or chlorambucil. Haematologica 2005; 90(7): 994–6
Weiss MA, Maślak PG, Jurcic JG, et al. Pentostatin and cyclophosphamide: an effective new regimen in previously treated patients with chronic lymphocytic leukemia. J Clin Oncol 2003; 21(7): 1278–84
Bosch F, Ferrer A, Lopez-Guillermo A, et al. Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukemia. Br J Haematol 2002; 119(4): 976–84
Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005; 23(18): 4070–8
Robak T, Moiseev S, Dmoszynska A, et al. Rituximab, fludarabine, and cyclophosphamide (R-FC) prolongs progression free survival in relapsed or refractory chronic lymphocytic leukemia (CLL) compared with FC alone: final results from the international randomized phase III REACH trial [abstract no. 157420]. Blood 2008; 112(11): LBA–1
Robak T, Smolewski P, Cebula B, et al. Rituximab plus cladribine with or without cyclophosphamide in patients with relapsed or refractory chronic lymphocytic leukemia. Eur J Haematol 2007; 79(2): 107–13
Lamanna N, Kalaycio M, Maslak P, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol 2006; 24(10): 1575–81
Karlsson C, Lundin J, Kimby E, et al. Phase II study of subcutaneous alemtuzumab without dose escalation in patients with advanced-stage, relapsed chronic lymphocytic leukaemia. Br J Haematol 2009; 144(1): 78–85
McCune SL, Gockerman JP, Moore JO, et al. Alemtuzumab in relapsed or refractory chronic lymphocytic leukemia and prolymphocytic leukaemia. Leuk Lymphoma 2002; 43(5): 1007–11
Rawstron AC, Kennedy B, Moreton P, et al. Early prediction of outcome and response to alemtuzumab therapy in chronic lymphocytic leukemia. Blood 2004; 103(6): 2027–31
Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2009; 27(24): 3994–4001
Kennedy B, Rawstron A, Carter C, et al. Campath-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukemia. Blood 2002; 99(6): 2245–7
Elter T, Borchmann P, Schulz H, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol 2005; 23(28): 7024–31
Wierda WG, O’Brien SM, Faderl SH, et al. Combined cyclophosphamide, fludarabine, alemtuzumab, and rituximab (CFAR), an active regimen for heavily treated patients with CLL [Abstract 31]. Blood 2006; 108: 14a
Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol 2003; 82(12): 759–65
Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma 2007; 48(12): 2412–7
Castro JE, Sandoval-Sus JD, Bole J, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008; 22(11): 2048–53
Dungarwalla M, Evans SO, Riley U, et al. High dose methylprednisolone and rituximab is an effective therapy in advanced refractory chronic lymphocytic leukemia resistant to fludarabine therapy. Haematologica 2008; 93(3): 475–6
Quinn JP, Mohamedbhai S, Chipperfield K, et al. Efficacy of rituximab in combination with steroids in refractory chronic lymphocytic leukemia. Leuk Lymphoma 2008; 49(10): 1995–8
Robak T. Novel drugs for chronic lymphoid leukemias: mechanism of action and therapeutic activity. Curr Med Chem 2009; 16(18): 2212–34
Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase I-II study. Blood 2008; 111(3): 1094–100
Byrd JC, O’Brien SO, Flinn IW, et al. Phase 1 study of lumiliximab with detailed pharmacokinetic and pharmacodynamic measurements in patients with relapsed or refractory chronic lymphocytic leukemia. Clin Cancer Res 2007; 13 (15 Pt 1): 4448–55
O’Brien SM, Cunningham CC, Golenkov AK, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide in patients with advanced chronic lymphocytic leukemia. J Clin Oncol 2005; 23(30): 7697–702
Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood 2007; 109(2): 399–404
Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol 2006; 24(34): 5343–9
Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 2008; 111(11): 52–61
