Abstract
Background
A substantial portion of patients at risk for acute coronary syndrome (ACS) are >65 years old. Prasugrel is a novel antiplatelet agent approved for the treatment of ACS patients undergoing percutaneous coronary intervention, and will be used in this population.
Objective
This study assessed the effect of age ≥65 years on the pharmacokinetics (PK) and pharmacodynamics (PD) of the active metabolite (R-138727) of prasugrel in healthy subjects taking aspirin (acetylsalicylic acid).
Methods
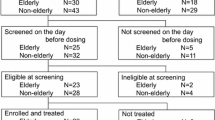
This was an open-label, single-sequence trial conducted in a single clinical research centre in the UK. A total of 17 subjects aged 65–80 years and 15 subjects aged 20–39 years received a prasugrel 5-mg once-daily maintenance dose for 10 days followed by 10-mg once daily maintenance doses for 10 days. All subjects also received aspirin 75 mg daily. Serial blood samples were collected pre-dose and at various times post-dose for measurement of the active metabolite of prasugrel in plasma on days 10 and 20, following the last 5- and 10-mg prasugrel dose, respectively. PK parameters of the active metabolite of prasugrel included area under the plasma concentration-time curve (AUC) from time zero to the time of the last quantifiable concentration (AUClast), maximum plasma concentration (Cmax) and time to Cmax (tmax). Maximal platelet aggregation (MPA), assessed by light transmission aggregometry using adenosine diphosphate (ADP) 20 µmol/L, was assessed at baseline and on day 10 (5-mg maintenance dose) and day 20 (10-mg maintenance dose). Bleeding times (BTs) were determined on days −5, 1, 10, 11, 20 and 21 using a modified Ivy technique.
Results
AUClast did not differ significantly between age groups. The steady-state trough MPA to ADP 20 µmol/L during 10-mg maintenance dosing was 30.6% and 26.6% in elderly and young subjects, respectively. Mean MPA was consistently higher in elderly subjects compared with young subjects; however, differences were generally less than ten percentage points. BTs did not differ between the two populations during 5-mg maintenance dosing; however, during 10-mg maintenance dosing, BTs were up to 67% longer in young compared with elderly subjects. A higher frequency of minor bleeding during 10-mg maintenance dosing was observed in elderly subjects compared with young subjects.
Conclusions
These data indicate that prasugrel PK and MPA were similar in healthy subjects regardless of age. Compared with younger subjects, elderly subjects had shorter BTs but a greater frequency of mild bleeding-related adverse events.







Similar content being viewed by others
References
Sharis PJ, Cannon CP, Loscalzo J. The antiplatelet effects of ticlopidine and clopidogrel. Ann Intern Med 1998; 129(5): 394–405
Algaier I, Jakubowski JA, Asai F, et al. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost 2008; 6(11): 1908–14
Yusuf S, Zhao F, Mehta SR, et al., on behalf of the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345(7): 494–502
Steinhubl SR, Berger PB, Mann JT III, et al., on behalf of the Clopidogrel for the Reduction of Events During Observation (CREDO) Investigators. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288(19): 2411–20
Popma JJ, Berger P, Ohman EM, et al. Antithrombotic therapy during percutaneous coronary intervention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126Suppl. 3: 576–99S
Rehmel JL, Eckstein JA, Farid NA, et al. Interactions of two major metabolites of prasugrel, a thienopyridine anti-platelet agent, with the cytochromes P450. Drug Metab Dispos 2006; 34(4): 600–7
Williams ET, Jones KO, Ponsler GD, et al. The biotransformation of prasugrel, a new thienopyridine prodrug, by the human carboxylesterases 1 and 2. Drug Metab Dispos 2008; 36(7): 1227–32
Farid NA, Smith RL, Gillespie TA, et al. The disposition of prasugrel, a novel thienopyridine, in humans. Drug Metab Dispos 2007; 35(7): 1096–104
Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 2007; 153(1): 66.e9–16
Farid NA, Payne CD, Small DS, et al. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther 2007; 81(5): 735–41
Payne CD, Li YG, Small DS, et al. Increased active metabolite formation explains the greater platelet inhibition with prasugrel compared to high-dose clopidogrel. J Cardiovasc Pharmacol 2007; 50(5): 555–62
Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur Heart J 2006; 27(10): 1166–73
Wiviott SD, Antman EM, Winters KJ, et al., for the JUMBO-TIMI 26 Investigators. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005; 111(25): 3366–73
Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12 receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J 2008; 29(1): 21–30
Wiviott SD, Braunwald E, McCabe CH, et al., on behalf of the TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357(20): 2001–15
Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 2007; 5(12): 2429–36
Mega JL, Close SL, Wiviott et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009; 119(19): 2553–60
Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360(4): 354–62
Farid NA, Payne CD, Ernest CS 2nd, et al. Prasugrel, a new thienopyridine antiplatelet drug, weakly inhibits cytochrome P450 2B6 in humans. J Clin Pharmacol 2008; 48(1): 53–9
Sugidachi A, Asai F, Ogawa T, et al. The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. Br J Pharmacol 2000; 129(7): 1439–46
Farid NA, McIntosh M, Garofolo F, et al. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2007; 21(2): 169–79
Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol 2008; 48(4): 475–84
Small DS, Farid NA, Li YG, et al. Effect of ranitidine on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. Curr Med Res Opin 2008; 24(8): 2251–7
Data on file, Daiichi Sankyo, Inc. and Eli Lilly and Company, 2007
Boldt J, Huttner I, Suttner S, et al. Changes of haemostasis in patients undergoing major abdominal surgery: is there a difference between elderly and younger patients?. Br J Anaesth 2001; 87(3): 435–40
Boldt J, Haisch G, Kumle B, et al. Does coagulation differ between elderly and younger patients undergoing cardiac surgery?. Intensive Care Med 2002; 28(4): 466–71
Acknowledgements
Funding for this study was supported by Daiichi Sankyo Company, Limited, Tokyo, Japan and Eli Lilly and Company, Indianapolis, IN, USA. Appreciation is expressed to employees of Eli Lilly and Company: Keri Poi, Ph.D. for writing assistance and Jan Short for formatting and submission of the manuscript. David S. Small, Rebecca E. Wrishko, C. Steven Ernest II, Lan Ni, Kenneth J. Winters, Nagy A. Farid, Ying G. Li and Chris Payne are employees of Eli Lilly and Company. Daniel E. Salazar is an employee of Daiichi Sankyo, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Small, D.S., Wrishko, R.E., Ernest, C.S. et al. Effect of Age on the Pharmacokinetics and Pharmacodynamics of Prasugrel during Multiple Dosing. Drugs Aging 26, 781–790 (2009). https://doi.org/10.2165/11315780-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11315780-000000000-00000