Abstract
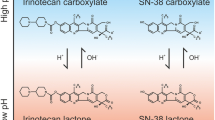
Hepatic metastases occur in about half of patients with colorectal cancer. Since hepatic metastases are often not accessible for surgery, chemotherapy of metastases is important. The most commonly used chemotherapy drugs for hepatic metastases are fluorouracil, irinotecan, and oxaliplatin. Several enzymes are known to be involved in the catabolism and anabolism of these drugs, and the activity of these enzymes varies greatly between individuals. The causes of this variation include genetic polymorphisms, different regulation between normal and cancer tissue, and the influence of chemotherapy on enzyme expression. The varying enzyme activity may have an important effect on the outcome of chemotherapy. Several studies confirm the influence of the activity of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase on the outcome of fluorouracil therapy for colorectal cancer, with higher enzyme activities predicting lower treatment efficacy. Although fewer studies are available regarding therapy of hepatic metastases, the same relationship between thymidylate synthase activity and outcome of fluorouracil therapy observed for primary colorectal cancer was found. For the other two enzymes, only a few studies are available, but the results indicate similarly that higher enzyme activity seems to be disadvantageous. The enzymes responsible for the activation, metabolism and mechanism of action of irinotecan, namely carboxylesterase 2, cytochrome P450 (CYP) 3A4, uridine diphosphate glucuronosyltransferase isoform 1A1 (UGT1A1), and topoisomerase-I, also exhibit variable inter-individual activity. Thus, there may be an association between enzyme activity and response to therapy. For instance, in patients with colorectal cancer, higher enzyme activity of topoisomerase-I seems to be predictive of a better response to irinotecan. CYP3A4 and UGT1A1 activity levels might be predictive of irinotecan toxicity rather than efficacy. The degradation of oxaliplatin is independent of potentially varying enzyme activity, but for this drug, the DNA repair enzyme ERCC1 may influence the survival time after chemotherapy. Taken together, the available data indicate the importance of the different enzyme activities on the outcome of chemotherapy of hepatic metastases in colorectal cancer. More information is needed, especially for the newer drugs irinotecan and oxaliplatin. However, the existing data are very promising in respect to the potential to guide dose and drug selection for more efficient and less toxic chemotherapy of hepatic metastases.





Similar content being viewed by others
References
Taylor I. Liver metastases from colorectal cancer: lessons from past and present clinical studies. Br J Surg 1996; 83(4): 456–60
Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol 2003; 14Suppl. 2: ii13–6
Adjei AA. A review of the pharmacology and clinical activity of new chemotherapy agents for the treatment of colorectal cancer. Br J Clin Pharmacol 1999; 48(3): 265–77
Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003; 3(5): 330–8
Metzger R, Danenberg K, Leichman CG, et al. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to 5-fluorouracil. Clin Cancer Res 1998; 4(10): 2371–6
Moghaddam A, Zhang HAT, Fan TP, et al. Thymidine phosphorylase is angiogenic and promotes tumor growth. Proc Natl Acad Sci U S A 1995 Feb; 92: 998–1002
Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet 1989; 16(4): 215–37
Young AM, Daryanani S, Kerr DJ. Can pharmacokinetic monitoring improve clinical use of fluorouracil? Clin Pharmacokinet 1999 Jun; 36(6): 391–8
Kornmann M, Link KH, Lenz HJ, et al. Thymidylate synthase is a predictor for response and resistance in hepatic artery infusion chemotherapy. Cancer Lett 1997; 118(1): 29–35
Guimbaud R, Guichard S, Dusseau C, et al. Dihydropyrimidine dehydrogenase activity in normal, inflammatory and tumour tissues of colon and liver in humans. Cancer Chemother Pharmacol 2000; 45(6): 477–82
Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol 1997; 15(10): 3223–9
Aschele C, Debernardis D, Casazza S, et al. Immunohistochemical quantitation of thymidylate synthase expression in colorectal cancer metastases predicts for clinical outcome to fluorouracil-based chemotherapy. J Clin Oncol 1999; 17(6): 1760–70
Corsi DC, Ciaparrone M, Zannoni G, et al. Predictive value of thymidylate synthase expression in resected metastases of colorectal cancer. Eur J Cancer 2002; 38(4): 527–34
Gonen M, Hummer A, Zervoudakis A, et al. Thymidylate synthase expression in hepatic tumors is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol 2003; 21(3): 406–12
Kawakami K, Salonga D, Park JM, et al. Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res 2001 Dec; 7: 4096–101
Marsh S, McKay JA, Cassidy J, et al. Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol 2001; 19(2): 383–6
