Abstract
Background and Objective
Drugs undergoing enterohepatic circulation (EHC) are associated with typical pharmacokinetic characteristics such as multiple-peak phenomenon in the plasma concentration-time profile and prolongation of the apparent elimination half-life (t1/2). Currently, versatile pharmacokinetic models are lacking that could test the hypothesis of an EHC for observed multiple-peak phenomenon in pharmacokinetic profiles and its quantitative contribution. The aim of this analysis was to accomplish a model that is able to describe typical plasma concentration-time profiles of compounds undergoing EHC using data from intravenous studies of tesofensine and meloxicam. In addition, the developed model should be able to quantify the contribution of an EHC to the pharmacokinetics by determining the influence of interrupting the EHC of tesofensine and meloxicam to various extents.
Methods
Two studies were investigated retrospectively for model development and model evaluation. Twentyone healthy subjects received a single 6-hour infusion of tesofensine (0.3, 0.6, 0.9, 1.2 mg) in a double-blind, randomized, placebo-controlled, single rising-dose study. Twelve healthy subjects were treated in a randomized, crossover study with meloxicam 30 mg as a single dose given intravenously (bolus) either alone or concomitantly with cholestyramine. The EHC model was developed based on data from the tesofensine study, where EHC is suspected. Model evaluation was performed with data from the meloxicam trial. Modelling and simulation analyses were performed using the software programs NONMEM, SAS and Berkeley Madonna.
Results
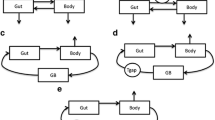
Plasma concentration-time profiles of tesofensine were best described by a three-compartment model (absorption, central and gallbladder) with first-order elimination. The release of the bile compartment was controlled by a sine function model, switching the bile compartment periodically on and off using the actual clock time as the control element. A four-compartment model (absorption, central, peripheral and gallbladder) with first-order elimination and the sine function for gallbladder control described the meloxicam data best. Coadministration of cholestyramine resulted in a predicted 56% withdrawal of meloxicam from the EHC process causing a reduction in the t1/2 from ∼19 hours to ∼12 hours.
Conclusion
A quantitative EHC model was successfully developed that was capable of describing the multiple peaks in plasma concentration-time profiles of tesofensine and meloxicam very well. Additionally, the model successfully quantified the observed results for an interruption of the meloxicam EHC. The model offers an in silico method to support an EHC hypothesis using standard pharmacokinetic data and might help to guide dosing recommendations of compounds undergoing EHC.









Similar content being viewed by others
References
Roberts MS, Magnusson BM, Burczynski FJ, et al. Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin Pharmacokinet 2002; 41: 751–90
Gabrielsson J, Weiner D. Pharmacokinetic/pharmacodynamic data analysis: concepts and applications. 3rd ed. Stockholm: Taylor & Francis, 2000
Mahmood I, Sahajwalla C. Interspecies scaling of biliary excreted drugs. J Pharm Sci 2002; 91: 1908–14
Mahmood I. Interspecies scaling of biliary excreted drugs: a comparison of several methods. J Pharm Sci 2005; 94: 883–92
Steimer JL, Plusquellec Y, Guillaume A, et al. A time-lag model for pharmacokinetics of drugs subject to enterohepatic circulation. J Pharm Sci 1982; 71:297–302
Hoglund P, Ohlin M. Effect modelling for drugs undergoing enterohepatic circulation. Eur J Drug Metab Pharmacokinet 1993; 18: 333–8
Khalil FA, Hanocq M, Dubois J. A new method to estimate parameters of pharmacokinetics with enterohepatic circulation. Eur J Drug Metab Pharmacokinet 1993; 18: 131–9
Funaki T. Enterohepatic circulation model for population pharmacokinetic analysis. J Pharm Pharmacol 1999; 51: 1143–8
Ezzet F, Krishna G, Wexler DB, et al. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther 2001; 23: 871–85