O’Brien SM, Claxton DF, Crump M, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood 2009; 113(2): 299–305
Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med 2008; 359(6): 613–26
Robak T. Novel monoclonal antibodies for the treatment of chronic lymphocytic leukemia. Curr Cancer Drug Targets 2008; 8(2): 156–71
Robak T. Ofatumumab, a human monoclonal antibody for lymphoid malignancies and autoimmune disorders. Curr Opin Mol Ther 2008; 10(3): 294–309
Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs 2009; 18(4): 491–500
Osterborg A, Kipps T, Mayer J, et al. Ofatumumab (HuMax-CD20), a novel CD20 monoclonal antibody, is an active treatment for patients with CLL refractory to fludarabine and alemtuzumab or bulky fludarabine-refractory disease: results from the planned interim analysis of an international pivotal trial [abstract no. 328]. Blood 2008; 112(11): 126
Robak T. GA-101, a third-generation, humanized and glyco-engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. Curr Opin Investig Drugs 2009; 10(6): 588–96
Umana P, Moessner E, Bruenker P, et al. GA101, a novel humanized type II CD20 antibody with glycoengineered Fc and enhanced cell death induction, exhibits superior anti-tumor efficacy and superior tissue B cell depletion in vivo [abstract no. 2348]. Blood 2007; 110(11): 694a
Salles GA, Morschhauser F, Cartron G, et al. A phase I/II study of RO5072759 (GA101) in patients with relapsed/refractory CD20+ malignant disease [abstract no. 234]. Blood 2008; 112(11): 93
Pathan NI, Chu P, Hariharan K, et al. Mediation of apoptosis by and antitumor activity of lumiliximab in chronic lymphocytic leukemia cells and CD23+ lymphoma cell lines. Blood 2008; 111(3): 1594–602
Pathan N, Byrd J, Hariharan K, et al. Lumiliximab (anti-CD23 antibody) mediates apoptosis and antitumor activity in chronic lymphocytic leukemia (CLL) cells and CD23+ lymphoma cell lines. 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 2007; 25 (18S; Jun 20 Suppl.): 3039
Byrd JC, Castro J, O’Brien S, et al. Comparison results from a phase 1/2 study of lumiliximab (anti-CD23) in combination with FCR for patients with relapsed CLL with published FCR results [abstract no. 32]. Blood 2006; 108(11): 14a
Grdisa M. Influence of CD40 ligation on survival and apoptosis of B-CLL cells in vitro. Leuk Res 2003; 27(10): 951–6
Hsu SJ, Esposito LA, Aukerman SL, et al. HCD122 an antagonist human anti-CD40 monoclonal antibody, inhibits tumor growth in xeno-graft models of human diffuse large B-cell lymphoma, a subsets of non-Hodgkin’s lymphoma [abstract no. 2519]. Blood 2006; 108(11): 713
Tong X, Aukerman SL, Lin K, et al. A non-internalizing anti-CD40 antibody CHIR-0.12.12, blocks CD40 L-induced cytokine production and mediated greater ADCC than rituximab in primary CLL cells [abstract no. 2964]. Blood 2005; 106(11): 831a
Byrd JC, Flinn IW, Khan KD, et al. Pharmacokinetics and pharmacodynamics from a first-in-human phase 1 dose escalation study with antagonist anti-CD40 antibody HCD122 (formerly CHIR-12.12) in patients with relapsed and refractory chronic lymphocytic leukemia [abstract no. 2837]. Blood 2006; 108(11): 803a
Kelley SK, Gelzleichter T, Xie D, et al. Preclinical pharmacokinetics, pharmacodynamics, and activity of a humanized anti-CD40 antibody (SGN-40) in rodents and non-human primates. Br J Pharmacol 2006; 148(8): 1116–23
Law CL, Gordon KA, Collier J, et al. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res 2005; 65(18): 8331–9
Tai YT, Catley LP, Mitsiades CS, et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res 2004; 64(8): 2846–52
Stockmeyer B, Schiller M, Repp R, et al. Enhanced killing of B lymphoma cells by granulocyte colonystimulating factor-primed effector cells and Hu 1D10-a humanized human leucocyte antigen DR antibody. Br J Hematol 2002; 118(4): 959–67