Etienne MC, Chazal M, Laurent-Puig P, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol 2002; 20(12): 2832–43
Umekita N, Tanaka S, Abe H, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase activity in a metastatic liver tumor from colorectal cancer. Gan To Kagaku Ryoho 2000; 27(12): 1883–5
Katona C, Kralovanszky J, Rosta A, et al. Putative role of dihydropyrimidine dehydrogenase in the toxic side effect of 5-fluorouracil in colorectal cancer patients. Oncology 1998; 55(5): 468–74
van Kuilenburg AB, Haasjes J, Richel DJ, et al. Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res 2000; 6(12): 4705–12
Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res 2000; 6(4): 1322–7
Graf W, Pahlman L, Bergstrom R, et al. The relationship between an objective response to chemotherapy and survival in advanced colorectal cancer. Br J Cancer 1994 Sep; 70(3): 559–63
Ridge SA, Sludden J, Brown O, et al. Dihydropyrimidine dehydrogenase pharmacogenetics in Caucasian subjects. Br J Clin Pharmacol 1998 Aug; 46(2): 151–6
Etienne MC, Lagrange JL, Dassonville O, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol 1994 Nov; 12(11): 2248–53
Miyamoto S, Ochiai A, Boku N, et al. Discrepancies between the gene expression, protein expression, and enzymatic activity of thymidylate synthase and dihydropyrimidine dehydrogenase in human gastrointestinal cancers and adjacent normal mucosa. Int J Oncol 2001; 18(4): 705–13
Aschele C, Debernardis D, Tunesi G, et al. Thymidylate synthase protein expression in primary colorectal cancer compared with the corresponding distant metastases and relationship with the clinical response to 5-fluorouracil. Clin Cancer Res 2000; 6(12): 4797–802
van Triest B, Pinedo HM, Blaauwgeers JL, et al. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res 2000; 6(3): 1063–72
Kawakami K, Ishida Y, Danenberg KD, et al. Functional polymorphism of the thymidylate synthase gene in colorectal cancer accompanied by frequent loss of heterozygosity. Jpn J Cancer Res 2002; 93: 1221–9
Uchida K, Hayashi K, Kawakami K, et al. Loss of heterozygosity at the thymidylate synthase (TS) locus on chromosome 18 affects tumor response and survival in individuals heterozygous for a 28-bp polymorphism in the TS gene. Clin Cancer Res 2004; 10: 433–9
Luccioni C, Beaumatin J, Bardot V, et al. Pyrimidine nucleotide metabolism in human colon carcinomas: comparison of normal tissues, primary tumors and xenografts. Int J Cancer 1994; 58(4): 517–22
McLeod HL, Sludden J, Murray GI, et al. Characterization of dihydropyrimidine dehydrogenase in human colorectal tumours. Br J Cancer 1998; 77(3): 461–5
Johnston SJ, Ridge SA, Cassidy J, et al. Regulation of dihydropyrimidine dehydrogenase in colorectal cancer. Clin Cancer Res 1999; 5(9): 2566–70
Ikeguchi M, Hirooka Y, Makino M, et al. Dihydropyrimidine dehydrogenase activity of cancerous and non-cancerous tissues in liver and large intestine. Oncol Rep 2001 May–Jun; 8(3): 621–5
Wang TL, Diaz LA, Romans K, et al. Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc Natl Acad Sci U S A 2004; 101(9): 3089–94
Aschele C, Debernardis D, Bandelloni R, et al. Thymidylate synthase protein expression in colorectal cancer metastases predicts for clinical outcome to leucovorin-modulated bolus or infusional 5-fluorouracil but not methotrexate-modulated bolus 5-fluorouracil. Ann Oncol 2002; 13(12): 1882–92
Bastian G, Scitz JF, Moutardier V, et al. Impact of UFT on tumoral TS and DPD levels in colorectal cancer. Oncology (Huntingt) 2000 Oct 14; 10Suppl. 9: 35–7
Punt CJ. New drugs in the treatment of colorectal carcinoma. Cancer 1998; 83(4): 679–89
Saltz LB, Douillard JY, Pirotta N, et al. Irinotecan plus fluorouracil/leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist 2001; 6(1): 81–91
Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Curr Med Chem 2003; 10(1): 41–9
Luo FR, Paranjpe PV, Guo A, et al. Intestinal transport of irinotecan in Caco-2 cells and MDCK II cells overexpressing efflux transporters Pgp, cMOAT, and MRP1. Drug Metab Dispos 2002; 30(7): 763–70
Chu XY, Suzuki H, Ueda K, et al. Active efflux of CPT-11 and its metabolites in human KB-derived cell lines. J Pharmacol Exp Ther 1999; 288(2): 735–41
Guemei AA, Cottrell J, Band R, et al. Human plasma carboxylesterase and butyrylcholinesterase enzyme activity: correlations with SN-38 pharmacokinetics during a prolonged infusion of irinotecan. Cancer Chemother Pharmacol 2001; 47(4): 283–90
Rivory LP, Bowles MR, Robert J, et al. Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochem Pharmacol 1996 Oct 11; 52(7): 1103–11
Dodds HM, Haaz MC, Riou JF, et al. Identification of a new metabolite of CPT-11 (irinotecan): pharmacological properties and activation to SN-38. J Pharmacol Exp Ther 1998; 286(1): 578–83
Santos A, Zanetta S, Cresteil T, et al. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin Cancer Res 2000; 6(5): 2012–20
Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11): role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 1998; 101(4): 847–54
