Abstract
Immune globulin subcutaneous 20% is a new high-concentration (200 g/L) solution of highly purified human IgG (≥98%) indicated in the EU and the US for antibody replacement therapy in patients with primary immunodeficiency with antibody deficiency, and in the EU for replacement therapy in humoral immunodeficiency secondary to myeloma or chronic lymphocytic leukaemia.
Immune globulin subcutaneous 20% is formulated with L-proline, which imparts long-term stability at room temperature and a relatively low viscosity.
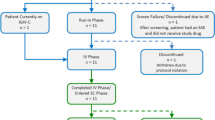
In two pivotal phase III trials in stably treated patients with primary immunodeficiency, immune globulin subcutaneous 20% at weekly subcutaneous dosages either equivalent to each patient’s previous intravenous or subcutaneous replacement therapy, or providing equivalent systemic exposure to previous intravenous therapy, produced mean serum IgG trough levels equal to or greater than pre-study levels. In each trial, there were no serious bacterial infections during treatment throughout the 28-week or 12-month efficacy periods. The rates of infectious episodes, days missed from work/school, days hospitalized or days with antibiotics were low.
Immune globulin subcutaneous 20% was generally well tolerated. A high proportion of patients experienced local infusion-site reactions, but infusion-related systemic adverse events were relatively infrequent. Most adverse events were of mild or moderate intensity and did not interfere with therapy.


Similar content being viewed by others
References
Merck & Co. The Merck manual online: overview of immunodeficiency disorders. 2008 Sep [online]. Available from URL: http://www.merckmanuals.com/professional/immunology_allergic_disorders/immunodeficiency_disorders/overview_of_immunodeficiency_disorders.html [Accessed 2012 May 6]
Jolles S, Sleasman JW. Subcutaneous immunoglobulin replacement therapy with Hizentra®, the first 20% SCIG preparation: a practical approach. Adv Ther 2011; 28 (7): 521–33
Jolles S, Bernatowska E, de Gracia J, et al. Efficacy and safety of Hizentra® in patients with primary immunodeficiency after a dose-equivalent switch from intravenous or subcutaneous replacement therapy. Clin Immunol 2011; 141 (1): 90–102
Berger M. Choices in IgG replacement therapy for primary immune deficiency diseases: subcutaneous IgG vs. intravenous IgG and selecting an optimal dose. Curr Opin Allergy Clin Immunol 2011; 11 (6): 532–8
Gardulf A, Nicolay U. Replacement IgG therapy and self-therapy at home improve the health-related quality of life in patients with primary antibody deficiencies. Curr Opin Allergy Clin Immunol 2006 Dec; 6 (6): 434–42
Berger M. L-proline-stabilized human IgG: Privigen® 10% for intravenous use and Hizentra® 20% for subcutaneous use. Immunother 2011; 3 (2): 163–76
Maeder W, Lieby P, Sebald A, et al. Local tolerance and stability up to 24 months of a new 20% proline-stabilized polyclonal immunoglobulin for subcutaneous administration. Biologicals 2011; 39 (1): 43–9
European Medicines Agency. Hizentra (human normal immunoglobulin [SCIg]): summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002127/WC500107057.pdf [Accessed 2012 May 4]
CSL Behring. Hizentra, immune globulin subcutaneous (human), 20% liquid: US prescribing information [online]. Available from URL: http://www.hizentra.com/docs/hizentraPI.pdf [Accessed 2012 May 4]
Stucki M, Schäfer W, Hostettler T, et al. Pathogen safety of a new 20% liquid immunoglobulin product [abstract no. 331]. J Allergy Clin Immunol 2009; 123 (2 Suppl. 1): S89
Stucki M, Boschetti N, Schäfer W, et al. Investigations of prion and virus safety of a new liquid IVIG product. Biologicals 2008 Jul; 36 (4): 239–47
Berger M. Incidence of infection is inversely related to steady-state (trough) serum IgG level in studies of subcutaneous IgG in PIDD. J Clin Immunol 2011; 31 (5): 924–6
Orange JS, Grossman WJ, Navickis RJ, et al. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol 2010 Oct; 137 (1): 21–30
Wasserman RL, Melamed I, Nelson RP, et al. Pharmaco-kinetics of subcutaneous IgPro 20 in patients with primary immunodeficiency. Clin Pharmacokinet 2011; 50 (6): 405–14
Berger M, Rojavin M, Kiessling P, et al. Pharmacokinetics of subcutaneous immunoglobulin and their use in dosing of replacement therapy in patients with primary immunodeficiencies. Clin Immunol 2011; 139 (2): 133–41
Thépot S, Malphettes M, Gardeur A, et al. Immunoglobulin dosage and switch from intravenous to subcutaneous immunoglobulin replacement therapy in patients with primary hypogammaglobulinemia: decreasing dosage does not alter serum IgG levels. J Clin Immunol 2010; 30 (4): 602–6
Hagan JB, Fasano MB, Spector S, et al. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro 20, in patients with primary immunodeficiency. J Clin Immunol 2010; 30 (5): 734–45
Nelson RP, Melamed I, Stein MR, et al. Safety, tolerability, and efficacy of Hizentra over an extended period for the treatment of primary immunodeficiency disease [abstract no. 314]. J Allergy Clin Immunol 2012; 129 (2 Suppl.): AB83. Plus poster presented at the American Academy of Allergy, Asthma & Immunology Annual Meeting; 2012 Mar 2–6; Orlando (FL)
Jones CA, Rojavin M, Baggish JS. Patients with primary immunodeficiency receiving subcutaneous immune globulin Hizentra maintain health-related quality of life and treatment satisfaction in a multicentre extension study of efficacy, tolerability and safety. J Pharm Health Serv Res 2012 Mar; 3 (1): 41–7
Conley ME, Notarangelo LD, Etzioni A, representing PA-GID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Diagnostic criteria for primary immunodeficiencies. Clin Immunol 1999 Dec; 93 (3): 190–7
Borte M, Pac M, Serban M, et al. Efficacy and safety of Hizentra®, a new 20% immunoglobulin preparation for subcutaneous administration, in pediatric patients with primary immunodeficiency. J Clin Immunol 2011; 31 (5): 752–61
Quevedo TG, Mannhardt-Laakmann W, Bernatowska E, et al. Health-related quality of life of patients with primary immunodeficiency switching from intravenous IgG to a new 20% subcutaneous IgG [abstract no. F39]. Clin Immunol 2010; 135 Suppl.: S87. Plus poster presented at the 10th Annual Meeting of the Federation of Clinical Immunology Societies; 2010 Jun 24–27; Boston (MA)
Acknowledgements and Disclosures
The manuscript was reviewed by: A. Fasth, Department of Pediatrics, Institute of Clinical Sciences, University of Gothenburg, Gothenburg, Sweden; R. Wasserman, DallasAllergyImmunology, Medical City Children’s Hospital, Dallas, TX, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCormack, P.L. Immune Globulin Subcutaneous (Human) 20%. Drugs 72, 1087–1097 (2012). https://doi.org/10.2165/11209490-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209490-000000000-00000