Lin TS, Stock W, Lucas MS, et al. A phase 1 dose escalation study of apolizumab (Hu1D10) using a stepped up dosing schedule in patients with chronic lymphocytic leukemia (CLL) and acute lymphocytic leukemia (ALL) [abstract no. 3167]. Blood 2002; 100(11): 802a
Hegde U, White T, Stetler-Stevenson M, et al. Phase I study of combination rituximab (CD20) and apolizumab (Hu1D10) monoclonal antibody therapy in previously treated: B-cell lymphoma and chronic lymphocytic leukemia [abstract no. 1389]. Blood 2002; 100(11): 358a
Czuczman MS, Thall A, Witzig TE, et al. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J Clin Oncol 2005; 23: 4390–8
Hariharan K, Anderson D, Leigh B, et al. Therapeutic activity of IDEC-114 (anti-CD80) and rituximab (Rituxan) in B cell lymphoma [abstract no. 2549]. Blood 2001; 98(11): 608a
Hariharan KH, Murphy T, Clanton D, et al. Combining galiximab with the chemotherapeutic agents fludarabine or doxorubicin improves efficacy in animal models of lymphoma. 2007 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 2007; 25: (18S; Jun 20 Suppl.): 3040
Leonard J, Friedberg J, Younes A, et al. A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory follicular lymphoma. Ann Oncol 2007; 18(7): 1216–23
Zhao XB, Biswas S, Mone A, et al. Novel anti-CD37 small modular immunopharmaceutical (SMIP) induces B-cell-specific, caspase-independent apoptosis in human CLL cells [abstract no. 2515]. Blood 2004; 104: 689a
Cerveny CG, Grosmaire L, Nilsson C, et al. In vitro and in vivo anti-B cell lymphoma activities of TRU-016. J Clin Oncol2008; 26 (May 20 Suppl.): 3074
Zhao XB, Trupti J, Lapalombella R, et al. NK cells contribute significantly to the innate immune effector role of CD37-specific SMIP in CLL and NHL [abstract no. 135]. Blood 2006; 108(11): 44a
Lapalombella R, Zhao XB, Baum PR, et al. Role of CD37SMIP a novel engineered small modular immunopharmaceutical in the treatment of CLL [abstract no. 801]. Blood 2007; 110(11): 246a
Andritsos L, Furman R, Flinn IW, et al. A phase I trial of TRU-016, an anti-CD37 small modular immunopharmaceutical (SMIP) in relapsed and refractory CLL [abstract no. 3017]. J Clin Oncol 2009; 27(15s): 26
Chanan-Khan A, Porter CW. Immunomodulating drugs for chronic lymphocytic leukemia. Lancet Oncol 2006; 7(6): 80–8
Ferrajoli A, O’Brien S, Wierda W, et al. Lenalidomide as initial treatment of elderly patients with chronic lymphocytic leukemia (CLL) [abstract no. 45]. Blood 2008; 112(11): 23
Chanan-Khan A, Miller KC, Takeshita K, et al. Results of a phase 1 clinical trial of thalidomide in combination with fludarabine as initial therapy for patients with treatment-requiring chronic lymphocytic leukemia (CLL). Blood 2005; 106(10): 3348–52
Laurenti L, Piccioni P, Tarnani M, et al. Low-dose thalidomide in combination with oral fludarabine and cyclophosphamide is ineffective in heavily pretreated patients with chronic lymphocytic leukemia. Leuk Res 2007; 31(2): 253–6
Chen C, Paul H, Xu W, et al. A phase II study of lenalidomide in previously untreated, symptomatic chronic lymphocytic leukemia (CLL) [abstract no. 44]. Blood 2008; 112(11): 23
Andritsos LA, Johnson AJ, Lozanski G, et al. Higher doses of lenalidomide are associated with unacceptable toxicity including life threatening tumor flare in patients with chronic lymphocytic leukemia. J Clin Oncol 2008; 26(15): 2519–25
Robak T, Lech-Maranda E, Korycka A, et al. Purine nucleoside analogs as immunosuppressive and antineoplastic agents: mechanism of action and clinical activity. Curr Med Chem 2006; 13(26): 3165–89
Lech-Maranda E, Korycka A, Robak T. Clofarabine as a novel nucleoside analogue approved to treat patients with haematological malignancies: mechanism of action and clinical activity. Mini Rev Med Chem 2009; 9(7): 805–12