Sanghani SP, Quinney SK, Fredenburg TB, et al. Carboxylesterase expressed in human colon tumor tissue and their role in CPT-11 hydrolysis. Clin Cancer Res 2003; 9: 4983–91
Hosokawa M, Endo T, Fujisawa M, et al. Interindividual variation in carboxylesterase levels in human liver microsomes. Drug Metab Dispos 1995 Oct; 23(10): 1022–7
Canal P, Gay C, Dezeuze A, et al. Pharmacokinetics and pharmacodynamics of irinotecan during a phase II clinical trial in colorectal cancer. Pharmacology and Molecular Mechanisms Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol 1996; 14(10): 2688–95
Guichard S, Terret C, Hennebelle I, et al. CPT-11 converting carboxylesterase and topoisomerase activities in tumour and normal colon and liver tissues. Br J Cancer 1999; 80(3–4): 364–70
Sy SK, Ciaccia A, Li W, et al. Modeling of human hepatic CYP3A4 enzyme kinetics, protein, and mRNA indicates deviation from log-normal distribution in CYP3A4 gene expression. Eur J Clin Pharmacol 2002; 58(5): 357–65
Massaad L, de Waziers I, Ribrag V, et al. Comparison of mouse and human colon tumors with regard to phase I and phase II drug-metabolizing enzyme systems. Cancer Res 1992; 52(23): 6567–75
Iyer L, Hall D, Das S, et al. Phenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther 1999; 65(5): 576–82
Innocenti F, Grimsley C, Das S, et al. Haplotype structure of the UDP-glucuronosyltransferase 1A1 promoter in different ethnic groups. Pharmacogenetics 2002; 12: 725–33
Ando Y, Ueoka H, Sugiyama T, et al. Polymorphisms of UDP-glucuronosyltransferase and pharmacokinetics of irinotecan. Ther Drug Monit 2002; 24(1): 111–6
Jansen WJ, Zwart B, Hulscher ST, et al. CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants. Int J Cancer 1997; 70(3): 335–40
Saltz L, Danenberg K, Paty P. High TS expression does not preclude activity of CPT-11 in colorectal cancer [abstract]. Proc Am Soc Clin Oncol 1998; 17:1080
Relling MV, Pui CH, Sandlund JT, et al. Adverse effect of anticonvulsants on efficacy of chemotherapy for acute lymphoblastic leukaemia. Lancet 2000; 356(9226): 285–90
Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 1999; 17(5): 1516–25
von Hoff DD, Rothenberg ML, Pitot HC, et al. [abstract 803]. Proc Am Soc Clin Oncol 1997; 16: 228a
Mathijssen RH, Verweij J, de Bruijn P, et al. Effects of St John’s wort on irinotecan metabolism. J Natl Cancer Inst 2002; 94(16): 1247–9
Gupta E, Lestingi TM, Mick R, et al. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhoea. Cancer Res 1994; 54(14): 3723–5
Ando Y, Saka H, Ando M, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 2000; 60(24): 6921–6
Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 2004; 8: 1382–8
Sata F, Sapone A, Elizondo G, et al. CYP3A4 allelic variants with amino acid substitutions in exons 7 and 12: evidence for an allelic variant with altered catalytic activity. Clin Pharmacol Ther 2000; 67(1): 48–56
Evans WE, McLeod HL. Pharmacogenomics-drug disposition, drug targets, and side effects. N Engl J Med 2003; 348(6): 538–49
Ando Y, Saka H, Asai G, et al. UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 1998 Aug; 9(8): 845–7
Iyer L, Das S, Janisch L, et al. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2002; 2(1): 43–7
Premawardhena A, Fisher CA, Liu YT, et al. The global distribution of length polymorphisms of the promoters of the glucuronosyltranferase 1 gene (UGT1A1): hematologic and evolutionary implications. Blood Cells Mol Dis 2003; 31(1): 98–101
Giovanella BC, Stehlin JS, Wall ME, et al. DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science 1989; 246(4933): 1046–8
Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 2003; 21(11): 2059–69
Graham MA, Lockwood GF, Greenslade D, et al. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 2000; 6(4): 1205–18
Jansman FG, Sleijfer DT, Coenen JL, et al. Risk factors determining chemotherapeutic toxicity in patients with advanced colorectal cancer. Drug Saf 2000; 23(4): 255–78
Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 2001; 19(23): 4298–304
Arnould S, Hennebelle I, Canal P, et al. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur J Cancer 2003; 39(1): 112–9
Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000; 25: 169–93
Petty RD, Nicolson MC, Kerr KM, et al. Gene expression profiling in non-small cell lung cancer: from molecular mechanisms to clinical application. Clin Cancer Res 2004; 10: 3237–48
Toffoli G, Cecchin E, Corona G, et al. Pharmacogenetics of irinotecan. Curr Med Chem Anti-Canc Agents 2003 May; 3(3): 225–37
Acknowledgments
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lentz, F., Tran, A., Rey, E. et al. Pharmacogenomics of Fluorouracil, Irinotecan, and Oxaliplatin in Hepatic Metastases of Colorectal Cancer. Am J Pharmacogenomics 5, 21–33 (2005). https://doi.org/10.2165/00129785-200505010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129785-200505010-00002