Wajima T, Yano Y, Oguma T. A pharmacokinetic model for analysis of drug disposition profiles undergoing enterohepatic circulation. J Pharm Pharmacol 2002; 54: 929–34
Wang YMC, Reuning RH. An experimental design strategy for quantitating complex pharmacokinetic models: enterohepatic circulation with time-varying gallbladder emptying as an example. Pharm Res 1992; 9: 169–77
Huntjens DRH, Strougo A, Chain A, et al. Population pharmacokinetic modelling of the enterohepatic recirculation of diclofenac and rofecoxib in rats. Br J Pharmacol 2008; 153: 1072–84
Jiao Z, Ding JJ, Shen J, et al. Population pharmacokinetic modelling for enterohepatic circulation of mycophenolic acid in healthy Chinese and the influence of polymorphisms in UGT1A9. Br J Clin Pharmacol 2008; 65: 893–907
Lehr T, Staab A, Tillmann C, et al. Contribution of the active metabolite M1 to the pharmacological activity of tesofensine in vivo: a pharmacokineticpharmacodynamic modelling approach. Br J Pharmacol 2008; 153: 164–74
Thatte U. NS-2330 NeuroSearch. Curr Opin Investig Drugs 2001; 2: 1592–4
Bara-Jimenez W, Dimitrova T, Sherzai A, et al. Effect of monoamine reuptake inhibitor NS 2330 in advanced Parkinson’s disease. Mov Disord 2004; 19:1183–6
Lehr T, Staab A, Tillmann C, et al. Population pharmacokinetic modelling of NS2330 (tesofensine) and its major metabolite in patients with Alzheimer’s disease. Br J Clin Pharmacol 2007; 64: 36–48
Busch U, Heinzel G, Narjes H. The effect of cholestyramine on the pharmacokinetics of meloxicam, a new nonsteroidal anti-inflammatory drug (NSAID), in man. Eur J Clin Pharmacol 1995; 48: 269–72
Türck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol 1996; 35: 13–6
Türck D, Busch U, Heinzel G, et al. Clinical pharmacokinetics of meloxicam. Arzneimittelforschung 1997; 47: 253–8
Davies NM, Skjodt NM. Clinical pharmacokinetics of meloxicam: a cyclooxygenase-2 preferential nonsteroidal anti-inflammatory drug. Clin Pharmacokinet 1999; 36: 115–26
Gates BJ, Nguyen TT, Setter SM, et al. Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety. Expert Opin Pharmacother 2005; 6: 2117–40
Beal SL, Sheiner LB. NONMEM users guide: conditional estimation methods. San Francisco (CA): University of California, 1998
Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control 1974; 19: 716–23
Jonsson EN, Karlsson MO. Xpose-An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 1999; 58: 51–64
Sheiner LB. Analysis of pharmacokinetic data using parametric models: III, hypothesis tests and confidence intervals. J Pharmacokinet Biopharm 1986; 14: 539–55
Montgomery DC, Peck EA, Vinters HV. Introduction to linear regression analysis. Chichester (NY): Wiley, 2001
Parke J, Holford NHG, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 1999; 59: 19–29
Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 1997; 37: 486–95
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN): a Perl module for NONMEM related programming. Comput Methods Programs Biomed 2004; 75: 85–94
Post TM, Freijer JI, Ploeger BA, et al. Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn 2008; 35: 185–202
Shaffer EA. Control of gall-bladder motor function. Aliment Pharmacol Ther 2000; 14: 2–8
Fisher RS, Stelzer F, Rock E, et al. Abnormal gallbladder emptying in patients with gallstones. Digest Dis Sci 1982; 27: 1019–24
Rozman B. Clinical pharmacokinetics of leflunomide. Clin Pharmacokinet 2002; 41:421–30
Acknowledgements
The authors wish to thank Ole Østerberg for his very helpful contribution. This study was financially supported by Boehringer Ingelheim Pharma GmbH and Co. KG, Biberach an der Riss, Germany. Charlotte Kloft received research funding from Boehringer Ingelheim Pharma. Thorsten Lehr, Alexander Staab, Christiane Tillmann, Dirk Trommeshauser, Hans-Guenter Schaefer are current employees at Boehringer Ingelheim Pharma GmbH and Co. KG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lehr, T., Staab, A., Tillmann, C. et al. A Quantitative Enterohepatic Circulation Model. Clin Pharmacokinet 48, 529–542 (2009). https://doi.org/10.2165/11313370-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11313370-000000000-00000