Galmarini CM. Drug evaluation: forodesine-PNP inhibitor for the treatment of leukemia, lymphoma and solid tumor. IDrugs 2006; 9(10): 712–22
Korycka A, Lech-Marańda E, Robak T. Novel purine nucleoside analogues for hematological malignancies. Recent Pat Anticancer Drug Discov 2008; 3(2): 123–36
Cheson BD. Oblimersen for the treatment of patients with chronic lymphocytic leukemia. Ther Clin Risk Manag 2007; 3(5): 855–70
Klasa RJ, Gillum AM, Klem RE, et al. Oblimersen Bcl-2 antisense: facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev 2002; 12(3): 193–213
O’Brien S, Moore JO, Boyd TE, et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol 2007; 25(9): 1114–20
O’Brien S, Moore JO, Boyd TE, et al. 5-Year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. Epub 2009 Sep 8
Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel bcl-2 homology domain-3 mimetic GX15-070 (obatoclax). Cancer Res 2008; 68(9): 3413–20
Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A 2007; 104(49): 19512–7
Paoluzzi L, Gonen M, Bhagat G, et al. The BH3-only mimetic ABT-737 synergizes the antineoplastic activity of proteasome inhibitors in lymphoid malignancies. Blood 2008; 112(7): 2906–16
Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable bcl-2 family inhibitor. Cancer Res 2008; 68(9): 3421–8
Wilson WH, Tulpule A, Levine AM, et al. A phase 1/2a study evaluating the safety, pharmacokinetics and efficacy of ABT-263 in subjects with refractory or relapsed lymphoid malignancies [abstract no. 1371]. Blood 2007; 110(11): 412a
Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed nuclear cell death delimits platelet life span. Cell 2007; 128(6): 1173–86
Paoluzzi L, Gonen M, Gardner JR, et al. Targeting Bcl-2 family members with the BH3 mimetic AT-101 markedly enhances the therapeutic effects of chemotherapeutic agents in in vitro and in vivo models of B-cell lymphoma. Blood 2008; 111(11): 5350–8
Kline MP, Rajkumar SV, Timm MM, et al. R-(−)-gossypol (AT-101) activates programmed cell death in multiple myeloma cells. Exp Hematol 2008; 36(5): 568–76
James DF, Mervis R, Mosadeghi R, et al. AT-101, a pan-inhibitor of Bcl-2 family anti-aoptotic proteins antagonizes the protective effect conferred by nurse-like cells on primary chronic lymphocytic leukemia cells [abstract no. 2100]. Blood 2006; 108(11): 595a
Castro J, Olivier L, Rober AA, et al. A phase II, open label study of AT-101 in combination with rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia [abstract no. 2838]. Blood 2006; 108(11): 803a
Christian BA, Grever MR, Byrd JC, et al. Flavopiridol in the treatment of chronic lymphocytic leukemia. Curr Opin Oncol 2007; 19(6): 573–8
Kitada S, Zapata JM, Andreeff M, et al. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood 2000; 96(2): 393–7
Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leuk Res 2005; 29(11): 1253–7
Byrd JC, Peterson BL, Gabrilove J, et al. Cancer and Leukemia Group B. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805. Cancer Res 2005; 11(11): 4176–81
Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood 2009 19; 113(12): 2637–45
Lin TS, Herema NA, Lozanski G. Flavopiridol (alvocidib) induces durable responses in relapsed chronic lymphocytic leukemia (CLL) patients with high-risk cytogenetic abnormalities [abstract no. 46]. Blood 2008; 112(11): 23
Mutter R, Wills M. Chemistry and clinical biology of the bryostatins. Bioorg Med Chem 2000; 8(8): 1841–60
Roberts JD, Smith MR, Feldman EJ, et al. Phase I study of bryostatin 1 and fludarabine in patients with chronic lymphocytic leukemia and indolent (non-Hodgkin’s) lymphoma. Clin Cancer Res 2006; 12(19): 5809–16
Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk 5. Eur J Biochem 1997; 243(1–2): 527–36
Hahntow IN, Schneller F, Oelsner M, et al. Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia 2004; 18(4): 747–55
Alvi AJ, Austen B, Weston VJ, et al. Novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by down-regulation of genes involved in transcription regulation and survival. Blood 2005; 105(11): 4484–91
Chen YB, LaCasce AS. Enzastaurin. Expert Opin Investig Drugs 2008; 17(6): 939–44
Ma S, Rosen ST. Enzastaurin. Curr Opin Oncol 2007; 19(6): 590–5
Robertson MJ, Kahl BS, Vose JM, et al. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2007; 25(13): 1741–6
Morschhauser F, Seymour JF, Kluin-Nelemans HC, et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann Oncol 2008; 19(2): 247–53
Costa LJ. Aspects of mTOR biology and the use of mTOR inhibitors in non-Hodgkin’s lymphoma. Cancer Treat Rev 2007; 33(1): 78–84
Janus A, Robak T, Smolewski P. The mammalian target of the rapamycin (mTOR) kinase pathway: its role in tumouri-genesis and targeted antitumour therapy. Cell Mol Biol Lett 2005; 10(3): 479–98
Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005; 4(12): 988–1004
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006; 441(7092): 424–30
Cohen EE. mTOR: the mammalian target of replication. J Clin Oncol 2008; 26(3): 348–9
Smolewski P. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anticancer Drugs 2006; 17(5): 487–94
Aleskog A, Norberg M, Nygren P, et al. Rapamycin shows anticancer activity in primary chronic lymphocytic leukemia cells in vitro, as single agent and in drug combination. Leuk Lymphoma 2008; 49(12): 2333–43
Decker T, Sandherr M, Goetze K, et al. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann Hematol 2009; 88(3): 221–7
Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res 2006; 12(17): 5165–73
Kirchner GI, Meier-Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin Pharmacokinet 2004; 43(2): 83–95
Mita MM, Mita AC, Chu QS, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol 2008; 26(3): 361–7
Rizzieri DA, Feldman E, Dipersio JF, et al. A phase 2 clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res 2008; 14(9): 2756–62
Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent tensirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol 2005; 23(23): 5347–56
Gora-Tybor J, Robak T. Targeted drugs in chronic myeloid leukemia. Curr Med Chem 2008; 15(29): 3036–51
Lin K, Glenn MA, Harris RJ, et al. c-Abl expression in chronic lymphocytic leukemia cells: clinical and therapeutic implications. Cancer Res 2006; 66(15): 7801–9
Aloyz R, Grzywacz K, Xu Z, et al. Imatinib sensitizes CLL lymphocytes to chlorambucil. Leukemia 2004; 18(3): 409–14
Veldurthy A, Patz M, Hagist S, et al. The kinase inhibitor dasatinib induces apoptosis in chronic lymphocytic leukemia cells in vitro with preference for a subgroup of patients with unmutated IgVH genes. Blood 2008; 112(4): 1443–52
Amrein L, Hernandez TA, Ferrario C, et al. Dasatinib sensitizes primary chronic lymphocytic leukaemia lymphocytes to chlorambucil and fludarabine in vitro. Br J Haematol 2008; 143(5): 698–706
Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J Clin Invest 2005; 115(2): 369–78
Lee M, Kim JY, Koh WS. Apoptotic effect of PP2 a Src tyrosine kinase inhibitor, in murine B cell leukemia. J Cell Biochem 2004; 93(3): 629–38
Amrein PC, Attar EC, Akvorian T, et al. A phase II study of dasatinib in relapsed and refractory chronic lymphocytic leukemia (CLL/SLL) [abstract no. 3126]. Blood 2007; 110(11): 920a
Bergsagel PL. Syk to death. Blood 2009; 113(11): 2371
Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia 2009; 23(4): 686–97
