Abstract
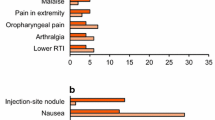
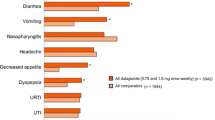
Exenatide is the first incretin mimetic, introduced into type 2 diabetes mellitus therapy in 2005, with first approval in the US. It is a glucagon-like peptide-1 (GLP-1) receptor agonist that can be used for treatment by twice-daily injection. A long-acting release formulation for once-weekly injection is in clinical development. Clinical studies and postmarketing experience with exenatide have shown a significant and sustained reduction in glycosylated haemoglobin (HbA1c) by approximately 1% together with other gylcaemic parameters without an intrinsic risk for hypoglycaemias, and a reduction in bodyweight by 5.3 kg in 82 weeks. Blood pressure and lipids are also favourably affected, but hard cardiovascular endpoints are not yet available. Animal studies show an improvement of β-cell function and an increase in β-cell mass after exenatide treatment. The most frequent adverse events associated with exenatide therapy are nausea and antibody formation (both approximately 40%). Nausea, mostly mild and transient, was responsible for a 6% dropout rate in clinical studies. A recent review on the association of acute pancreatitis with exenatide treatment showed no increased risk (relative risk 1.0; 95% CI 0.6, 1.7). This review gives a benefit-risk assessment of exenatide.





Similar content being viewed by others
References
Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12: 75–80
Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 2006; 116: 1802–12
Mitrakou A, Kelley D, Mokan M, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992; 326: 22–9
Matthews DR, Cull CA, Stratton IM, et al. UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med 1998; 15: 297–303
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427–43 99
Gallwitz B. Therapies for the treatment of type 2 diabetes mellitus based on incretin action. Minerva Endocrinol 2006; 31: 133–47
Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16: 75–85
Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993; 91: 301–7
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696–705
Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002; 359: 824–30
Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–65
Gallwitz B. Exenatide in type 2 diabetes: treatment effects in clinical studies and animal study data. Int J Clin Pract 2006; 60: 1654–61
Eng J, Kleinman WA, Singh L, et al. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom: further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267: 7402–5
Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 2007; 30: 1487–93
Gedulin BR, Smith P, Prickett KS, et al. Dose-response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia 2005; 48: 1380–5
Gallwitz B. Liraglutide. Drugs Future 2008; 33: 1–8
Vilsboll T, Knop FK. Long-acting GLP-1 analogs for the treatment of type 2 diabetes mellitus. BioDrugs 2008; 22: 251–7
Giannoukakis N. BIM-51077, a dipeptidyl peptidase-IV-resistant glucagon-like peptide-1 analog. Curr Opin Investig Drugs 2007; 8: 842–8
Pratley RE. Overview of glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Medscape J Med 2008; 10: 171
Goke R, Fehmann HC, Linn T, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 1993; 268: 19650–5
Holst JJ. Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia 2006; 49: 253–60
Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005; 62: 173–81
Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 2003; 26: 2370–7
Yoo BK, Triller DM, Yoo DJ. Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother 2006; 40: 1777–84
Bray GM. Exenatide. Am J Health Syst Pharm 2006; 63: 411–8
Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003; 88: 3082–9
Zheng D, Ionut V, Mooradian V, et al. Exenatide sensitizes insulin-mediated whole-body glucose disposal and promotes uptake of exogenous glucose by the liver. Diabetes 2009; 58: 352–9
Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 2007; 92: 1249–55
Meier JJ. Beta cell mass in diabetes: a realistic therapeutic target? Diabetologia 2008; 51: 703–1
Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005; 90: 5991–7
Bunck MC, Diamant M, Corner A, et al. Beta-cell function and glycemic control following one year exenatide therapy, and after 12 week wash out, in patients with type 2 diabetes [abstract]. Diabetes 2008; 57 Suppl. 1: A32
Byetta(exenatide injection): US prescribing information. San Diego (CA): Amylin Pharmaceuticals, 2007
Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004; 27: 2628–35
DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28: 1092–100
Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–91
Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006; 8: 436–47
Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab 2006; 8: 419–28
Zinman B, Hoogwerf BJ, Duran Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007; 146: 477–85
Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled 100 type 2 diabetes: a randomized trial. Ann Intern Med 2005; 143: 559–69
Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007; 50: 259–67
Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008; 372: 1240–50
Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24: 275–86
FDA alert: information for healthcare professionals — exenatide (marketed as Byetta). Rockville (MD): Food and Drug Administration [online]. Available from URL: http://www.fda.gov/cder/drug/InfoSheets/HCP/exenatide2008HCP.htm [Accessed 2008 Aug 18]
Cure P, Pileggi A, Alejandro R. Exenatide and rare adverse events. N Engl J Med 2008; 358: 1969–70, discussion 1971–2
Ahmad SR, Swann J. Exenatide and rare adverse events. N Engl J Med 2008; 358: 1970–1, discussion 1971–2
Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009; 25: 1019–27
US FDA, US Department of Health & Human Services. Byetta (exenatide): renal failure [online]. Available from URL: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm188703.htm [Accessed 2009 Dec 2]
Russell-Jones D. Molecular, pharmacological and clinical aspects of liraglutide, a once-daily human GLP-1 analogue. Mol Cell Endocrinol 2009; 297: 137–40
Nauck MA, Ratner RE, Kapitza C, et al. Treatment with the human once-weekly GLP-1 analogue taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes mellitus inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care 2009; 32: 1237–43
Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in anti-diabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30: 1448–60
Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29: 139–53
Barnett A. Exenatide. Expert Opin Pharmacother 2007; 8: 2593–608
Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006; 12: 694–9
Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109: 962–5
Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009; 58: 975–83
Mussig K, Oncu A, Lindauer P, et al. Effects of intravenous glucagon-like peptide-1 on glucose control and hemodynamics after coronary artery bypass surgery in patients with type 2 diabetes. Am J Cardiol 2008; 102: 646–7
Hirata K, Kume S, Araki S, et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun 2009; 380: 44–9
Matthaei S, Bierwirth R, Fritsche A, et al. Medikamentöse antihyperglykämische Therapie des Diabetes mellitus Typ2. Diabetologie 2009; 4: 1–33
Matthaei S, Bierwirth R, Fritsche A, et al. Medical anti-hyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes 2009; 117: 522–57
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203
Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473–81
Vilsboll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 2007; 30: 1608–10
Acknowledgements
Professor Gallwitz has been paid honoraria for lectures on various topics related to diabetes by Eli Lilly. He has been participating and is currently involved as investigator or principal investigator in clinical studies sponsored by Eli Lilly. No sources of funding were used to assist in the preparation of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallwitz, B. Benefit-Risk Assessment of Exenatide in the Therapy of Type 2 Diabetes Mellitus. Drug-Safety 33, 87–100 (2010). https://doi.org/10.2165/11319130-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319130-000000000-00000