Baudot AD, Jeandel PY, Mouska X, et al. The tyrosine kinase Syk regulates the survival of chronic lymphocytic leukemia B cells through PKCdelta and proteasome-dependent regulation of Mcl-1 expression. Oncogene 2009; 28(37): 3261–73
Buchner M, Fuchs S, Prinz G, et al. Spleen tyrosine kinase (SYK) is overexpressed and represents a potential therapeutic target in chronic lymphocytic leukaemia [abstract no. 543]. Blood 2008; 112(11): 203
Friedberg JW, Sharman J, Schaefer-Cutillo J, et al. Fostamatinib disodium (FosD), an oral inhibitor of Syk, is well-tolerated and has significant clinical activity in diffuse large B cell lymphoma (DLBCL) and chronic lymphocytic leukemia (SLL/CLL) [abstract no. 3]. Blood 2008; 112(11): 3
Gribben JG. Stem cell transplantation in chronic lymphocytic leukemia. Biol Blood Marrow Transplant 2008; 15 (1 Suppl.): 53–8
Tam CS, Khouri I. The role of stem cell transplantation in the management of chronic lymphocytic leukaemia. Hematol Oncol 2009; 27(2): 53–60
Milligan DW, Fernandes S, Dasgupta R, et al. Results of the MRC pilot study show autografting for younger patients with chronic lymphocytic leukemia is safe and achieves a high percentage of molecular response. Blood 2005; 105(1): 397–404
Michallet M, Archimbaud E, Bandini G, et al. HLA identical sibling bone marrow transplants for chronic lymphocytic leukemia. Ann Intern Med 1996; 124(3): 311–5
Michallet M, Michallet AS, Le QH, et al. Conventional HLA-identical sibling bone marrow transplantation is able to cure chronic lymphocytic leukemia [abstract no. 1729]. Blood 2003; 102(11): 474a
Khouri IF. Reduced-intensity regimens in allogenic stem-cell transplantation for non-Hodgkin lymphoma and chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2007; 390–7
Sorror ML, Maris MB, Sandmaier BM, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol 2005; 23(16): 3819–29
Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol 2008; 26(30): 4912–20
Dreger P, Brand R, Milligan D, et al. Reduced-intensity conditioning lowers treatment-related mortality of allogeneic stem cell transplantation for chronic lymphocytic leukemia: a population-matched analysis. Leukemia 2005; 19(6): 1029–33
Peres E, Braun T, Krijanovski O, et al. Reduced intensity versus full myeloablative stem cell transplant for advanced CLL. Bone Marrow Transplant. Epub 2009 Mar 23
Dreger P, Corradini P, Kimby E, et al. Indication for allogenic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia 2007; 21(1): 12–7
Palma M, Adamson L, Hansson L, et al. Development of a dendritic cell-based vaccine for chronic lymphocytic leukemia. Cancer Immunol Immunother 2008; 57(11): 1705–10
Kater AP, van Oers MH, Kipps TJ. Cellular immune therapy for chronic lymphocytic leukemia. Blood 2007; 110(8): 2811–8
Wierda WG, Kipps TJ, Keating MJ. Novel immune-based treatment strategies for chronic lymphocytic leukemia. J Clin Oncol 2005; 23(26): 6325–32
Biagi E, Rousseau R, Yvon E, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin Cancer Res 2005; 11 (19 Pt 1): 6916–23
Hus I, Rolinski J, Tabarkiewicz J, et al. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia 2005; 19(9): 1621–7
Acknowledgements
This work was supported in part by a grant from the Medical University of Lodz (No. 503-1093-1) and by the Foundation for the Development of Diagnostics and Therapy, Warsaw, Poland. Professor Tadeusz Robak has received honoraria for consulting from Hoffmann-LaRoche, GlaxoSmithKline and Celgene, and research grants from Hoffmann-LaRoche and GlaxoSmithKline. Dr Krzysztof Jamroziak and Dr Pawel Robak have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robak, T., Jamroziak, K. & Robak, P. Current and Emerging Treatments for Chronic Lymphocytic Leukaemia. Drugs 69, 2415–2449 (2009). https://doi.org/10.2165/11319270-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319270-000000000-00000